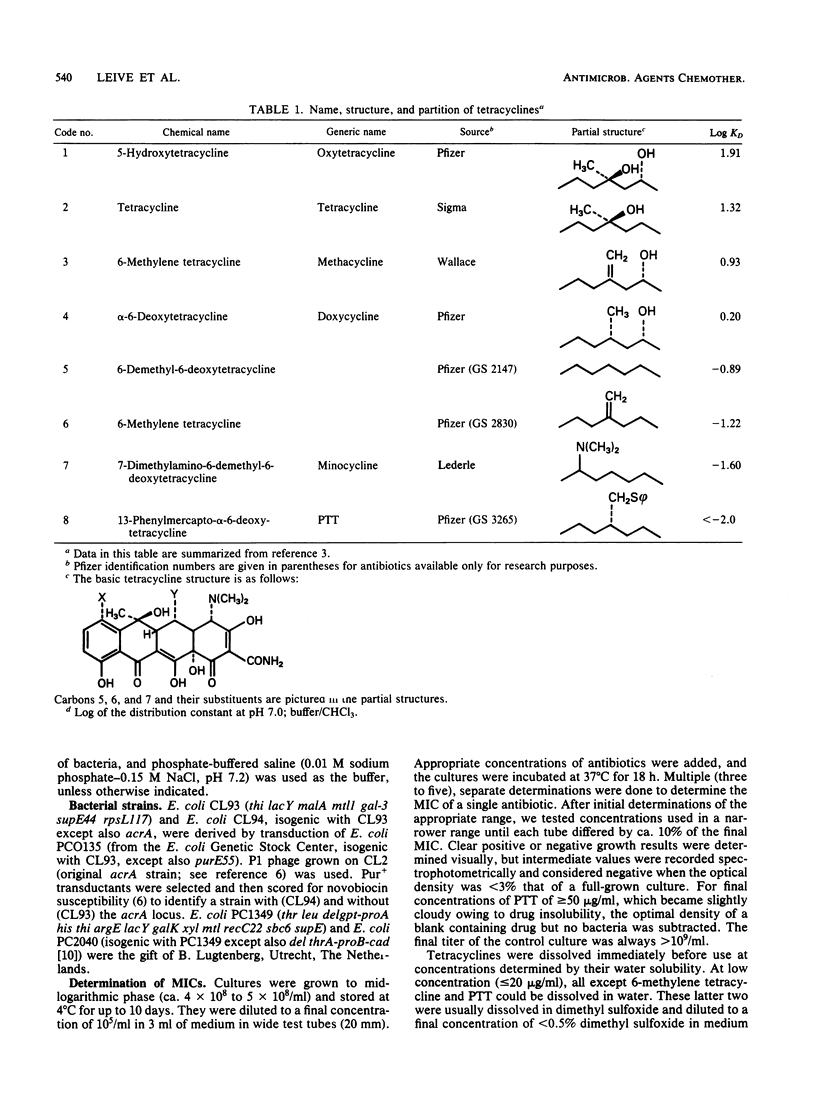
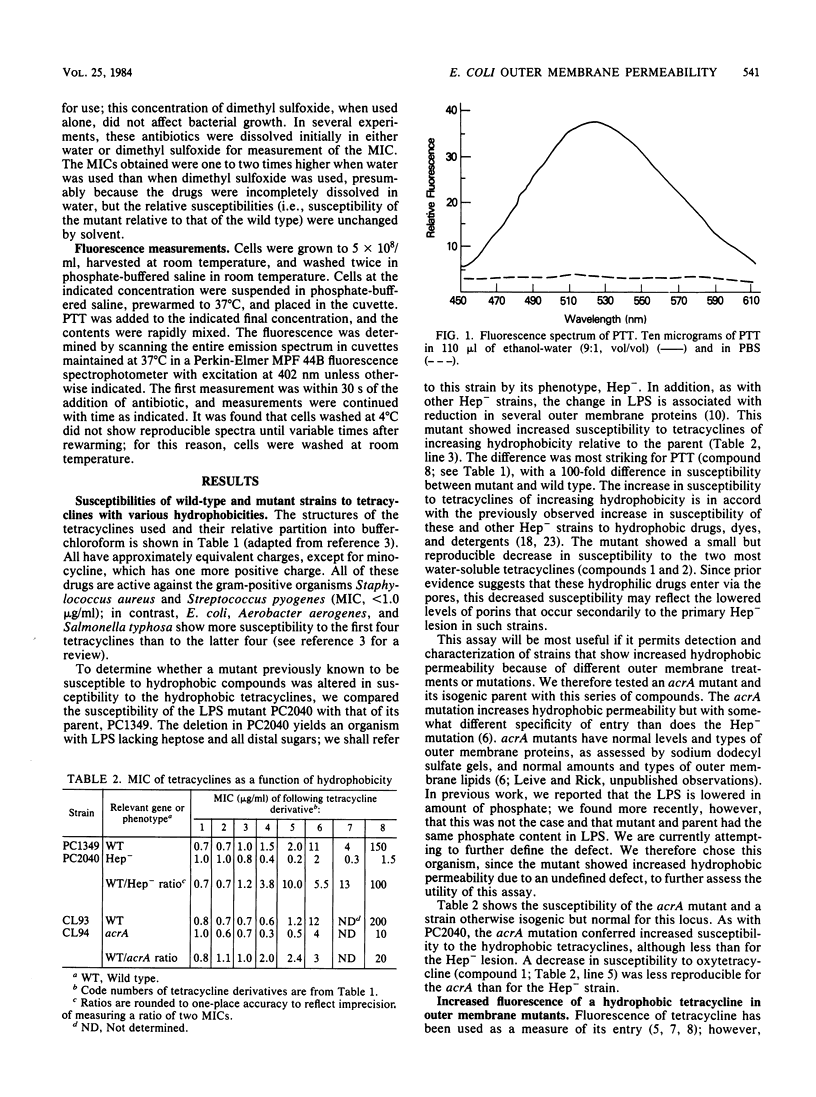
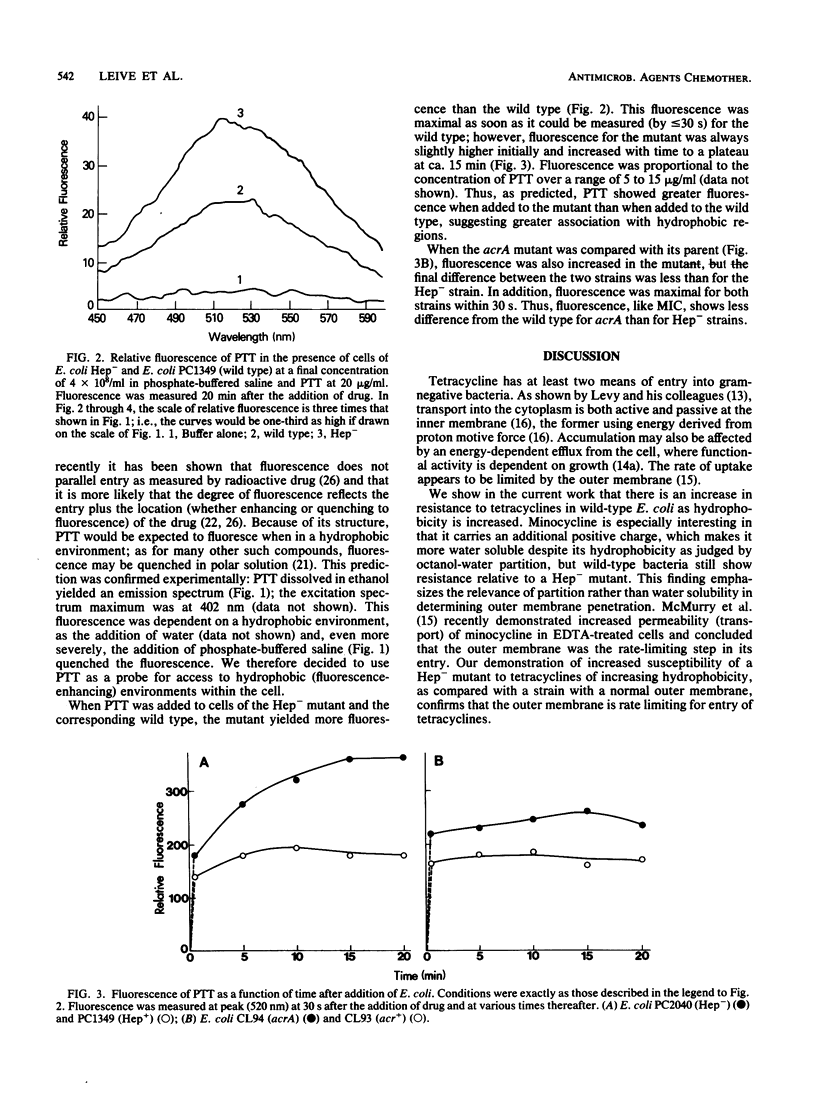
Abstract
The outer membrane of gram-negative cells excludes hydrophobic molecules and is responsible for the resistance of these cells to a number of dyes, detergents, and antibiotics. We describe a test for hydrophobic permeability in which a series of tetracyclines with various hydrophobicities are used. Normal Escherichia coli cells became more resistant as hydrophobicity was increased in this series, but mutants altered in outer membrane permeability remained susceptible. A mutant lacking all polysaccharide except 2-keto-3- deoxyoctonic acid in its lipopolysaccharide is virtually as susceptible to the hydrophobic drug 13- phenylmercapto -alpha-6- deoxytetracycline as to oxytetracycline (MIC 100 times lower than that of the wild type), and a mutant with another, as yet undefined outer membrane defect, acrA , also shows increased, although somewhat lesser, susceptibility (MIC 20 times lower than that of the wild type). Increased susceptibility to this tetracycline derivative is associated with greater fluorescence of the derivative when added to the cells, which we interpret as increased interaction of the derivative with hydrophobic domains, such as membranes, in the mutants. This series of tetracyclines may provide an assay for measuring the permeability of gram-negative organisms and their mutants to hydrophobic molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Chopra I., Eccles S. J. Diffusion of tetracycline across the outer membrane of Escherichia coli K-12: involvement of protein Ia. Biochem Biophys Res Commun. 