Abstract
The genome of Myxococcus xanthus encodes lipolytic enzymes in three different families: patatin lipases, α/β hydrolases, and GDSL lipases. One member of each family was characterized. The protein encoded by MXAN_3852 contains motifs characteristic of patatins. MXAN_5522 encodes a protein with the G-X-S-X-G motif characteristic of the lipase subfamily of α/β hydrolases. MXAN_4569 encodes a member of the GDSL family of lipolytic enzymes. Strains with deletions of MXAN_5522 and MXAN_4569 undergo faster development and earlier myxospore formation than the wild-type strain. The MXAN_5522 mutation results in spore yields substantially higher than those seen for wild-type cells. Gene expression analysis using translational lacZ fusions indicates that while all three genes are expressed during development, only MXAN_5522 and MXAN_4569 are expressed during vegetative growth. The proteins encoded by these genes were overexpressed using a T7 RNA polymerase transcription (pET102/D-TOPO) system in Escherichia coli BL21 Star (DE3) cells. The substrate specificities of the purified enzymes were investigated using p-nitrophenyl esters with chain lengths from C2 to C16. These enzymes preferentially hydrolyzed esters of short-chain fatty acids, yielding the highest activity with p-nitrophenyl acetate.
The myxobacteria differ from other members of the δ Proteobacteria in that they have a highly social life cycle. Myxococcus xanthus, like other myxobacteria, glides on surfaces either as single cells or, more typically, in groups feeding cooperatively. When nutrients are depleted, approximately 100,000 cells aggregate to form a multicellular fruiting body. Inside the fruiting bodies, the long, thin vegetative rods differentiate into ovoid myxospores with a thick coat that are resistant to prolonged starvation, desiccation, and high temperatures. Spores germinate when nutrients become available (for reviews, see references 16, 17, and 60). Development requires the exchange of signals (59) and the expression of a large number of genes (24, 29, 34, 35). Some of the signaling pathways have been described previously; others remain to be elucidated.
Lipids play a central role in the M. xanthus life cycle. Lipids containing the fatty acid c16:1ω5c are among the most abundant lipids in M. xanthus but are rare in other bacteria (12). They are chemoattractants and may play a role in development (32). Branched-chain fatty acids also appear to play a role in development (15). Lipids containing the fatty acid c18:1ω9c are not found in M. xanthus (12) and appear to serve as chemoattractants for detecting prey bacteria. Fatty acids are consumed for carbon and energy during growth (37) along with protein that is locked in the prey cytoplasm. M. xanthus may remove the membrane barrier with lipolytic enzymes that not only release fatty acids but empty the cytoplasmic contents of the prey.
Lipases (triacylglycerol acylhydrolases; EC 3.1.1.3) and esterases (carboxylic ester hydrolases; EC 3.1.1.1), collectively known as “lipolytic enzymes,” hydrolyze hydrophobic carboxylic acid esters, liberating fatty acids and glycerol. Esterases act on water-soluble substrates with a preference for short fatty acid chains, whereas lipases work at the water-lipid interface, and their major substrates are long-chain triacylglycerols (4, 26). Lipases from Candida, Pseudomonas, Bacillus, and Rhizopus have been extensively studied for biotechnology (47). In addition to their role in catalyzing ester hydrolysis, lipases function as biocatalysts for transesterification, alcoholysis, acidolysis, and aminolysis reactions and are widely used in the food, detergent, chemical, and biochemical industries (47). Lipases and phospholipases secreted by pathogenic microorganisms are virulence factors in animals (13) and plants (66). The colonization and persistence by Propionibacterium acnes may be enhanced by a lipase that hydrolyzes triacylglycerides on human skin (21).
Esterases, like lipases, are found in animals, plants, and microorganisms (45). The physiological functions of bacterial esterases are less clear, although some may be involved in plant pathogenicity, carbon source provision, and biocide detoxification (33). Nevertheless, esterase enzymes play important roles as catalysts in biotechnology. In addition to ester hydrolysis, esterases catalyze interesterification, aminolysis, and peracid formation (8, 18). These biochemical reactions are used in the pharmaceutical and food industries (18).
The three-dimensional structures of some lipases and esterases show a characteristic α/β hydrolase fold, with a central, predominantly parallel β-sheet flanked by α-helical connections (8). For this reason, these enzymes have been classified as members of a large superfamily called the α/β hydrolase superfamily (41, 46). Esterase and lipase members of the superfamily also share a characteristic sequence motif, G-X-S-X-G, called the nucleophilic elbow (41, 46). The serine residue in this motif constitutes a catalytic triad with aspartate and histidine residues that are placed in this specific order (i.e., serine-aspartate-histidine). Recently, several other sequence motifs have also been found with this superfamily (4, 57).
Some lipases and esterases do not exhibit the conventional pentapeptide G-X-S-X-G but rather display a G-D-S-(L) [Gly-Asp-Ser-(Leu)] consensus sequence containing the active-site serine residue (4). These enzymes have an α/β-tertiary fold that differs substantially from the α/β hydrolase fold (8). Unlike the common lipases, GDSL enzymes do not have the nucleophilic elbow (1). GDSL enzymes have five consensus sequence blocks (I to V) and four invariant catalytic residues, Ser, Gly, Asn, and His, in blocks I, II, III, and V, respectively (1).
