Abstract
Our previous studies demonstrated that a putative Staphylococcus aureus glycoprotease (Gcp) is essential for bacterial survival, indicating that Gcp may be a novel target for developing antibacterial agents. However, the biological function of Gcp is unclear. In order to elucidate the reason that Gcp is required for growth, we examined the role of Gcp in bacterial autolysis, which is an important biological process for bacterial growth. Using both a spacp-regulated gcp expression strain and a TetR-regulated gcp antisense expression strain, we found that the down-regulation of gcp expression can effectively inhibit Triton X-100-induced lysis, eliminate penicillin- and vancomycin-caused cell lysis, and dramatically increase tolerance to hydrolases. Moreover, we determined whether resistance to lysis is due to a defect in murein hydrolase activity by using a zymogram analysis. The results showed that the cell lysate of a down-regulated gcp expression mutant displayed several bands of decreased murein hydrolytic activity. Furthermore, we explored the potential mechanism of Gcp's involvement in autolysis and demonstrated that Gcp may function independently from several key autolysins (Atl, LytM, and LytN) and regulators (ArlRS, Mgr/Rat, and CidA). Taken together, the above results indicate that the essential Gcp is involved in the modification of substrates of murein hydrolases as well as in the regulation of expression and/or activity of some murein hydrolases, which, in turn, may play important roles in bacterial viability.
Staphylococcus aureus is a major animal and human pathogen that causes a wide range of infections (23). The emergence of multidrug-resistant staphylococcal isolates, especially methicillin-resistant S. aureus, is generating enormous public health concern and highlights an urgent need for new, alternative agents for treating multidrug-resistant pathogens. Our previous studies demonstrated that a putative glycoprotease (Gcp) is essential for the viability of S. aureus and indicated that Gcp may be a potential target for developing novel antibacterial agents (49).
Various glycoprotease homologues have been found in many gram-positive and gram-negative pathogens, including Bacillus anthracis, Streptococcus pyogenes, Pasteurella haemolytica A1 (31), and Escherichia coli (29), which have >42% amino acid identity (49). Glycoproteases have a variety of functions. The first discovered glycoprotease of P. haemolytica A1 is highly specific for O-glycosylated glycoproteins (1). The Gcp homolog in E. coli may be involved in the modulation of a macromolecular operon (29). However, in the cyanobacterium Synechocystis sp., mutation of the glycoprotease gene results in a reduction of salt tolerance and alters pigmentation and cyanophycin accumulation (50). For S. aureus, although we demonstrated that Gcp is required for bacterial survival, Gcp function and the reasons that Gcp is required for growth are still unclear.
Our preliminary microarray analysis indicated that the down-regulation of gcp expression may affect the expression of genes associated with bacterial autolysis (unpublished data). Therefore, we predicted that Gcp may be involved in modulating autolysis of S. aureus. Bacterial autolysis plays important physiological roles in cell separation and ongoing peptidoglycan remodeling (6, 25). It has been demonstrated that peptidoglycan (murein) hydrolases are involved in the lysis of bacteria by hydrolyzing either the glycan or the peptide moieties of peptidoglycan in the gram-positive cell wall (38). The major murein hydrolases in staphylococci have been revealed, including N-acetyl muramidase, N-acetyl glucosaminidase, N-acetylmuramyl-l-alanine amidase, transglycosylases, and endopeptidase (36, 39, 40, 43, 44). Autolysin (Atl) is a bifunctional protein containing glucosaminidase and amidase domains, which are separated by proteolytic processing to generate two extracellular lytic enzymes, a 51-kDa glucosaminidase and a 62-kDa amidase, which cleave MurNAc(1-4)GlcNAc and GlcNAc(1-4)MurNAc, respectively (30, 41). More importantly, these murein hydrolases are also associated with other important biological processes in cell division and growth, including cell wall biosynthesis, daughter cell separation, and peptidoglycan recycling (2, 4, 16, 47, 48).
Because murein hydrolases are important for the maintenance of bacterial cell integrity and growth, their activity must be tightly controlled. In S. aureus, the expression of the murein hydrolases is coordinately regulated at the transcriptional level by different regulators, including different two-component signal transduction systems. The two-component system lytSR is involved in the repression of peptidoglycan hydrolases, as the mutation of lytS increases hydrolysis and autolysis (8). The lytSR system positively regulates the expression of lrgA and lrgB, whose products are similar to bacteriophage murein hydrolase transporter proteins (known as holins) that are able to inhibit murein hydrolases (9, 15). Another two-component system, arlRS, has a negative effect on autolysis (12). Our recent data indicate that the arlRS system may function through positive regulation of lytSR, lrgA, and lrgB expression (22). In addition, some murein hydrolase activities are repressed by transcriptional regulators, including sar (13) and rat (also known as mgr) (17), but are positively regulated by agr (13) and the cidAB operon (37). Moreover, Clp protease activity seems to have a positive impact on the expression of regulators related to murein hydrolases, as the mutation of clpP down-regulates the expression of lytSR, lrgAB, arlRS, and rat (27). On the other hand, the activities of some murein hydrolases are mediated at the posttranslational level, including substrate modification, selective transport, interaction with lipoteichoic acids, etc. (5, 10, 25, 45).
In this study, we report that the essential putative glycoprotease appears to be involved in modifying the substrate (peptidoglycan) of murein hydrolases as well as in modulating the expression and/or activity of some murein hydrolases. Conditional mutation of gcp had a lethal effect on bacterial viability and dramatically reduced lysis induced by Triton X-100, penicillin, and vancomycin. Based on our results, we propose that Gcp functions as an important modulator involved in the cell wall biosynthesis pathway associated with the basic physiological process of cell autolysis in S. aureus, which, in turn, may play important roles in bacterial survival.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The S. aureus strains used in this study are listed in Table 1. The bacterial cells were incubated in Trypticase soy broth (TSB) at 37°C, with shaking, unless stated otherwise. E. coli cells were grown in Luria-Bertani (LB) medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| S. aureus strains | ||
| RN4220 | Laboratory strain; rsbU | 19 |
| RN6390 | Parental strain of KB350 | 33 |
| KB350 | RN6390 cidA::Erm Ermr | 37 |
| WCUH29 | Clinical human isolate; rsbU+ | 18 |
| 15981 | Clinical human isolate | 46 |
| ΔarlRS | 15981 with deletion of arlRS | 46 |
| RN4220/Pspac-gcp | spacp-regulated gcp mutant | 49 |
| RN4220/gcp-as | TetR-regulated gcp antisense mutant | 49 |
| WCUH29/gcp-as | TetR-regulated gcp antisense mutant | This study |
| ΔarlRS/gcp-as | TetR-regulated gcp antisense mutant | This study |
| Plasmids | ||
| pYH4 | Shuttle vector; Ermr | 49 |
| pYH4/gcp-as | Shuttle vector with gcp antisense construct; Ermr | 49 |
| pSB2025 | Shuttle vector containing luxABCDE; Cmr | 34 |
| pCY806 | pYH4/gcp-as with promoterless luxABCDE; Ermr | This study |
| pLZ106 | pCY806 with cidA promoter-lux reporter fusion; Ermr | This study |
Construction of TetR-regulated gcp antisense expression strains.