1978 Jul 28;83(2):550–557. doi: 10.1016/0006-291x(78)91025-2. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S., Ball P. Tetracycline resistance determinants from groups A to D vary in their ability to confer decreased accumulation of tetracycline derivatives by Escherichia coli. J Gen Microbiol. 1982 Apr;128(4):689–692. doi: 10.1099/00221287-128-4-689. [DOI] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockter M. E., Magnuson J. A. Characterization of the active transport of chlorotetracycline in staphylococcus aureus by a fluorescence technique. J Supramol Struct. 1974;2(1):32–44. doi: 10.1002/jss.400020105. [DOI] [PubMed] [Google Scholar]
- Dockter M. E., Magnuson J. A. Membrane phase transitions and the transport of chlortetracycline. Arch Biochem Biophys. 1975 May;168(1):81–88. doi: 10.1016/0003-9861(75)90230-1. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- McMurry L. M., Aronson D. A., Levy S. B. Susceptible Escherichia coli cells can actively excrete tetracyclines. Antimicrob Agents Chemother. 1983 Oct;24(4):544–551. doi: 10.1128/aac.24.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Petrucci R. E., Jr, Levy S. B. Active uptake of tetracycline by membrane vesicles from susceptible Escherichia coli. Antimicrob Agents Chemother. 