A new family of bacterial lipolytic enzymes, called patatin-like proteins, has recently been proposed (5). Patatin is the major soluble storage protein in potato tubers. This protein displays lipid acyl hydrolase and acyl transferase activities (2, 48, 52). The role of patatin in potato tubers remains unclear. It has been speculated that the lipase activity might confer resistance to pathogens (23). Patatin homologues are highly represented in some animal pathogen and plant pathogen/symbiont genomes, suggesting that they confer an advantage (5).
In spite of the fact that lipids have a prominent role in the M. xanthus life cycle, there have been few attempts to examine them in detail (61). The recent completion of the M. xanthus genome sequence (20) enables a comprehensive examination of putative lipase genes beginning with a bioinformatics approach. M. xanthus has a large number of putative lipase genes in three main families: patatin lipases, α/β hydrolases, and GDSL lipases. In this work, we focused on one member of each family. The phenotypes of deletion strains are characterized, gene expression is examined, and the proteins, expressed in Escherichia coli, are characterized in vitro.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. All Myxococcus xanthus strains were grown in CYE broth [10 g of Difco Casitone per liter, 5 g of yeast extract per liter, 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS; pH 7.6), and 4 mM MgSO4] (9) with vigorous shaking (300 rpm) at 32°C. M. xanthus strains were maintained using CYE plates supplemented with 40 μg ml−1 kanamycin when necessary. Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) broth (54), which was supplemented with ampicillin (100 μg ml−1) or kanamycin (25 μg ml−1) when needed.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype/phenotype or relevant feature/genea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | F′ [traD36 proAB+lacIqlacZΔM15] recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) | 71 |
| BL21 Star (DE3) | F−ompT hsdSB(rB− mB−) gal dcm rne-131 (DE3) | Invitrogen |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| M. xanthus | ||
| DK1622 | Wild type | 30 |
| LS2500 | ΔMXAN_3852 Galr Kms | This study |
| LS2501 | ΔMXAN_5522 Galr Kms | This study |
| LS2502 | ΔMXAN_4569 Galr Kms | This study |
| LS2530 | MXAN_3852-lacZ Kmr | This study |
| LS2531 | MXAN_5522-lacZ Kmr | This study |
| LS2532 | MXAN_4569-lacZ Kmr | This study |
| MxH2129 | aglU sglK Kmr | 72 |
| SW501 | difE Kmr | 70 |
| Plasmids | ||
| pBJ113 | galK Kmr | 28 |
| pET102/D-TOPO | Expression vector; Apr | Invitrogen |
| pKY481 | lacZY Kmr | 11 |
| pAMM6 | ΔMXAN_3852 Kmr | This study |
| pAMM7 | ΔMXAN_5522 Kmr | This study |
| pAMM10 | ΔMXAN_4569 Kmr | This study |
| pAMM11 | pET with MXAN_3852 Apr | This study |
| pAMM12 | pET with MXAN_5522 Apr | This study |
| pAMM15 | pET with MXAN_4569 Apr | This study |
| pAMM18 | MXAN_3852-lacZ Kmr | This study |
| pAMM19 | MXAN_5522-lacZ Kmr | This study |
| pAMM20 | MXAN_4569-lacZ Kmr | This study |
Kmr, Kms, Apr, and Galr refer to kanamycin resistance, kanamycin sensitivity, ampicillin resistance, and galactose resistance, respectively.
Construction of in-frame mutants.
The in-frame deletion strains were generated by allelic exchange using the GalK selection method (64). Briefly, sequences upstream and downstream of the genes MXAN_3852, MXAN_5522, and MXAN_4569 were amplified from wild-type chromosomal DNA by use of the high-fidelity polymerase Pfu (Stratagene) with PCR using the primers listed in Table 2. The amplified products were then cloned into HindIII- and EcoRI-digested pBJ113 (28) to obtain plasmids pAMM6, pAMM7, and pAMM10, respectively. The resulting nonreplicating plasmids carrying the deletion were introduced into wild-type M. xanthus by electroporation (31). Chromosomal integration was selected for by plating cells onto CYE plates containing 80 μg ml−1 kanamycin (positive selection). Individual Kmr transformants were diluted and plated onto CYE plates containing 1% galactose for negative selection. PCR analysis was used to screen the Kms Galr colonies for the loss of the wild-type allele. Verified MXAN_3852, MXAN_5522, and MXAN_4569 deletion mutants were designated LS2500, LS2501, and LS2502, respectively.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Purpose | Sequence (5′→3′)a |
|---|---|---|
