Abstract
The mechanisms underlying the persistence of the Staphylococcus aureus small-colony variant (SCV) are not fully elucidated. In this study, clinical thymidine-dependent SCVs displayed altered expression of citB, clpC, and arcA genes, reduced acetate catabolization, and enhanced survival. These results implicate the importance of changes in tricarboxylic acid cycle and acetic acid metabolism in SCV survival and persistence.
The persistence of Staphylococcus aureus in cystic fibrosis (CF) and other chronic diseases, such as device-related infections and osteomyelitis, has been associated with the isolation of S. aureus small-colony variants (SCVs) (9, 15, 18, 24). SCVs are slow-growing subpopulations that cause recurrent and persisting infections even in the presence of bactericidal antimicrobial therapy (16). Isolates of clinical SCVs are auxotrophic for substrates, such as menadione, hemin, thiamine, or thymidine (16). While previous work has focused on metabolic and phenotypic aspects of menadione- and/or hemin-dependent SCVs (2, 12, 14, 19, 25), most clinical SCVs isolated from the airways of CF patients were found to be thymidine-dependent SCVs (TD-SCVs) (11). SCVs display enhanced survival inside host cells, reducing susceptibility towards antimicrobial peptides; thus, they escape from innate host defense mechanisms (1, 4, 11, 13, 23). However, bacterial life or death is not only determined by host defense; rather, the intrinsic long-term growth and survival characteristics of SCVs may be pertinent for the course of disease and clinical outcome. Thus, we determined growth and survival of clinical TD-SCVs along with the corresponding metabolic and gene expression profiles.
Thymidine auxotrophism impairs exponential-phase growth but enhances stationary-phase survival in the TD-SCV.
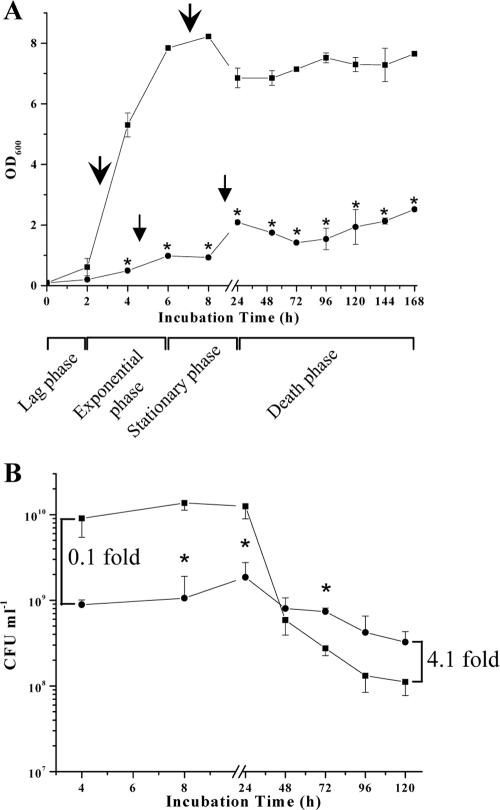
A TD-SCV S. aureus strain and its isogenic normal (non-TD) strain (strain pair 4 in reference 10), both isolated from the airways of a CF patient, were cultured in brain heart infusion (BHI) (DIFCO, Germany) broth at 37°C and aerated by shaking at 160 rpm for 1 to 7 days (168 h) (10). Normal S. aureus exhibited a short lag phase (until 2 h), followed by a rapid increase in cell density (optical density at 600 nm) during the exponential phase (2 to 6 h) (Fig. 1A). Between 8 h and 24 h (stationary phase), the cell density steadily decreased and remained constant for up to 7 days. In contrast, the TD-SCV displayed an extended lag phase throughout the exponential growth phase of the normal strain. The cell density of the TD-SCV increased between 8 h and 24 h, even though it was very low at 168 h compared to the isogenic normal strain (Fig. 1A). To assess stationary-phase survival of the TD-SCV and normal S. aureus, aliquots of bacteria (200 μl) were withdrawn at the indicated time intervals to determine viable bacterial counts on BHI agar plates (incubated at 37°C for 24 h) (Fig. 1B). CFU values of normal S. aureus coincided with growth curve results, demonstrating elevated viable bacterial counts during the exponential growth phase (4 h) through the stationary phase (8 h and 24 h), followed by a rapid decrease in viability, indicating the entrance into the death phase (24 to 120 h). In contrast, the TD-SCV displayed a significantly reduced number of viable bacterial counts (0.1-fold compared to the normal S. aureus value at 4 h). However, considering this initial growth “deficit” and relating it to the enhanced CFU number of the TD-SCV in the post-stationary phase (4.1-fold at 120 h), the relative “survival advantage” of the TD-SCV over the normal isolate could be calculated as 41.6-fold, hence a delayed entrance into the death phase.