1981 Sep;20(3):307–313. doi: 10.1128/aac.20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Levy S. B. Two transport systems for tetracycline in sensitive Escherichia coli: critical role for an initial rapid uptake system insensitive to energy inhibitors. Antimicrob Agents Chemother. 1978 Aug;14(2):201–209. doi: 10.1128/aac.14.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Permeability of the outer membrane of bacteria. Angew Chem Int Ed Engl. 1979 May;18(5):337–350. doi: 10.1002/anie.197903373. [DOI] [PubMed] [Google Scholar]
- Samra Z., Krausz-Steinmetz J., Sompolinsky D. Transport of tetracyclines through the bacterial cell membrane assayed by fluorescence: a study with susceptible and resistant strains of Staphylococcus aureus and Escherichia coli. Microbios. 1978;21(83):7–21. [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Antibiotica-Empfindlichkeit bei S- und R-Formen von Salmonella minnesota. Naturwissenschaften. 1968 Oct;55(10):494–495. doi: 10.1007/BF00599721. [DOI] [PubMed] [Google Scholar]
- Shales S., Chopra I. Outer membrane composition in Escherichia coli and the poor activity of hydrophobic antibiotics against enteric bacteria. J Antimicrob Chemother. 1982 Apr;9(4):325–327. doi: 10.1093/jac/9.4.325. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. C., Chopra I. Limitations of a fluorescence assay for studies on tetracycline transport into Escherichia coli. Antimicrob Agents Chemother. 1983 Jan;23(1):175–178. doi: 10.1128/aac.23.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