| MXAN3852HindUp | Amplification upstream of MXAN_3852 (pAMM6) | CGAAGCTTGCTGGCGTTGCTGCGCCTCT |
| MXAN3852PstUp | Amplification upstream of MXAN_3852 (pAMM6) | ACCTGCAGTTCCATGCACCACCGGCCGG |
| MXAN3852PstDw | Amplification downstream of MXAN_3852 (pAMM6) | CCCTGCAGGCCTAGACCGCCGCGGGCTT |
| MXAN3852EcoDw | Amplification downstream of MXAN_3852 (pAMM6) | AGGAATTCGGCGCGGCGGAAGTGGGGCC |
| MXAN5522HindUp | Amplification upstream of MXAN_5522 (pAMM7) | CAAAGCTTTGACGGATGCCTATGCGGCC |
| MXAN5522PstUp | Amplification upstream of MXAN_5522 (pAMM7) | ACCTGCAGTCGCATGAGGAGGGGCTCCT |
| MXAN5522PstDw | Amplification downstream of MXAN_5522 (pAMM7) | GGCTGCAGCTCTAGACGGCCGCGCGCGA |
| MXAN5522EcoDw | Amplification downstream of MXAN_5522 (pAMM7) | TCGAATTCCACCGCCTGCTGCCGCAGGG |
| MXAN4569HindUp | Amplification upstream of MXAN_4569 (pAMM10) | TGAAGCTTGCCAGGACTGCTACTTCGCG |
| MXAN4569PstUp | Amplification upstream of MXAN_4569 (pAMM10) | GGCTGCAGTCCCATTCCCATTCCTCCCG |
| MXAN4569PstDw | Amplification downstream of MXAN_4569 (pAMM10) | AGCTGCAGCTCTAGCCGCGCCCCCGCGC |
| MXAN4569EcoDw | Amplification downstream of MXAN_4569 (pAMM10) | TCGAATTCGGCATGCGCAACACCGGGTA |
| pET3852_5′ | Amplification of MXAN_3852 (pAMM11) | CACCATGGAAGTTGCCGTCCGAGGT |
| pET3852_3′ | Amplification of MXAN_3852 (pAMM11) | GGCATGCATGGGGGGCTCCG |
| pET5522_5′ | Amplification of MXAN_5522 (pAMM12) | CACCATGCGAAACGCTGTCCGGACA |
| pET5522_3′ | Amplification of MXAN_5522 (pAMM12) | GAGGCCGAGCCCCTTCAGGC |
| pET4569_5′ | Amplification of MXAN_4569 (pAMM15) | CACCATGGGAATGAAGCCGGGGTGG |
| pET4569_3′ | Amplification of MXAN_4569 (pAMM15) | GAGGCCGCCCTCTTCGCGCA |
| 3852ZKpn | Amplification upstream of MXAN_3852 (pAMM18) | CGGGTACCGCTGGCGTTGCTGCGCCTCT |
| 3852ZBam | Amplification upstream of MXAN_3852 (pAMM18) | GAGGATCCATGGCACGCCTCCTGGGAAA |
| 5522ZKpn | Amplification upstream of MXAN_5522 (pAMM19) | CAGGTACCTGACGGATGCCTATGCGGCC |
| 5522ZBam | Amplification upstream of MXAN_5522 (pAMM19) | CGGGATCCATGAGGAGGGGCTCCTGTTC |
| 4569ZKpn | Amplification upstream of MXAN_4569 (pAMM20) | TGGGTACCGCCAGGACTGCTACTTCGCG |
| 4569ZBam | Amplification upstream of MXAN_4569 (pAMM20) | TCGGATCCATTCCCATTCCTCCCGGCTT |
Underlined are the restriction sites used in cloning and the sequence CACC for cloning into pET102/D-TOPO vector.
Fruiting body formation.
Fruiting bodies were obtained on CF and TPM agars. CF medium contains 10 mM Tris-HCl (pH 7.6), 1 mM KH2PO4-K2HPO4 (pH 7.6), 8 mM MgSO4, 0.02% (NH4)2SO4, 0.015% Bacto Casitone, 0.2% sodium citrate (pH 7.6), 0.1% sodium pyruvate (pH 7.6), and 15 g of Bacto agar per liter (22). TPM medium contains 10 mM Tris-HCl (pH 7.6), 1 mM KH2PO4-K2HPO4 (pH 7.6), 8 mM MgSO4 and 15 g of Bacto agar per liter (35). Cells were grown in CYE broth to approximately 5 × 108 cells ml−1 and resuspended to 8 × 109 cells m−1 in CYE broth. Ten microliters of each suspension was spotted onto agar plates containing the appropriate medium and incubated at 32°C with observation on a Wild Heerbrugg dissecting microscope over 5 days.
Sporulation assay.
M. xanthus cells were grown in CYE broth to approximately 5 × 108 cells ml−1 and concentrated to 1 × 1010 cells ml−1 in CYE broth. Five drops of 10 μl each were spotted on CF or TPM agar and incubated at 32°C. At different times, the fruiting bodies of one plate were harvested, resuspended in 250 μl of TPM buffer, heated at 50°C for 2 h, and then sonicated at a 40% duty cycle for 4× 5 s on an ultrasonic processor (Heat Systems-Ultrasonic) to kill vegetative cells. Spore production was determined by direct count of refractile myxospores with a Petroff-Hausser counting chamber.
Predation assay.
Strains DK1622, LS2500, LS201, and LS2502 were grown in CYE broth to approximately 5 × 108 cells ml−1 and diluted to 1 × 108 cells ml−1 in CYE broth. E. coli was grown to 1 × 109 cells ml−1. M. xanthus cells were mixed with E. coli cells at a ratio of 1 to 10. Ten drops of 20 μl each were spotted on TPM agar and incubated at 32°C for 5 h. Cells were removed from TPM and suspended in 2% glutaraldehyde for 12 h or more. Glutaraldehyde-fixed cells were subjected to sonic oscillation for 30 seconds to disrupt cell clumps, and the cells were counted in a Petroff-Hausser counting chamber. Cell size and morphology were used to distinguish M. xanthus cells from E. coli cells.
Construction and assay of strains harboring translational lacZ fusions.