FIG. 1.
(A) Long-term growth analysis of normal (non-TD) S. aureus and TD-SCV. Growth curves (optical density at 600 nm [OD600]) of normal S. aureus (▪) and TD-SCV (•) were determined in BHI medium under aerobic conditions. Arrows indicate the time points for total RNA extraction. The data shown are means ± standard errors of the means (error bars) for values obtained from three independent experiments. Values that are significantly different from the values for normal S. aureus (P < 0.001 by t test) are indicated (*). (B) Thymidine limitation in TD-SCV S. aureus enhances post-stationary-phase survival. Cultural aliquots were removed at different time intervals of normal S. aureus (▪) and TD-SCV (•) from the growth analysis cultures, plated on BHI agar plates, and incubated at 37°C for 24 h to 48 h. The values (CFU per milliliter) were determined in triplicate. The data shown are means ± standard deviations (error bars) for values obtained from two independent experiments. The indicated increases (0.1-fold and 4.1-fold increases) relate CFU counts of the TD-SCV with those of normal S. aureus at respective time points (4 h and 120 h) and refer to differences in viability of the TD-SCV versus normal S. aureus (please refer also to the calculated “survival advantage” in “Thymidine auxotrophism impairs exponential-phase growth but enhances stationary-phase survival in the TD-SCV.”). The comparison of the two curves as a whole shows that the curves are significantly different referring to the population as well as the incubation time (two-factor analysis of variance, P ≤ 0.001), with significant differences at select time points (*, P ≤ 0.05 compared to normal S. aureus [t test]).
Thymidine limitation delays pH recovery and ammonia accumulation in the TD-SCV.
We measured the pH of the culture supernatant of normal (non-TD) S. aureus and TD-SCV because acidic pH is an indicator of acetic acid production during normal growth in S. aureus (6, 7, 22). During the exponential growth phase, the pH of the supernatant of the normal S. aureus decreased to a pH of 6.5 (effect of glycolysis) (Fig. 2A), followed by an increase during late exponential phase, and reaching a pH of 8.5 to 9.0 during stationary phase (24 h) (consequence of amino acid catabolism generating ammonia [Fig. 2B]). In contrast, the pH of the supernatant of the TD-SCV decreased more slowly and reached a nadir pH of 6.2 at 8 h (Fig. 2A). The culture medium of TD-SCVs experienced delayed ammonia accumulation until 8 h (Fig. 2B), indicating reduced amino acid catabolism. The level of ammonia subsequently increased during the stationary phase, accompanied by a gradual increase of pH, even though these changes did not affect the final growth and survival of the TD-SCVs. However, as the reduced growth of TD-SCVs (Fig. 1A) coincides with these metabolic characteristics, the respective contributions of amino acid catabolism, reduced tricarboxylic acid (TCA) cycle metabolism (shown below) and decreased cell numbers, cannot be separated.
FIG. 2.
Determination of external pH, glucose concentration, and levels of metabolites (ammonia, acetate, and lactate) in the culture supernatant. At different time intervals, supernatants of normal (non-TD) S. aureus (▪) and TD-SCV (•), cultivated in BHI medium, were analyzed for (A) external pH and (B) ammonia, (C) glucose, (D) acetate, and (E) lactate concentrations. External pH data are means ± standard deviations (error bars) for values obtained from three independent experiments. Values that are significantly different from the values for normal S. aureus (t test) are indicated (*, P < 0.01; **, P < 0.001). Ammonia, glucose, acetate, and lactate data are representative results of at least two independent experiments.
TD-SCVs lack acetate catabolism and concomitantly display decreased citB and clpC transcription.