In order to examine the effect of Gcp on autolysis in the wild-type S. aureus isolate, the TetR-regulated gcp antisense expression vector, pYH4/gcp-as (49), and the control vector, pYH4, were electroporated into strain WCUH29 as described previously (18), resulting in strains WCUH29/gcp-as and WCUH29/pYH4, respectively. In order to determine the effect of the arlRS regulator on Gcp function, we utilized the same method and introduced the TetR-regulated gcp antisense expression vector, pYH4/gcp-as, into the arlRS null mutant and its parent strain, 15981 (46), resulting in strains ΔarlRS/gcp-as and 15981/gcp-as, respectively.
Triton X-100-induced autolysis assays.
Autolysis assays were performed as previously described (12). RN4220/Pspac-gcp cells were grown in TSB containing 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and appropriate antibiotics at 37°C, with shaking, to an optical density at 600 nm (OD600) of 1.2 to 1.3. WCUH29/pYH4 and WCUH29/gcp-as cells were grown in TSB containing 5 μg/ml of erythromycin (Erm) at 37°C, with shaking, to an OD600 of 1.2 to 1.3. The bacterial cultures were then diluted 1:100 with fresh TSB containing 1 M NaCl, with or without inducer (1 mM IPTG for RN4220/Pspac-gcp and 500 ng/ml anhydrotetracycline [ATc] for WCUH29/pYH4 and WCUH29/gcp-as), and incubated to an OD580 of 0.6 to 0.8 at 37°C. The bacterial cells were harvested by centrifugation at 4,000 × g and resuspended in the same volume of buffer containing 50 mM Tris-HCl (pH 7.5) and 0.1% Triton X-100. The bacterial cells were then incubated at 30°C with shaking, and the changes in OD580 were measured. Results were normalized to the OD580 at time zero (OD0), i.e., percent lysis at time t = [(OD0 − OD at time t)/OD0] × 100. All experiments were repeated at least three times.
Penicillin and vancomycin tolerance assay.
To assess the sensitivity of the gcp conditional mutants to penicillin and vancomycin, the mutants were incubated in TSB in the presence of the inducer IPTG or the antisense inducer ATc. RN4220/Pspac-gcp cells were grown in TSB containing 1 mM IPTG and appropriate antibiotics at 37°C, with shaking, to an OD600 of 1.2 to 1.3. WCUH29/pYH4 and WCUH29/gcp-as cells were grown in TSB containing 5 μg/ml Erm at 37°C, with shaking, to an OD600 of 1.2 to 1.3. The bacterial cultures were then inoculated at 1% with fresh TSB in the absence or presence of inducer (1 mM IPTG for RN4220/Pspac-gcp and 500 ng/ml ATc for WCUH29/pYH4 and WCUH29/gcp-as) and grown at 37°C, with shaking, to reach exponential phase (OD600 of ∼0.5). Penicillin G was added to a final concentration of 8 μg/ml (20× MIC), and vancomycin was added to a final concentration of 16 μg/ml (1× MIC). Cultures were incubated continuously, and the OD600 values for cultures were measured every hour for 8 h.
Hydrolase-induced autolysis assay.
The bacterial cell's susceptibility to extracellular hydrolases was determined as described previously (12). Briefly, the spacp-regulated gcp mutant was grown in TSB, with or without the inducer IPTG, at 37°C with shaking to reach the exponential phase (OD600 of ∼0.5). The bacterial cells were heat killed at 121°C for 15 min, collected by centrifugation, and washed with H2O. The heat-killed cells were resuspended in fresh, filter-sterilized supernatants of overnight cultures of RN4220. The OD600 of resuspended dead cells was adjusted to 0.5, and the dead cells were incubated at 37°C with shaking. The OD600 was measured every 30 min.
Zymographic analysis.
In order to detect the presence of extracellular and intracellular murein hydrolases, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-based zymographic analyses were performed as described previously (17). Briefly, various strains were grown in TSB, with or without inducers, for 16 h at 37°C with shaking. The extracellular murein hydrolases were isolated from the cultures by centrifugation at 10,000 × g for 15 min at 4°C. The supernatants were collected, filter sterilized, and concentrated 100-fold by ethanol precipitation overnight at 4°C. To obtain intracellular and cell wall-associated murein hydrolases, the bacterial cell pellets were washed with phosphate-buffered saline (PBS) and resuspended with PBS. The bacterial cells were lysed with lysostaphin, and the total proteins were evaluated by running the samples in 10% SDS-PAGE. The concentration of total proteins in each sample was determined by using the Bradford assay (Pierce Biotech) according to the manufacturer's instructions. A total of 10 μg of proteins from each sample was resolved in a 10% SDS-PAGE gel containing 0.2% autoclaved and lyophilized S. aureus RN4220 wet cells. After electrophoresis, gels were washed with water and incubated overnight in 25 mM Tris-Cl, pH 7.0, containing 1% Triton X-100 at 37°C to allow hydrolysis of the embedded bacterial cells. After incubation, gels were scanned (HP Scanjet 4570c). The zones of hydrolysis appeared as white bands in the gels, but black bands indicate regions of murein hydrolase activity in the figures.
RNA isolation and purification.
Overnight cultures of S. aureus were inoculated in 1% TSB and grown to the mid-exponential (3 h) phase of growth. Cells were harvested by centrifugation, and the RNAs were isolated with an RNAPrep kit (Promega, Madison, WI). Contaminating DNA was removed with a DNA-free kit (Ambion), and the RNA yield was determined spectrophotometrically at 260 nm.
Quantitative real-time RT-PCR analysis.