The lacZ fusion plasmids were constructed using vector pKY481 (11). By use of Pfu polymerase (Stratagene), fragments encompassing the upstream MXAN_3852, MXAN_5522, and MXAN_4569 regions were amplified from wild-type chromosomal DNA by use of oligonucleotide pairs 3852ZKpn-3852ZBam, 5522ZKpn-5522ZBam, and 4569ZKpn-4569ZBam, respectively (Table 2). The BamHI site in the primers was introduced at the start codon of the genes and in frame with the BamHI site existing in the lacZ gene of plasmid pKY481. PCR products were digested with KpnI and BamHI and ligated to vector pKY481 digested with the same enzymes. The resulting plasmids, pAMM18 (MXAN_3852-lacZ), pAMM19 (MXAN_5522-lacZ), and pAMM20 (MXAN_4569-lacZ), were introduced into M. xanthus wild-type cells by electroporation to generate kanamycin-resistant transformants. The resultant strains harboring the fusions, LS2530 (MXAN_3852-lacZ), LS2531 (MXAN_5522-lacZ), and LS2532 (MXAN_4569-lacZ), were confirmed by PCR analysis.
Strains LS2530, LS2531, and LS2532 were grown in CYE broth to approximately 5 × 108 cells ml−1 and concentrated to 8 × 109 cells ml−1 in TM buffer (10 mM Tris-HCl [pH 7.6], 1 mM MgSO4). For preparation of cell extracts during development, 200 μl of concentrated cultures was spotted on TPM and CF agar and incubated at 32°C. At different times, fruiting bodies were harvested, resuspended in 200 μl of glass beads equilibrated in TM buffer, and sonicated as described previously (40). Cell debris and glass beads were removed by centrifugation. Preparation of cell extracts during vegetative growth followed the same procedure except for the use of CYE agar and the absence of glass beads during sonication. The amounts of protein in the supernatants were determined by using the Bio-Rad protein assay (Bio-Rad, Inc.) with bovine serum albumin as a standard. β-Galactosidase activity was determined as described by Kroos et al. (35). Specific activity is expressed as nmol of o-nitrophenol produced per min per mg of protein. The results are the averages of three different experiments.
Expression of lipolytic enzymes in E. coli.
For high-level expression of MXAN_3852, MXAN_5522, and MXAN_4569 in E. coli, the genes were PCR amplified with the primer pairs pET3852_5′-pET3852_3′, pET5522_5′-pET5522_3′, and pET4569_5′-pET4569_3′, respectively (Table 2). In all the cases, the forward primers contained CACC at their 5′ termini for cloning in the correct orientation with the pET102/D-TOPO (Invitrogen) expression vector. The purified PCR products were cloned in the vector according to the manufacturer's instruction, used to transform E. coli TOP10 competent cells, and plated on LB agar plates (100 mg ml−1 ampicillin). The resultant plasmids harboring the correct constructions, pAMM11 (pET with MXAN_3852), pAMM12 (pET with MXAN_5522), and pAMM15 (pET with MXAN_4569), were free of nucleotide substitutions. The recombinant plasmids were transformed into the expression strain E. coli BL21 Star (DE3) (Invitrogen). The fusion proteins were expressed as follows. Transformed BL21 Star (DE3) cells were grown in LB medium (containing 100 μg ml−1 ampicillin) on a rotary shaker (250 rpm) at 37°C until the optical density at 600 nm reached 0.5 to 0.7. Fifteen minutes before induction, the flasks were shifted to 15°C. Induction was performed by the addition of 1 mM of isopropyl-β-d-thiogalactopyranoside (IPTG). Induced cultures were incubated with shaking at 15°C for 6 h in the case of pET with MXAN_3852 and for 24 h in the case of pET with MXAN_5522 and pET with MXAN_4569 before the cells were harvested.
Purification of recombinant proteins.
Recombinant proteins were purified using the ProBond purification system (Invitrogen). Briefly, the bacterial pellets were resuspended in 8 ml of native binding buffer (50 mM NaH2PO4, 0.5 M NaCl [pH 8.0]), 1 mg ml−1 lysozyme, incubated on ice for 30 min before sonication, and cleared by centrifugation. The supernatants were then incubated for 1 h with ProBond nickel-chelating resin (Invitrogen). After being washed with 16 column volumes of native wash buffer (50 mM NaH2PO4, 0.5 M NaCl, 20 mM imidazole [pH 8.0]), bound proteins were eluted with native elution buffer containing 100, 300, or 500 mM imidazole (50 mM NaH2PO4, 0.5 M NaCl, with 100 mM, 300 mM, or 500 mM imidazole [pH 8.0], respectively). Fractions eluting with 300 mM imidazole were used in this work.
Western blot analysis.
Purified recombinant enzymes were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred onto nitrocellulose for Western blotting. The primary antibody was HisProbe-HRP (Pierce) and was used at a 1:5,000 dilution according to the manufacturer's instructions. The immunoblot was developed with an ECL luminescence detection kit (Amersham Pharmacia, Piscataway, NJ).
Determination of lipolytic activity.
Recombinant proteins were assayed using p-nitrophenyl (pNP) esters of fatty acids (Sigma) as substrates (50). The purified enzymes were added to a mixture containing 0.4 mM pNP ester, 2% Triton X-100, and 50 mM phosphate buffer (pH 7.0) and incubated at 37°C for 1 h. The release of pNP was measured spectrophotometrically at 405 nm. One unit of lipolytic activity was defined as the amount of enzyme that caused an increase of 0.01 absorbance units at 37°C for 1 h. Protein concentrations were determined using a bicinchoninic acid protein assay (Pierce). Activities on each substrate are expressed as the percentage of activity with pNP acetate (C2), which was taken as 100%.