Previously, we have shown that an S. aureus clpC mutant exhibited an enhanced stationary-phase survival due to the lack of a functional TCA cycle which is required to facilitate the catabolism of secondary metabolites (e.g., acetate) during post-exponential-phase growth (5, 20, 22). On the basis of these observations and on the fact that TD-SCV demonstrated enhanced post-stationary-phase survival, we hypothesized that this delayed entrance into the death phase was dependent upon a delayed catabolism of secondary metabolites. To test this hypothesis, we (i) determined glucose and acetate concentrations in the supernatant of the normal (non-TD) and TD-SCV cultures and (ii) determined expression levels of the genes encoding aconitase (citB) (the enzyme involved in the first step of the TCA cycle) and the ClpC ATPase (clpC) (encoding a heat shock protein; shown to be involved in maintaining/regulating the TCA cycle [5]). In accordance with published data (22), glucose was completely consumed in the normal S. aureus by 4 h (Fig. 2C). The TD-SCV catabolized glucose more rapidly when the value was calculated as glucose consumed per CFU count. Derepression of the TCA cycle occurs upon depletion of readily catabolizable carbon source(s) like glucose and/or glutamate coinciding with the depletion of acetate from the culture medium (22). Consequently, the normal S. aureus cells started to catabolize acetate after 4 h, and by 7 h, acetate was completely depleted from the culture medium (Fig. 2D). In striking contrast, the TD-SCVs failed to catabolize acetate until 120 h (Fig. 2D), suggesting that thymidine auxotrophy resulted in delayed TCA cycle function. Moreover, lactate was only briefly detected between 2 and 8 h (Fig. 2E), excluding induction of a fermentative metabolism as seen in a hemB SCV (12).
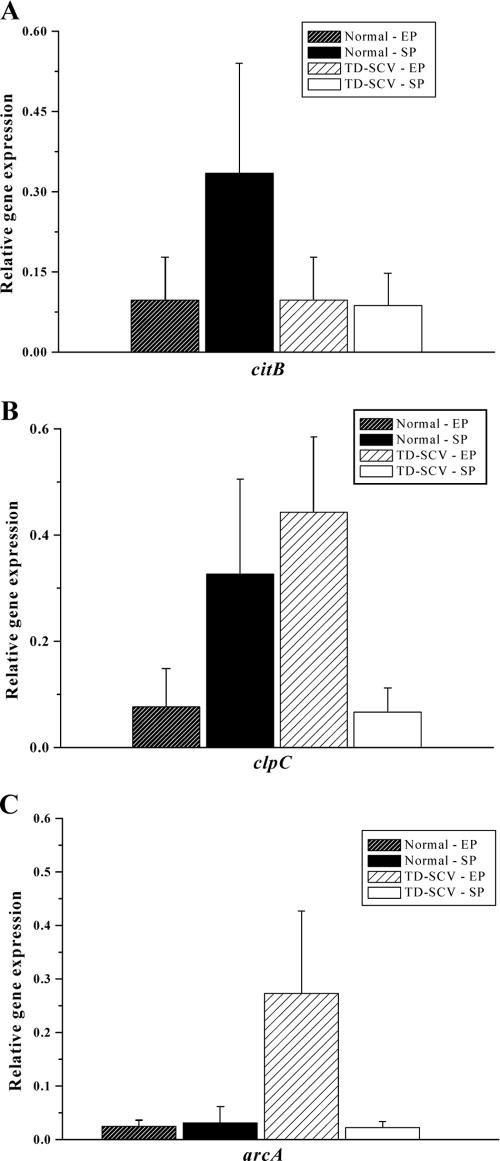
mRNA transcript levels of citB and clpC relative to gyrB expression were determined by real-time RT-PCR as previously described (5). Total RNA of normal (non-TD) S. aureus and TD-SCV was isolated as indicated by the growth curve (Fig. 1A), and the same amount of total RNA (8 μg) for each strain was used for synthesis of cDNA, both at exponential phase and stationary phase. Exponential-phase transcription of citB was repressed in both strains as shown previously (5, 12, 22) (Fig. 3A). During stationary phase, the increase in transcription of citB in normal S. aureus was associated with a decrease in acetate levels (Fig. 2D). In contrast, relative transcript levels of citB in TD-SCV were reduced fourfold during the stationary growth phase compared to the normal strain. In contrast to citB, clpC transcription levels were up-regulated during exponential-phase growth in the TD-SCV probably due to thymidine limitation stress (Fig. 3B). Interestingly, during stationary phase, clpC gene transcription was reduced fivefold in the S. aureus TD-SCV versus the isogenic normal strain (Fig. 3B), suggesting yet another mechanism (i.e., reduced maintenance of aconitase activity) contributing to impaired TCA cycle function.