In order to examine whether down-regulation of gcp expression has any impact on the expression of genes involved in autolysis, we employed quantitative real-time reverse transcription-PCR (RT-PCR) to compare the RNA levels, as described previously (22). The first-strand cDNAs were synthesized using reverse transcriptase with a SuperScript III Platinum two-step qRT-PCR kit (Invitrogen). For each RNA sample, we performed duplicate reverse transcription reactions, as well as a control reaction without reverse transcriptase, in order to determine the levels of DNA contamination. PCRs were set up in triplicate by using SYBR green PCR master mix (Stratagene). Real-time sequence-specific detection and relative quantitation were performed with the Stratagene Mx3000P real-time PCR system. Gene-specific primers were designed to yield ∼100-bp specific products (Table 2). Relative quantification of the product was calculated using the comparative cycle threshold method, as described for the Stratagene Mx3000P system. The housekeeping 16S rRNA gene was used as an endogenous control (22). All samples were analyzed in triplicate and normalized against 16S rRNA gene expression. The experiments were repeated at least three times.
TABLE 2.
Primers used in this study
| N315 open reading frame | Gene | Primer orientation | Primer sequence (5′-3′)a |
|---|---|---|---|
| SA1854 | gcp | Forward | TTGGCGCGCCGTTTAAAGCTATTCAA |
| Reverse | GGGATAGTTAATTGAATGTC | ||
| SA1248 | arlR | Forward | TGACAAAGTTGCTGGGCTTGATTAC |
| Reverse | TGTGGCTGACGACGTAAAATTGC | ||
| SA0252 | lrgA | Forward | TGAAACAACAAAAAGACGCATCAAAACCAG |
| Reverse | ACTTCGCCTAACTTAACAGCACCAG | ||
| SA0250 | lytS | Forward | GCATGGTTCTATCGTCGGTACATTG |
| Reverse | ACTTACTTTGCGTTTCGGCTTCAC | ||
| SA2329 | cidA | Forward | GTCTTTTTCTTCATACCGTCAGT |
| Reverse | TCATTCATAAGCGTCTACACCT | ||
| SA0641 | rat/mgr | Forward | CGCAGATGATTATATGCAAAAC |
| Reverse | CGACAGCATCTTGCCAAGTC | ||
| SA0905 | atl | Forward | GCTGGTTATAGTTTAGTTGATGATG |
| Reverse | GGTTGTGCTGAAGCGCTAAAAG | ||
| SA0265 | lytM | Forward | GCAGGAGATAACAATGACTACAC |
| Reverse | TTACTTGCTGATCCACCATTTTG | ||
| SA1090 | lytN | Forward | AGCTGAACCTGGGGACTTAG |
| Reverse | CAACTTTATGTGCAACCTCTGC | ||
| SA1206 | femA | Forward | TCATCGATTACAGACGAAGACAC |
| Reverse | TCTTTTAGTTTAGACGGCGCAACC | ||
| 16S rRNA | Forward | CTGTTGCACATCTTGACGGTA | |
| Reverse | TCAGCGTCAGTTACAGACCA | ||
| lux | Forward | TAGAATTC GCGGCCGC GGCCGGCC AGTACT AGGTAGGTAATAGGAGGACTCTCTATGAAATTTGGAAACTT (EcoRI, NotI, FseI, and ScaI, respectively) | |
| Reverse | TAGAATTCTCAACTATCAACGCTTCGGTTAAGCT (EcoRI) | ||
| SA2329 | cidA promoter | Forward | AAGCGGCCGCCCACTTTGCCAGCTGATCATCA (NotI) |
| Reverse | ACAGTACTAAATATGTCTAAAATGTTACAATAACT (ScaI) | ||
| SA0905 | atl promoter | Forward | AAGCGGCCGCTGATTTTGTTAGCATGTGGAGGAA (NotI) |
| Reverse | TTAGTACTTGTGCGTATTTAACCAATTTGTA (ScaI) |
Restriction sites are indicated in bold, with the corresponding enzyme(s) shown in parentheses after the sequence.
Construction of cidA promoter-lux reporter fusion system.
In order to confirm whether the conditional mutation of gcp has an impact on cidA expression, we created a cidA promoter-lux reporter fusion system by using a TetR-regulated gcp antisense expression vector (49). First, the luxABCD genes were obtained from pSB2025 by a PCR using the primers listed in Table 1. They were digested with EcoRI and ligated into the EcoRI site of pYH4/gcp-as vector, which resulted in plasmid pCY806. The cidA promoter region was amplified by a PCR using the primers listed in Table 1, digested with NotI and ScaI, and ligated into the NotI and ScaI sites of pCY806, which resulted in plasmid pLZ106, containing a cidA promoter-lux reporter fusion system. The resulting plasmid, pLZ106, was purified and electroporated into S. aureus WCUH29, resulting in strain WCUH29/pLZ106. lux expression was monitored with a Chiron luminometer.
Scanning electron microscopy.
For scanning electron microscopy, staphylococci were grown overnight in TSB on polystyrene chamber slides at 37°C. After the medium was decanted, the slides were washed three times with 1× PBS, mounted on aluminum stubs, and shadowed with gold. For visualization, a scanning electron microscope (Zeiss DSM962) was used at 15 kV.
RESULTS
Conditional mutation of gcp results in bacteriostatic phenotype.
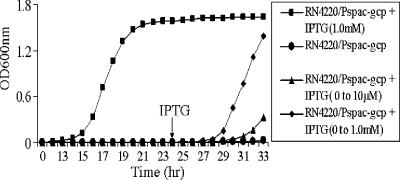
Using a spacp-regulated gcp mutant and a TetR-regulated gcp antisense mutant, we demonstrated that the down-regulation of gcp expression can have a lethal effect on bacterial growth (49). To investigate the reason that Gcp is essential for survival, we first examined whether the down-regulation of Gcp is bactericidal or bacteriostatic by using the spacp-regulated gcp expression strain. The results showed that in the absence of the inducer IPTG, no growth was detected during a 30-h period of incubation; in contrast, in the presence of IPTG, the spacp-regulated gcp expression strain reached the early log phase and stationary phase of growth at 8 and 12 h, respectively (Fig. 1). To test whether the addition of the inducer IPTG was able to revive the bacteria that were originally inoculated and grown in TSB without IPTG, we added different concentrations of IPTG to the 24-h culture and incubated the culture. The capacity of bacterial growth was recovered in a dose-dependent manner after the addition of the inducer (Fig. 1). Interestingly, after 32 h of incubation without the inducer IPTG, no cell lysis was observed (Fig. 1). In addition, we counted viable CFU from the 24-h culture without inducer to further confirm the impact of gcp on bacterial survival. The results showed that with IPTG induction, the bacteria were still viable (data not shown). Taken together, the above data indicate that the down-regulation of Gcp leads to a bacteriostatic effect.
FIG. 1.