RESULTS
Myxococcus xanthus has a large number of putative lipase genes in three main families: patatin lipases, α/β hydrolases, and GDSL lipases. Specifically, the M. xanthus genome contains 7 open reading frames (ORFs) that encode patatin-like lipases, 25 ORFs that encode α/β fold family members, and 4 ORFs that encode GDSL-like lipases. One member of each family was examined in detail.
Sequence analyses of MXAN_3852, MXAN_5522, and MXAN_4569.
MXAN_3852 encodes a 314-amino-acid protein. The start codon was chosen based on codon usage (25) and the presence of a putative ribosome-binding site (AGGAGG) 6 bases upstream. This protein lacks both predicted signal peptide and transmembrane domains. BLAST (55) results indicate homology with patatin lipases, members of a multigene family of plant vacuolar glycoproteins. Patatins represent 40% of total soluble potato tuber protein (49) and, while considered to be storage proteins, show lipid acyl hydrolase activity (2), possibly as a defense against plant parasites (62). Potato patatin B2 and human cytosolic phospholipase A2 share conserved domains of protein homology (23), including an active-site dyad instead of the more common Ser-His-Asp (or Glu) triad of lipolytic enzymes (53, 56).
Bacterial patatin-like proteins possess four conserved domains (blocks I to IV) similar to those in potato patatin B2 (5). All these conserved domains are present in the MXAN_3852 protein (Fig. 1A). Block I consists of a glycine-rich region with a conserved Arg or Lys (Lys16 in the MXAN_3852 protein) residue, which probably serves as an oxyanion hole. Block II is located in proximity to block I (about 10 to 20 amino acids away) and comprises the hydrolase motif G-X-S-X-G with the putative active-site Ser (Gly40-X-Ser42-X-Gly44) (56). Block III contains a conserved Ser (Ser144), which may be an important structural element due to the capacity to form hydrogen bonds or serve as a potential phosphorylation site. The adjacent highly conserved Pro residues (in blocks III and IV) (Pro148 and Pro153) may be important for the proper conformation of the protein. Block IV contains the active-site Asp (Asp161) residue that forms the catalytic Ser-Asp dyad.
FIG. 1.
Protein sequence alignments of conserved blocks of Myxococcus xanthus enzymes. (A) Alignment of the M. xanthus MXAN_3852 protein with conserved patatin domains. (B) Comparison of the MXAN_5522 protein with characterized α/β hydrolases. (C) Alignment of the MXAN_4569 protein with GDSL enzymes. Shown on gray and black backgrounds are identical amino acids, and functionally similar amino acids are shown in black letters. Residues necessary for catalytic activity are shown on a black background. The numbers between blocks indicate the numbers of amino acids preceding or following the conserved blocks.
MXAN_5522 encodes a 308-amino-acid protein with a putative signal peptide for the translocation across the cytoplasmic membrane. The predicted cleavage site of the signal peptide is located between Ala24 and Asn25 according to the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP/) (6). The deduced amino acid sequence of the MXAN_5522 protein resembles that of the lipase subfamily of α/β hydrolases, enzymes with a catalytic triad usually formed by Ser, His, and Asp residues (46). In the amino acid sequences of α/β hydrolases, the three conserved residues follow the order Ser-Asp-His. The serine residue is common to both the pentapeptide and the catalytic triad (4).
MXAN_5522 encodes a Gly-His-Ser-His-Gly sequence (positions 105 to 109) (Fig. 1B), which matches with the consensus sequence conserved in the active center of lipases. In addition, some functionally important residues are strictly conserved in the MXAN_5522 protein, such as residues Cys208 and Cys259, which form a disulfide bond, two conserved Asp residues for coordinating a Ca2+ ion (Asp235 and Asp277), and the catalytic triad Ser107 Asp253 His275 (Fig. 1B).
The MXAN_5522 protein has significant identity to triacylglycerol lipases from organisms such as Pseudomonas aeruginosa, Acinetobacter calcoaceticus, and Burkholderia cepacia. Triacylglycerol lipases cleave ester bonds on all three glycerol sn positions. Bacterial α/β hydrolases are divided into two evolutionarily distinct classes on the basis of the codon for the active-site serine, which can be either AGY or TCN (3). The MXAN_5522 protein belongs to the first group; the catalytic serine corresponds to the codon AGC. This first group is further divided into four subgroups (26) based on the mechanism of secretion and, more interestingly, the presence of a lipase chaperone gene next to the lipase gene. The chaperone functions in folding the lipase in the periplasm. The first subgroup is characterized by Xcp-dependent secretion and the presence of the lipase chaperone in an operon with the lipase gene itself. The MXAN_5522 protein likely falls into this first subgroup, as MXAN_5523 encodes a lipase chaperone separated by 27 base pairs from MXAN_5522. Separation between lipase genes and their cognate chaperones of up to 35 base pairs is common (63). The M. xanthus lipase and lipase chaperone both have predicted signal secretion sequences, and the lipase chaperone has a predicted transmembrane helix at the N terminus, used to anchor lipase chaperones to the inner membrane.