FIG. 3.
Real-time reverse transcription-PCR quantification. Gene expression of (A) citB (encoding aconitase), (B) clpC (encoding ClpC ATPase) and (C) arcA (encoding arginine deiminase) was determined in normal (non-TD) S. aureus and TD-SCV at exponential growth phase (EP) and stationary growth phase (SP) by real-time reverse transcription-PCR. The transcript quantities relative to the values for an internal control (gyrB) transcript expressed as changes in the increase (n-fold increase) are shown. Data are means plus standard errors of the means (error bars) for values obtained from three independent experiments. (EP for normal S. aureus is 2.5 h, while SP is 7 h; EP for TD-SCV is 4.5 h, while SP is 9 h).
TD-SCVs do not use the ADI pathway during stationary-phase metabolism.
Another pathway which is utilized by S. aureus and by a variety of other bacteria under acidic and decreased energy conditions is the arginine deiminase (ADI) pathway (3, 12, 17). Therefore, we ascertained whether TD-SCVs display increased transcription of genes of the ADI pathway, as seen in a hemB mutant with the SCV phenotype (12). Consistent with our hypothesis, arcA transcript levels were markedly increased in the TD-SCVs compared to the normal (non-TD) strain (Fig. 3C), but this was pertinent only during the exponential phase. During the stationary phase, arcA transcript levels in both the strains were down-regulated (Fig. 3C). The catabolism of arginine usually involves the ADI pathway; however, supplementation of TD-SCV cultures with l-arginine at a concentration of 5 mM could not restore their growth and viability (data not shown). These results implied that TD-SCVs, in contrast to the hemB mutant, did not utilize the ADI pathway during stationary-phase metabolism and survival.
Conclusions.
Our results suggest that several mechanisms may contribute to enhanced post-stationary-phase survival in TD-SCVs. Thymidine auxotrophism is accompanied by a delay in TCA cycle function, thus preventing entry into the death phase of TD-SCVs in a complex medium. Moreover, as a fully functioning TCA cycle is essential for amino acid catabolism (20), the absence of catabolism of nonpreferred carbon sources like acetate results in delayed amino acid catabolism. Furthermore, down-regulation of the heat shock protein ClpC ATPase was detected in the TD-SCVs during stationary phase; both ClpC and/or activated SigB (which is also decreased in TD-SCVs as we previously showed [10]) might be involved in the regulation of the TCA cycle under thymidine starvation conditions (5; this study).
The observed thymidine auxotrophism of our clinical SCVs suggests that the thymidine synthase pathway is compromised, and our sequence data for thyA (thymidylate synthase gene; annotated as SA1260 in the S. aureus N315 genome) in TD-SCV supports this assumption. Initial experiments using a thyA-complemented SCV strain suggest (partial) reversal of all phenotypes of the TD-SCV presented in this report (I. Chatterjee, unpublished data).
In contrast to the completely fermentative growth demonstrated for a hemB mutant with the SCV phenotype (12), the TD-SCV generates acetate under oxidative growth conditions during stationary phase. This indicates that TD-SCVs isolated from CF patients use different metabolic pathways for adaptation during stationary-phase survival in comparison to a laboratory-generated insertional inactivation hemB mutant with the SCV phenotype.
Inactivation of aconitase increases long-term survival and persistence of S. aureus and reduces production of formylated δ-toxin, a chemoattractant for human leukocytes (21). Moreover, the agr effector molecule RNAIII, which also encodes the δ-toxin message (hld) (8, 21), is lacking in post-stationary-phase S. aureus populations (10). Taken together, we propose that the combination of a decrease in citB transcription and reduced levels of δ-toxin may support enhanced persistence of TD-SCVs, owing to a bacterial survival advantage and suppression of the host immune response.