Growth curves for the spacp-regulated gcp mutant. The spacp-regulated gcp mutant was incubated overnight in TSB in the presence of 1 mM IPTG. Bacteria were diluted in fresh TSB and incubated in TSB, with or without IPTG, at 37°C with shaking. Different concentrations of IPTG were added to the bacterial cultures without IPTG 24 h after incubation. The bacterial cultures were continuously incubated at 37°C, with shaking, and the OD600 of the cultures was measured every hour.
To determine whether the down-regulation of Gcp has any morphological effect on bacterial cells, we performed light microscopic and electronic microscopic assays. No obvious morphological changes, including the size and appearance of the cell surface, were observed during the down-regulation of gcp expression (data not shown).
Down-regulation of gcp expression decreases Triton X-100-induced autolysis.
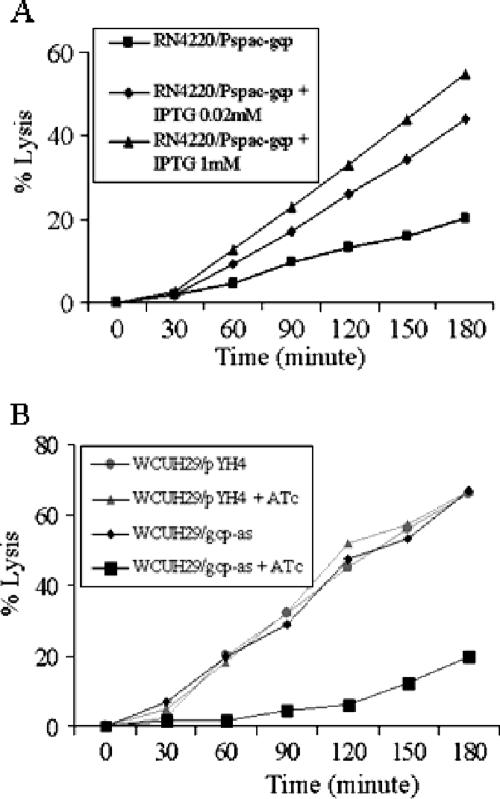
Our preliminary microarray data showed that the down-regulation of gcp expression has some impact on the transcription of arlRS and cidA, which are both involved in the regulation of bacterial autolysis (12, 37). Therefore, we hypothesized that Gcp may be involved in the modulation of autolysis and/or cell division pathways, which, in turn, may play important roles in bacterial viability. To test our hypothesis, we first examined the effect of Gcp on susceptibility to cell lysis induced by a nonionic detergent, Triton X-100, using the spacp-regulated gcp mutant strain. S. aureus strains were grown in TSB containing 1 M NaCl and resuspended in a medium containing 0.1% Triton X-100. In the presence of IPTG, >50% of the spacp-regulated gcp expression cells harvested from a mid-exponential-phase culture lysed within 3 h at 30°C in the presence of 0.1% Triton X-100 (Fig. 2A). Moreover, Triton X-100-induced cell lysis significantly increased in the presence of IPTG, in a dose-dependent manner (Fig. 2A). In contrast, fewer than 20% of the spacp-regulated gcp mutant cells lysed within 3 h in the absence of IPTG (Fig. 2A).
FIG. 2.
Triton X-100-induced autolysis of conditional gcp mutants. (A) spacp-regulated gcp mutant RN4220/Pspac-gcp; (B) gcp antisense mutant WCUH29/gcp-as. The indicated strains and the control, WCUH29/pYH4, were grown in TSB in the presence of different concentrations of IPTG (1 mM) or ATc (0.5 μg/ml). Results were normalized to the OD580 at time zero (OD0). The percent lysis was determined as follows: percent lysis at time t = [(OD0 − ODt)/OD0] × 100. The experiments were repeated at least three times. Each figure represents the results of one experiment.
To further confirm the effect of Gcp on autolysis, we transformed the gcp antisense expression vector into wild-type S. aureus WCUH29 and examined the Triton X-100-induced autolysis. The results showed that >70% of the control bacterial cells, including the control strain carrying the parent vector pYH4, with or without inducer, and the gcp antisense strain without the inducer, lysed within 3 h (Fig. 2B). In contrast, only 15% of the gcp antisense mutant cells lysed within 3 h with the induction of gcp antisense RNA (Fig. 2B). Taken together, the above results indicate that Gcp is involved in cell autolysis.
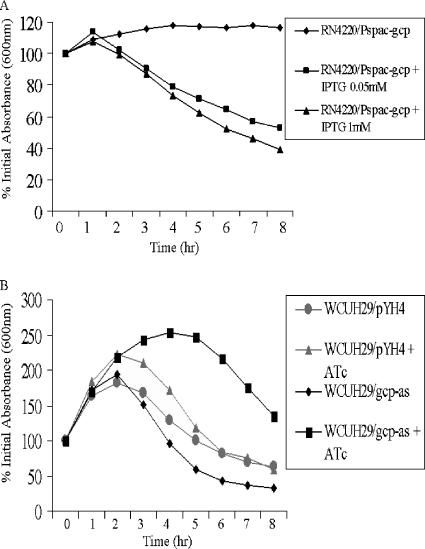
Down-regulation of gcp expression increases bacterial tolerance to penicillin- and vancomycin-induced autolysis.
The β-lactam moiety of penicillin binds to the transpeptidase to inhibit the transpeptidation reaction linking the pentaglycine bridge between two peptidoglycan chains (14). We examined the effect of Gcp on penicillin-induced cell lysis in the presence of 8 μg/ml of penicillin (20× MIC), using the spacp-regulated gcp mutant as described previously (17). In the presence of the inducer IPTG (1 mM), penicillin-induced cell lysis significantly increased in a dose-dependent manner (Fig. 3A). In contrast, in the absence of IPTG, the spacp-regulated gcp mutant cells were significantly resistant to penicillin-induced lysis (Fig. 3A). To further confirm the importance of Gcp in cell autolysis, we determined the effect of Gcp on penicillin-induced cell lysis by using the gcp antisense mutant created in the wild-type S. aureus isolate WCUH29. In the presence of ATc induction, the gcp antisense mutant was more resistant to penicillin-induced autolysis than either the mutant without ATc induction or the control strain, whether or not ATc was present in the culture (Fig. 3B). Moreover, the antisense effects shown in Fig. 3B resulted from the strong down-regulation of endogenous gcp expression (data not shown).
FIG. 3.