MXAN_4569 encodes a 424-amino-acid protein. The start codon was chosen based on codon usage and the presence of a putative ribosome-binding site (GGGAGGAA) 5 bases upstream of the ATG. A putative signal peptide cleavage site is located between Ala20 and Cys21. The MXAN_4569 protein shows similarities to GDS(L) lipases, a family of bacterial esterases with varied properties (10). GDS(L) proteins are characterized by a Gly-Asp-Ser-(Leu) [G-D-S-(L)] (Gly241-Asp-242-Ser243 in MXAN_4569) (Fig. 1C) motif containing the active-site serine residue in block I (4). Recently, a subgroup of this GDS(L) family was further classified as an SGNH hydrolase due to the presence of four strictly conserved residues, Ser, Gly, Asn, and His, in four conserved blocks, blocks I, II, III, and V, respectively (1). Each of the four residues plays a key role in the catalytic function of the enzyme. The catalytic Ser in block I (Ser243) serves as a nucleophile and a proton donor to the oxyanion hole. The Gly residue in block II (Gly278) and the Asn in block III (Asn308) serve as the proton donors to the oxyanion hole. The His residue in block V (His401) acts as a base to make the active-site Ser more nucleophilic by deprotonating the hydroxyl group. Another feature in block V is the presence of Asp (Asp398) located at the third amino acid preceding His (His401) (i.e., Asp-X-X-His serves as the third member of the catalytic triad). All of these conserved residues are present in the amino acid sequence of the MXAN_4569 protein (Fig. 1C).
Phenotypic characterization of in-frame deletion mutants.
To study the role of each gene in the life cycle of M. xanthus, markerless, in-frame deletion mutants for each of the lipolytic genes were created. The strains harboring the deletions in MXAN_3852, MXAN_5522, and MXAN_4569 were called LS2500, LS2501, and LS2502, respectively.
No differences were observed when mutant strains and the wild type were cultured in the rich liquid medium CYE. The generation time and cell density at the stationary phase were identical to those of the wild-type strain (data not shown). Moreover, the death phase also occurred with the same slope. The mutants showed wild-type motility on CYE agar (Fig. 2).
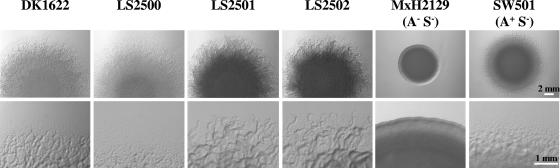
FIG. 2.
Comparison of the colony edges of Myxococcus xanthus DK1622 and two motility mutants with the in-frame deletion mutations (LS2500, ΔMXAN_3852; LS2501, ΔMXAN_5522; LS2502, ΔMXAN_4569). Cells were grown in liquid CYE to approximately 5 × 108 cells ml−1 and concentrated to 5 × 109 cells ml−1 in CYE. Ten microliters was spotted in CYE agar. Pictures were taken after 96 h of incubation at 32°C. All the pictures in the same row were taken at the same magnification. All three mutants exhibited normal motility. A, adventurous motility; S, social motility.
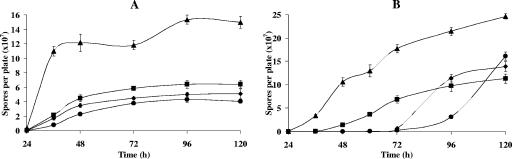
The deletion mutants were plated on TPM and CF agar to study their developmental phenotypes. On TPM there was little difference in the shape of fruiting bodies from that for the wild type (Fig. 3). However, strain LS2501 forms threefold more spores than the wild type after 120 h of incubation (see Fig. 5A). Neither LS2500 nor LS2502 showed differences in spore production relative to the wild type at 120 h, although differences in the timing of sporulation were observed (see Fig. 5A).
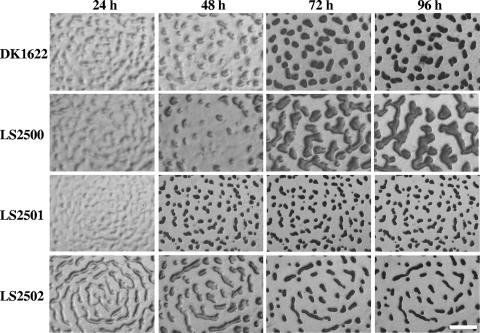
FIG. 3.
Morphologies of Myxococcus xanthus DK1622 and deletion mutant fruiting bodies on TPM agar (LS2500, ΔMXAN_3852; LS2501, ΔMXAN_5522; LS2502, ΔMXAN_4569). Cells were grown in liquid CYE to approximately 5 × 108 cells ml−1 and concentrated to 8 × 109 cells ml−1 in CYE. For analysis of aggregation, 10 μl was spotted on TPM agar and incubated at 32°C. Pictures were taken at indicated times. Bar, 1 mm.
FIG. 5.
Spore formation of Myxococcus xanthus DK1622 (diamonds) and of LS2500 (ΔMXAN_3852) (circles), LS2501 (ΔMXAN_5522) (triangles), and LS2502 (ΔMXAN_4569) (squares) deletion mutants on TPM (A) and CF (B) agars. The results shown are the averages of three different experiments. The error bars indicate standard deviations. Note differences in the scales.
On CF medium, LS2500 shows delayed aggregation and eventually makes larger fruiting bodies than the wild type (Fig. 4). LS2501 develops faster than the wild type, completing aggregation after 48 h compared with 72 h for the wild type. Moreover, the fruiting bodies formed by LS2501 are visibly smaller than fruiting bodies originated by the wild type. The differences between LS2502 and the wild type were even more pronounced on CF; mutant aggregation was largely complete after 24 h. Spore counts confirmed the accelerated development of LS2501 and LS2502 relative to the wild type (Fig. 5B). Again, the number of spores produced by LS2501 was threefold higher than that produced by the wild type.