Acknowledgments
This work received grant support from the Deutsche Forschungsgemeinschaft (Specialized Priority Programmes 1047 and 1130 and also grant HE 1850/8-1 to M.H.), the Medical Faculty of the University of Saarland (HOMFOR to M.H.), the Medical Faculty of the University of Münster (Innovative Research Grant KA-1-1-05-02 and DFG KA2249/1-3 to B.C.K.), and NIH (grant AI42072 to R.A.P.).
We are indebted to S. Gräber for statistical advice, to G. A. Somerville for helpful discussions, and to K. Hilgert for expert technical assistance.
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 2.Baumert, N., C. von Eiff, F. Schaaff, G. Peters, R. A. Proctor, and H. G. Sahl. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb. Drug Resist. 8:253-260. [DOI] [PubMed] [Google Scholar]
- 3.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouillette, E., G. Grondin, L. Shkreta, P. Lacasse, and B. G. Talbot. 2003. In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb. Pathog. 35:159-168. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee, I., P. Becker, M. Grundmeier, M. Bischoff, G. A. Somerville, G. Peters, B. Sinha, N. Harraghy, R. A. Proctor, and M. Herrmann. 2005. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 187:4488-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner, J. F., and J. Lascelles. 1962. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J. Gen. Microbiol. 29:157-164. [DOI] [PubMed] [Google Scholar]
- 7.Goldschmidt, M. C., and D. M. Powelson. 1953. Effect of the culture medium on the oxidation of acetate by Micrococcus pyogenes var. aureus. Arch. Biochem. Biophys. 46:154-163. [DOI] [PubMed] [Google Scholar]
- 8.Janzon, L., S. Lofdahl, and S. Arvidson. 1989. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol. Gen. Genet. 219:480-485. [DOI] [PubMed] [Google Scholar]
- 9.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 10.Kahl, B. C., G. Belling, P. Becker, I. Chatterjee, K. Wardecki, K. Hilgert, A. L. Cheung, G. Peters, and M. Herrmann. 2005. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect. Immun. 73:4119-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahl, B. C., A. Duebbers, G. Lubritz, J. Haeberle, H. G. Koch, B. Ritzerfeld, M. Reilly, E. Harms, R. A. Proctor, M. Herrmann, and G. Peters. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41:4424-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler, C., C. von Eiff, G. Peters, R. A. Proctor, M. Hecker, and S. Engelmann. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 185:6928-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo, S. P., A. S. Bayer, H. G. Sahl, R. A. Proctor, and M. R. Yeaman. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect. Immun. 64:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moisan, H., E. Brouillette, C. L. Jacob, P. Langlois-Begin, S. Michaud, and F. Malouin. 2006. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor, R. A., J. M. Balwit, and O. Vesga. 1994. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3:302-312. [PubMed] [Google Scholar]
- 16.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295-305. [DOI] [PubMed] [Google Scholar]
- 17.Resch, A., R. Rosenstein, C. Nerz, and F. Götz. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ Microbiol. 71:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert, H., H. Wisplinghoff, P. Schnabel, and C. von Eiff. 2003. Small colony variants of Staphylococcus aureus and pacemaker-related infection. Emerg. Infect. Dis. 9:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senn, M. M., M. Bischoff, C. von Eiff, and B. Berger-Bächi. 2005. σB activity in a Staphylococcus aureus hemB mutant. J. Bacteriol. 187:7397-7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 70:6373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somerville, G. A., A. Cockayne, M. Dürr, A. Peschel, M. Otto, and J. M. Musser. 2003. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 185:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somerville, G. A., B. Saïd-Salim, J. M. Wickman, S. J. Raffel, B. N. Kreiswirth, and J. M. Musser. 2003. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen interactions. Infect. Immun. 71:4724-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643-1647. [DOI] [PubMed] [Google Scholar]
- 24.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25:1250-1251. [DOI] [PubMed] [Google Scholar]
- 25.von Eiff, C., P. McNamara, K. Becker, D. Bates, X. H. Lei, M. Ziman, B. R. Bochner, G. Peters, and R. A. Proctor. 2006. Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with the small-colony-variant phenotype. J. Bacteriol. 188:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]