Effects of penicillin G on the growth of conditional gcp mutants. (A) spacp-regulated gcp mutant RN4220/Pspac-gcp; (B) gcp antisense mutant WCUH29/gcp-as. The indicated strains and the control, WCUH29/pYH4, were grown in TSB in the presence of different concentrations of inducer (either IPTG or ATc). Penicillin (20× MIC) was added to the exponential-phase cultures at a final concentration of 8 μg/ml. The bacterial cultures were continuously incubated, and the OD600 values for the cultures were measured every hour for 8 h.
To further determine whether Gcp is involved in the cell wall biosynthesis pathway, we examined the impact of Gcp on bacterial susceptibility to vancomycin by using the spacp-regulated gcp mutant. Vancomycin is a glycopeptide antibacterial agent that prevents the incorporation of N-acetylmuramic acid-peptide and N-acetylglucosamine-peptide subunits into the peptidoglycan matrix and inhibits peptidoglycan synthesis by binding to d-Ala-d-Ala (3). Bacterial autolysis plays a role in vancomycin-mediated killing in S. aureus (7). The results showed that the down-regulation of gcp expression increased bacterial tolerance to vancomycin-induced cell lysis (data not shown). Taken together, the above results indicate that Gcp may be involved in the cell wall peptidoglycan biosynthesis pathway.
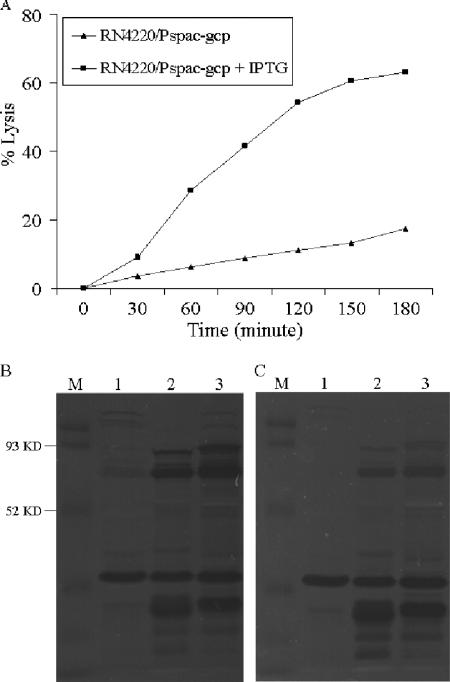
Down-regulation of gcp expression increases bacterial resistance to hydrolases.
In order to further investigate Gcp's association with cell wall synthesis, we examined the effect of Gcp on susceptibility to extracellular murein hydrolases. Bacterial cells were collected from log-phase (OD600 of ∼0.5) cultures of the spacp-regulated gcp mutant, with or without IPTG, and then killed. The susceptibility to hydrolases was analyzed using the heat-killed bacterial cells as described previously (12). The results showed that of the heat-killed cells from the culture with IPTG that were incubated with the supernatant collected from the culture of wild-type S. aureus, >60% lysed within 3 h (Fig. 4A). In contrast, <20% of the heat-killed cells from the culture without IPTG were lysed (Fig. 4A). We then performed zymographic analysis to compare the sensitivities to hydrolases, as described previously (17). Similarly, the heat-killed cells from the culture with IPTG were more sensitive to different hydrolases, especially to hydrolases of between 52 and 93 kDa (Fig. 4B and C). Collectively, these data demonstrate that Gcp is involved in the modification of the cell wall peptidoglycan biosynthesis pathway.
FIG. 4.
Effect of down-regulation of gcp expression on hydrolase activity. The spacp-regulated gcp mutant was grown in TSB, with or without the inducer IPTG (1 mM), and the bacterial cells were heat killed, collected by centrifugation, and washed with H2O. (A) The heat-killed cells were resuspended with fresh filter-sterilized supernatants of overnight cultures of RN4220. The optical density of resuspended dead cells was adjusted to 0.5 at 600 nm, and the dead cells were incubated at 37°C with shaking. The OD600 was measured every 30 min. (B and C) Zymogram analysis of the spacp-regulated gcp mutant. Equal amounts (10 μg) of proteins prepared from the supernatants of different cultures of S. aureus strains were loaded and separated in 10% SDS-PAGE gels containing 0.2% heat-killed cells harvested from culture with 1 mM IPTG (B) or without IPTG (C). Lytic bands appeared as dark zones after scanning. M, protein molecular size marker.
Down-regulation of gcp expression inhibits the expression and/or activity of autolysins.
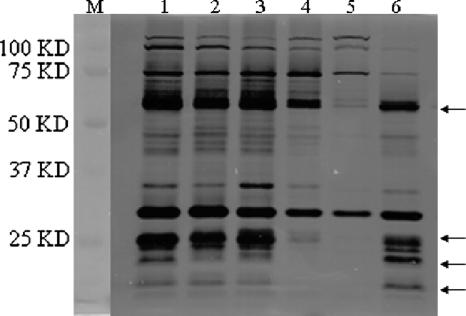
Finally, we determined whether resistant lysis resulted from a defect in murein hydrolase activity, using a zymogram analysis as described previously (17). No significant difference in zymographic patterns was observed for exported hydrolases that were isolated from the supernatants of stationary-phase cultures of either the control strain carrying the parent vector pYH4, with or without the inducer ATc, or the gcp antisense strain without inducer (Fig. 5, lanes 1 to 3). In contrast, using the exported hydrolases isolated from the supernatants of stationary-phase cultures of the gcp antisense mutant in the presence of the inducer ATc, the zymogram displayed several bands of reduced murein hydrolytic activity (Fig. 5, lane 4 [major changes are indicated by arrows]). This result was further confirmed by using the spacp-regulated gcp mutant (Fig. 5, lane 5 [without IPTG]).
FIG. 5.
Zymogram analysis of regulated gcp mutants. Equal amounts (10 μg) of proteins prepared from the supernatants of the gcp mutants were loaded and separated in 10% SDS-PAGE gels containing S. aureus RN4220 cells (0.2% [wet weight] heat-killed cells). Lytic bands appeared as clear zones on an opaque background but showed dark zones after scanning. Lane 1, RN4220/pYH4; lane 2, RN4220/pYH4 with ATc (500 ng/ml); lane 3, RN4220/gcp-as; lane 4, RN4220/gcp-as with ATc (500 ng/ml); lane 5, RN4220/Pspac-gcp without IPTG; lane 6, RN4220/Pspac-gcp with IPTG (1 mM). M, protein molecular size marker.
To explore the possibility that the decreased activities of hydrolases resulted from the impact of down-regulated Gcp on a translocation enzyme, we performed zymogram analyses using whole-cell lysates and examined the activity of the intracellular or cell wall-associated hydrolases. No obvious changes in intracellular hydrolase activity were detected after the down-regulation of gcp expression, using both the spacp-regulated gcp mutant and the gcp antisense mutant, compared to controls (data not shown). This suggests that Gcp is not involved in mediating the translocation of murein hydrolases.