FIG. 4.
Morphologies of Myxococcus xanthus DK1622 and deletion mutant fruiting bodies on CF agar (LS2500, ΔMXAN_3852; LS2501, ΔMXAN_5522; LS2502, ΔMXAN_4569). Cells were grown in CYE to approximately 5 × 108 cells ml−1 and concentrated to 8 × 109 cells ml−1 in CYE. Ten microliters was spotted on CF agar and incubated at 32°C. Pictures were taken at indicated times. Bar, 1 mm.
All three mutants were examined for their abilities to lyse E. coli cells in a predation assay. M. xanthus cells were mixed with E. coli cells at a ratio of 1 to 10 and incubated for 5 h on TPM agar. All three mutants consumed the E. coli cells at rates comparable to that of the wild type.
Expression of MXAN_3852, MXAN_5522, and MXAN_4569.
Gene expression was examined in M. xanthus strains harboring a translational fusion between the first codon of each gene and E. coli lacZ. Strains harboring the translational fusions in MXAN_3852, MXAN_5522, and MXAN_4569 were called LS2530, LS2531, and LS2532, respectively. β-Galactosidase activities in these strains were determined on both TPM and CF starvation agar as well as on the vegetative medium CYE agar.
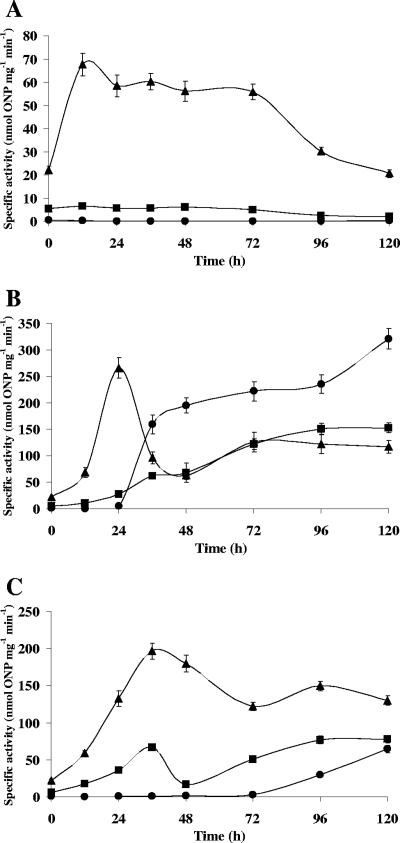
MXAN_3852 is not expressed during growth (Fig. 6A), and expression increases about 36 h after starvation on TPM (Fig. 6B) and 96 h on CF (Fig. 6C). Activity increased after that time in both media, but the levels of expression on TPM were sixfold higher than those on CF after 120 h.
FIG. 6.
Expression of Myxococcus xanthus lipolytic enzyme translational fusions with β-galactosidase. β-Galactosidase-specific activity (nmol of o-nitrophenol produced per min per mg of protein) of LS2530 (MXAN_3852-lacZ) (circles), LS2531 (MXAN_5522-lacZ) (triangles), and LS2532 (MXAN_4569-lacZ) (squares) strains during vegetative growth in CYE agar (A) and development on TPM agar (B) and CF agar (C). The results are the averages of three different experiments. The error bars indicate standard deviations. Note differences in the scales.
While MXAN_5522 is expressed at moderate levels during vegetative growth on CYE (Fig. 6A), expression increases during development. Expression peaks at 24 h on TPM agar (Fig. 6B) and 36 h on CF agar (Fig. 6C) when cells have aggregated into fruiting bodies.
MXAN_4569 was expressed at a low and constant level during vegetative growth (Fig. 6A). Expression was developmentally regulated and increased about 20-fold on TPM (Fig. 6B) and 10-fold on CF (Fig. 6C). Peak expression coincides with spore formation.
Characterization of recombinant enzymes.
In order to investigate the biochemical properties of the MXAN_3852, MXAN_5522, and MXAN_4569 proteins, these proteins were expressed with a six-histidine tag at their C termini in E. coli and purified to homogeneity using ProBond nickel-chelating resin (Invitrogen). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of purified proteins showed the enzymes to be of the estimated molecular masses. This result was confirmed by Western blot analysis using an anti-His antibody, which recognizes the carboxy-terminal six-histidine tag on the recombinant proteins (data not shown).
The substrate specificities of purified enzymes were determined using pNP esters with chain lengths ranging from C2 to C16. Studies of substrate specificity of lipolytic enzymes may give rise to artifacts when changes in physicochemical properties of the substrates are not controlled (23). To avoid undesired effects, the substrates were dissolved at low molarity in the inert detergent Triton X-100.
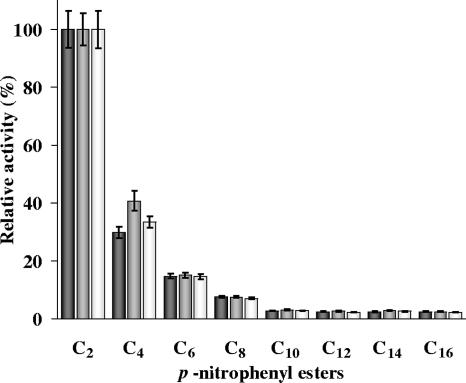
As shown in Fig. 7, the enzymes exhibited a marked preference for short-chain fatty acids, yielding the highest activity against pNP acetate (C2). Hydrolytic activity declined with increasing chain length. Hydrolytic activity toward pNPs with chain lengths ranging from C10 to C16 was less than 5%.