Down-regulation of Gcp has different effects on the expression of genes associated with autolysis.
To determine the mechanism by which Gcp is involved in bacterial autolysis, we examined the impact of Gcp on the expression of genes associated with autolysis in S. aureus by using quantitative RT-PCR. Several regulators, including LrgAB, LytSR, ArlRS, and Rat (Mgr), are repressors of autolytic activity in S. aureus, as the mutation of any these regulators has been shown to increase autolysis in S. aureus (8, 12, 15, 17, 22). In contrast, the cidAB operon is a positive regulator, since the mutation of cidA decreases autolysis in S. aureus (37). When gcp was down-regulated using the spacp-regulated gcp expression strain, we detected no significant changes in the expression of many genes, including rat, atl, lytM, lytN, and femA, whereas we observed an ∼2-fold increase in lrgA expression, a slight increase in arlR expression, and a >2-fold decrease in cidA expression (Table 3). Similar results were obtained using the regulated gcp antisense mutant (data not shown).
TABLE 3.
Real-time RT-PCR analysis of gene expression in mid-log phase of growth, using the spacp-regulated gcp expression strain
| N315 open reading frame | Gene | Fold changea |
|---|---|---|
| SA1854 | gcp | −16.1 |
| SA1248 | arlR | 1.1 |
| SA0252 | lrgA | 1.9 |
| SA0250 | lytS | NC |
| SA2329 | cidA | −2.1 |
| SA0641 | rat/mgr | NC |
| SA0905 | atl | NC |
| SA0265 | lytM | NC |
| SA1090 | lytN | NC |
| SA1206 | femA | NC |
Negative numbers represent genes that were down-regulated without the inducer IPTG (1 mM). NC, no change.
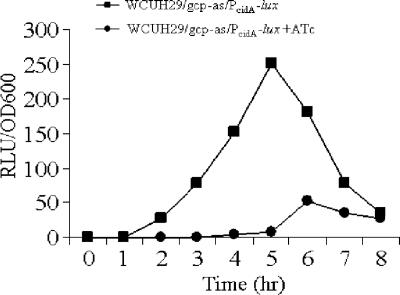
To further confirm the effect of Gcp on cidA expression, we constructed a transcriptional cidA promoter-lux reporter system by using the regulated gcp antisense expression vector. Bioluminescence activity was detected using a luminometer. No light signal was detected in the controls (the regulated gcp antisense strain carrying a promoterless lux reporter, with or without inducer). In contrast, in the presence of inducer, the luciferase activity significantly decreased in the log-phase cultures compared to that without gcp antisense expression (Fig. 6). However, no change in luciferase activity was detected during the down-regulation of gcp expression using an atl promoter-lux reporter system (data not shown).
FIG. 6.
Expression of lux driven by the cidA promoter in the gcp antisense expression strain WCUH29/gcp-as. Promoter activation was represented as the mean light intensity/OD600 ratio from triplicate readings at different times during growth. RLU, relative light units.
To investigate the possibility that Gcp may function through the regulation of cidA, we compared the autolysis profiles of the cidA null mutant and the spacp-regulated gcp mutant. The magnitudes of increased tolerance to lysis induced by Triton X-100, penicillin, and vancomycin were clearly different between the down-regulated gcp expression strain and the cidA null mutant (data not shown). In addition, zymographic patterns also showed obvious differences between the down-regulated gcp mutant and the cidA null mutant (data not shown). These data indicate that despite the fact that Gcp positively affects cidA expression, Gcp and CidA may function differently in the regulation of cell autolysis.
Mutation of arlRS has no impact on the role of Gcp in the regulation of bacterial autolysis.
The two-component system arlRS is involved in the negative regulation of autolysis in S. aureus (12), and our data above indicate that Gcp positively affects bacterial autolysis. To investigate whether arlRS has any impact on the role of Gcp in the regulation of autolysis, we introduced the regulated gcp antisense expression vector into an arlRS deletion mutant. The down-regulation of gcp expression significantly inhibited growth and penicillin-induced autolysis of the arlRS null mutant, similar to the case with the parental control (data not shown). In addition, the effects of the mutation in arlRS on bacterial susceptibilities to penicillin and hydrolase activities were not complementary to those of the down-regulated gcp mutant (data not shown). The above data indicate that mutation of arlRS has no influence on the function of Gcp in the regulation of autolysis.
DISCUSSION
The putative glycoprotease (Gcp) is a potential target for a new class of antibacterial agents because it is essential for bacterial survival. However, very little is known about the biological function of Gcp in S. aureus. A better understanding of the function of Gcp will allow us to elucidate the reason that Gcp is essential for bacterial survival and enable us to more comprehensively evaluate it as a drug target. It has been reported that murein hydrolases are important for bacterial growth because murein hydrolases target cell wall peptidoglycan during cell division and remodeling of the cell wall (28). In this study, we have demonstrated that Gcp is a critical mediator involved in the modification of cell wall biosynthesis and/or cell division as well as in the regulation of the activities of different murein hydrolases in S. aureus.
Our results showed that the down-regulation of gcp expression had a lethal effect on growth and dramatically increased the cell's resistance to autolysis induced by detergent and penicillin. It has been reported that cell wall modification and a decrease in autolysis can be produced with protein synthesis inhibitors, including tetracycline and chloramphenicol (20), and we reasoned that a Gcp-specific inhibitor should have a similar effect on autolysis and should not be used together with other cell wall-active antibacterial agents. However, using the MIC assay, we found that the down-regulation of gcp expression had no significant impact on bacterial susceptibility to penicillin, vancomycin, and other antibiotics (data not shown). Therefore, the Gcp-specific inhibitor may be a more potent antibacterial agent for the treatment of infections caused by methicillin- and vancomycin-resistant S. aureus.
Bacterial cells regulate autolysis through various mechanisms, including by modifying cell wall peptidoglycan (the substrate of murein hydrolases) and regulating the expression and activity of murein hydrolases (8, 15, 17, 26, 37). Our results clearly indicate that Gcp is associated with the modification of cell wall peptidoglycan synthesis, because the consequences of down-regulating gcp expression include increased bacterial tolerance to detergent-, penicillin-, and vancomycin-induced lysis. We confirmed a similar effect on autolysis by using both a spacp-regulated gcp mutant (which was created in the rsbU library strain RN4220) and a TetR-regulated gcp antisense expression strain (which was constructed in the rsbU+ wild-type strain WCUH29). We also found that the down-regulation of gcp expression led the heat-killed bacterial cells to be resistant to exported murein hydrolases (Fig. 4). On the other hand, our data show that Gcp in S. aureus also partially contributes to the positive regulation of different murein hydrolases. When gcp was down-regulated, the activities of certain murein hydrolases (size ranges of ∼60 kDa and 20 to 25 kDa) were either decreased or abolished in the conditional gcp mutants (Fig. 5). We now have studies in progress to investigate the cell wall composition when Gcp is depleted and to identify which hydrolases are affected by Gcp. In addition, we found an increased tolerance to lysis induced by penicillin and vancomycin after the down-regulation of gcp expression, suggesting that Gcp may play important roles in bacterial persistence after the bacterial population is exposed to antibiotics, although the mechanism of bacterial persistence is still unclear (21).
Since Gcp is essential for viability, we predicted that Gcp may be involved in the modulation of autolysins. In S. aureus, Atl is an important cell wall-associated autolysin involved in cell separation, cell lysis, and release of cell wall material at the cell surface, and it is essential for penicillin-induced autolysis (11, 40, 43). In addition, glycylglycine endopeptidase (LytM) and cell wall hydrolase (LytN) are two major secreted enzymes involved in cell lysis (35, 42). After down-regulating gcp expression, we found no significant impact on the expression of Atl, LytM, and LytN, and no difference in hydrolytic activity was observed in the size range of ∼37 kDa, the molecular mass of Gcp in S. aureus. However, we did find that the down-regulation of gcp expression inhibited several extracellular hydrolase activities (Fig. 5). These data indicate that Gcp is likely associated with extracellular hydrolase activity distinct from those of Atl, LytM, and LytN. However, we cannot rule out the possibility of the posttranslational regulation of hydrolase activity by Gcp.
In order to further understand the mechanisms of Gcp involvement in autolysis, we explored whether the impact of Gcp on autolysis may occur through the arlRS-regulated lytSR and lrgAB pathway. The two-component regulatory system LytSR negatively modulates the expression of murein hydrolases and positively regulates the expression of the lrgAB operon (9). The lrgA gene product, LrgA, seems to be analogous to an antiholin, which negatively controls extracellular murein hydrolase activity by inhibiting the transport of murein hydrolase across cell membranes (15). Our recent findings indicate that another two-component signal system, ArlSR, positively regulates lytSR expression and may be indirectly involved in mediating autolysis (22). Our studies have demonstrated that Gcp negatively affects the expression of lrgA, which is consistent with the decreased autolytic activities in the down-regulation of gcp expression mutants. However, we found that the mutation of the arlRS regulator had no influence on the growth-deficient phenotype and elevated tolerance to penicillin-induced lysis of the conditional gcp mutant. Another important global regulator, Mgr/Rat, has been demonstrated to negatively modulate autolytic activity by differentiated regulation of the expression of lytSR, lrgAB, arlRS, lytM, lytN, and cidA (17, 24), but our studies showed that the down-regulation of gcp expression had no obvious effect on rat expression. Taken together, these findings suggest that Gcp involved in the modulation of autolysis may function independently from arlRS and rat regulons.
The cidAB operon encodes the holin-like counterpart of the lrgAB operon and is a positive regulator of cell autolysis in S. aureus (37). The results reported here showed that Gcp positively affects cidA expression at the transcriptional level and led us to predict that Gcp's involvement in autolysis may be through the modulation of the cidAB operon. However, we found that the conditional gcp mutants displayed completely different magnitudes of resistance to autolysis and showed distinct zymographic patterns of murein hydrolases compared to the cidA mutant. Moreover, our studies indicate that Gcp plays critical roles in controlling cell lysis in the log phase of growth, whereas cidA has less of an effect on cell lysis during this period of growth, which is consistent with a previous report that a mutation in cidA inhibits cell lysis in the stationary phase but has only a minimal influence on survival (32). Thus, Gcp's involvement in the regulation of autolysis may be independent of the cidAB regulon. Moreover, a bioinformatic analysis of Gcp motifs indicated that Gcp is not a DNA-binding protein, because it has no helix-turn-helix DNA-binding structure (data not shown). These data suggest that Gcp may indirectly influence cidA expression.
Taking these results collectively, Gcp's involvement in the modification of cell wall biosynthesis is likely an important reason that it is essential for bacterial survival. We now have studies in progress to investigate the mechanism of Gcp's involvement in cell wall biosynthesis, using comprehensive genomic and proteomic approaches. The outcome of this research will provide additional insights into the complex mechanisms of bacterial cell autolysis as well as help us to elucidate the biological function of this essential glycoprotease in S. aureus.
Acknowledgments
This work was funded by NIH grant AI065740.
We thank Gilbert Ahlstrand for his assistance in scanning electronic microscopy analysis, Katherine Doll for her assistance in autolysis analysis, and Karin Matchett and reviewers for critical readings of the manuscript and for helpful suggestions.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Abdullah, K., E. Udoh, P. Shewen, and A. Mellors. 1992. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect. Immun. 60:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, A. R., I. C. Hancock, and C. R. Harwood. 1993. Cell wall structures, synthesis, and turnover, p. 381-410. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology and molecular genetics. ASM Press, Washington, DC.
- 3.Arthur, M., P. Reynolds, and P. Courvalin. 1996. Glycopeptide resistance in enterococci. Trends Microbiol. 4:401-407. [DOI] [PubMed] [Google Scholar]
- 4.Berry, A. M., R. A. Lock, D. Hansman, and J. C. Paton. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierbaum, G., and H. G. Sahl. 1987. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J. Bacteriol. 169:5452-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman, S. A., T. J. Smith, and S. J. Foster. 1998. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144:73-82. [DOI] [PubMed] [Google Scholar]
- 7.Boyle-Vavra, S., M. Challapalli, and R. S. Daum. 2003. Resistance to autolysis in vancomycin-selected Staphylococcus aureus isolates precedes vancomycin-intermediate resistance. Antimicrob. Agents Chemother. 47:2036-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunskill, E. W., and K. W. Bayles. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 178:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunskill, E. W., and K. W. Bayles. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178:5810-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleveland, R., J. Holtje, A. Wicken, A. Tomasz, L. Daneo-Moore, and G. D. Shockman. 1975. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem. Biophys. Res. Commun. 67:1128-1135. [DOI] [PubMed] [Google Scholar]
- 11.Foster, S. J. 1995. Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J. Bacteriol. 177:5723-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto, D. F., and K. W. Bayles. 1998. Opposing roles of the Staphylococcus aureus virulence regulators, Agr and Sar, in Triton X-100- and penicillin-induced autolysis. J. Bacteriol. 180:3724-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groicher, K., B. Firek, D. Fujimoto, and K. Bayles. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtje, J. V., and E. I. Tuomanen. 1991. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J. Gen. Microbiol. 137:441-454. [DOI] [PubMed] [Google Scholar]
- 17.Ingavale, S., W. van Wamel, and A. L. Cheung. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 48:1451-1466. [DOI] [PubMed] [Google Scholar]
- 18.Ji, Y., B. Zhang, S. van Horn, P. Warren, M. Burnham, G. Woodnutt, and M. Rosenberg. 2001. Identification of critical staphylococcal genes using conditional growth phenotypes generated by antisense RNA. Science 293:2266-2269. [DOI] [PubMed] [Google Scholar]
- 19.Kreiswirth, B., S. Lofdahl, M. Betley, M. O'Reilly, P. Schlievert, M. Bergdoll, and R. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 20.Ledala, N., B. Wilkinson, and R. Jayaswal. 2006. Effect of oxacillin and tetracycline on autolysis, autolysis processing and atl transcription in Staphylococcus aureus. Int. J. Antimicrob. Agents 27:518-524. [DOI] [PubMed] [Google Scholar]
- 21.Levin, B. R. 2004. Noninherited resistance to antibiotics. Science 305:1578-1579. [DOI] [PubMed] [Google Scholar]
- 22.Liang, X., L. Zheng, C. Landwehr, D. Lunsford, D. Holmes, and Y. Ji. 2005. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J. Bacteriol. 187:5486-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 24.Luong, T., P. Dunman, E. Murphy, S. Projan, and C. Lee. 2006. Transcriptional profiling of the mgrA regulon in Staphylococcus aureus. J. Bacteriol. 188:1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madiraju, M., D. Brunner, and B. Wilkinson. 1987. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna, A., S. Ingavale, M. Maloney, W. van Wamel, and A. Cheung. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J. Bacteriol. 186:5267-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel, A., F. Agerer, C. Hauck, M. Herrmann, J. Ullrich, J. Hacker, and K. Ohlsen. 2006. Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxidative stress response, autolysis, and DNA repair. J. Bacteriol. 188:5783-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesin, M., J. Lupski, P. Svec, and G. Godson. 1987. Possible new genes as revealed by molecular analysis of a 5-kb Escherichia coli chromosomal region 5′ to the rpsU-dnaG-rpoD macromolecular-synthesis operon. Gene 51:149-161. [DOI] [PubMed] [Google Scholar]
- 30.Oshida, T., M. Sugai, H. Komatsuzawa, Y. Hong, H. Suginaka, and A. Tomasz. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 92:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otulakowski, G., P. Shewen, E. Udoh, A. Mellors, and N. Wilkie. 1983. Proteolysis of sialoglycoprotein by Pasteurella haemolytica cytotoxic culture supernatant. Infect. Immun. 42:64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton, T., K. Rice, M. Foster, and K. Bayles. 2005. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 56:1664-1674. [DOI] [PubMed] [Google Scholar]
- 33.Peng, H. L., R. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qazi, S., E. Counil, J. Morrissey, C. Rees, A. Cockayne, K. Winzer, W. Chan, P. Williams, and P. Hill. 2001. agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infect. Immun. 69:7074-7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramadurai, L., and R. K. Jayaswal. 1997. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J. Bacteriol. 179:3625-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramadurai, L., K. Lockwood, M. Nadakavukaren, and R. K. Jayaswal. 1999. Characterization of a chromosomally encoded glycylglycine endopeptidase of Staphylococcus aureus. Microbiology 145:801-808. [DOI] [PubMed] [Google Scholar]
- 37.Rice, K., B. Firek, J. Nelson, S. Yang, T. Patton, and K. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shockman, G. D., and J. V. Holtje. 1994. Microbial peptidoglycan (murein) hydrolases, p. 131-168. In J. M. Ghuysen and R. Hackenbeck (ed.), Bacterial cell wall, vol. 27. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 39.Singer, H. J., E. Wise, and J. Park. 1972. Properties and purification of N-acetylmuramyl-l-alanine amidase from Staphylococcus aureus H. J. Bacteriol. 112:932-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugai, M., H. Komatsuzawa, T. Akiyama, Y. Hong, T. Oshida, and Y. Miyake. 1995. Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J. Bacteriol. 177:1491-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugai, M. 1997. Peptidoglycan hydrolases of the staphylococci. J. Infect. Chemother. 3:113-127. [Google Scholar]
- 42.Sugai, M., T. Fujiwara, H. Komatsuzawa, and H. Suginaka. 1998. Identification and molecular characterization of a gene homologous to epr (endopeptidase resistance gene) in Staphylococcus aureus. Gene 224:67-75. [DOI] [PubMed] [Google Scholar]
- 43.Sugai, M., S. Yamada, S. Nakashima, H. Komatsuzawa, A. Matsumoto, T. Oshida, and H. Suginaka. 1997. Localized perforation of the cell wall by a major autolysin: atl gene products and the onset of penicillin-induced lysis of Staphylococcus aureus. J. Bacteriol. 179:2958-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tipper, D. J. 1969. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J. Bacteriol. 97:837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobin, P., N. Mani, and R. Jayaswal. 1994. Effect of physiological conditions on the autolysis of Staphylococcus aureus strains. Antonie Leeuwenhoek 65:71-78. [DOI] [PubMed] [Google Scholar]
- 46.Toledo-Arana, A., N. Merino, M. Vergara-Irigaray, M. Débarbouillé, J. Penadés, and I. Lasa. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 187:5318-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong, W., A. Chatterjee, and F. Young. 1978. Regulation of bacterial cell walls: correlation between autolytic activity and cell wall turnover in Staphylococcus aureus. J. Bacteriol. 134:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada, S., M. Sugai, H. Komatsuzawa, S. Nakashima, T. Oshida, A. Matsumoto, and H. Suginaka. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 178:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng, L., J. Yang, C. Landwehr, F. Fan, and Y. Ji. 2005. Identification of an essential glycoprotease in Staphylococcus aureus. FEMS Microbiol. Lett. 245:279-285. [DOI] [PubMed] [Google Scholar]
- 50.Zuther, E., H. Schubert, and M. Hagemann. 1998. Mutation of a gene encoding a putative glycoprotease leads to reduced salt tolerance, altered pigmentation, and cyanophycin accumulation in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 180:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]