FIG. 7.
Relative activities of MXAN_3852 (black bars), MXAN_5522 (gray bars), and MXAN_4569 (white bars) enzymes towards pNP esters with different carbon chain lengths: C2, pNP acetate; C4, pNP butyrate; C6, pNP caproate; C8, pNP caprylate; C10, pNP caprate; C12, pNP laurate; C14, pNP myristate; and C16, pNP palmitate. Activities on each substrate are expressed as the percentages of activity relative to that with pNPC2, which was taken as 100%. Values are the averages of triplicate determinations, with standard deviations indicated.
DISCUSSION
Lipolytic enzymes govern the turnover of lipids and the biogenesis of membranes in all Bacteria. They also augment the life cycle of Myxococcus xanthus during predation and development. Though lysis of prey cells is observed following direct physical contact with M. xanthus (38, 58, 73), lytic enzymes can precede the arrival of the predator (36), potentially establishing gradients of hydrolytic products (7). Lipolytic enzymes would remove the barrier between the predator and the rich supply of nutrients inside the prey. In addition, fatty acids are rich sources of carbon and energy (37). During development, lipolytic enzymes may play a role in mobilizing the cellular lipid reserves and generating cell signals. For these reasons, putative lipases were examined in more detail. The M. xanthus genome contains genes for lipases belonging to three different protein families. One member of each of these families was examined.
The MXAN_3852 protein contains the conserved motifs that characterize patatins. This family of enzymes typically requires the previous action of other hydrolytic enzymes that produce a substantially degraded product. Patatin hydrolyzes mono- and diacyl phospholipids, monoacylglycerols, and pNPs but not diacylglycerols and triacylglycerols (2, 23). Since the action of this enzyme would require other hydrolytic enzymes to initiate degradation of triacylglycerols and diacylglycerols, it not surprising that the MXAN_3852 protein has minimal expression during vegetative growth and early development. On CF agar, all aspects of MXAN_3852 development were slightly delayed but eventually wild-type levels of spores were produced.
MXAN_5522 encodes an α/β hydrolase with identity to triacylglycerol lipases from other microorganisms. The presence of a chaperone lipase gene (MXAN_5523) next to the lipase gene is a characteristic of a subgroup of bacterial α/β hydrolases (3, 26). On CF agar, the MXAN_5522 mutant exhibited accelerated development relative to the wild-type strain. These results suggest that a checkpoint that delays development is missing in this mutant. MXAN_5522 is expressed during vegetative growth and early development. One possibility is that this checkpoint consists of consumption of lipid reserves that cannot be mobilized by the lipase mutant.
MXAN_5522 also produced threefold more spores than wild-type cells on CF agar. Higher spore yields were also evident on TPM agar, which produces more extreme cell starvation conditions. It is not clear why high levels of spores are produced, but this phenotype has rarely been reported. Fruiting body development produces cells with three different fates. The majority of the cells die (69), presumably aiding development through the release of nutrients or factors inducing sporulation (68). A smaller fraction, about 10 to 20% of the cells, never enter the fruiting body and develop a pattern of gene expression altered from that for developing cells (42-44). These peripheral rods are thought to absorb transient influxes of nutrients without the commitment to spore germination (43). It is also possible that peripheral rods protect the fruiting body from other organisms using hydrolytic enzymes and antibiotics. The increased spore yield must come from either the peripheral rods or the autolytic cells, which argues that MXAN_5522 plays a role in cell fate determination. Differences in spore yield relative to the wild type were smaller at lower cell densities (data not shown).
The MXAN_4569 protein contains the conserved residues that characterize SGNH hydrolases, a subgroup of the GDSL lipase family (1, 10). On CF agar, the MXAN_4569 mutant developed slightly faster than the wild type but formed normal levels of spores.
All in all, the developmental phenotypes of the three lipase mutants were minor and predation was indistinguishable from that seen for the wild type. One possibility for the lack of striking phenotypes is the fact that there are such a large number of putative lipase genes. It is possible that the lipases have redundant or overlapping functions.
Substrate specificities of purified enzymes were determined using pNPs of various chain lengths. All three proteins had optimal activity for short-chain (C2) pNP (pNPC2). These enzymes were able to hydrolyze medium-chain esters (pNPC4 to pNPC8) but not long-chain esters (pNPC10 to pNPC16) (Fig. 7). Lipases are, by definition, carboxylesterases that hydrolyze long-chain acylglycerols (≥C10), whereas esterases hydrolyze ester substrates with short-chain fatty acids (≤C10) (65). Sequence information of cloned esterase and lipase genes has been used to identify sequence-specific motifs. Most esterases display a conserved sequence motif (GESAG) around the central active-site serine residue. However, the GESAG motif is also found in some lipases, for example the lipases from Candida rugosa, Geotrichum candidum, and Yarrowia lipolytica. The ProSite esterase consensus active site is F-[GR]-G-x(4)-[LIVM]-x-[LIV]-x-G-S-[STAG]-G (S is the active-site residue). According to ProSite, lipases have a consensus motif of [LIV]-x-[LIVFY]-[LIVMST]-G-[HYWV]-S-x-G-[GSTAC] (S is the active-site residue). These motifs are so similar that differentiation between esterases and lipases must be determined biochemically. These M. xanthus enzymes could be considered esterases, since the preferred substrates were short-chain fatty acid esters (≤C10).
Esterases and other lipolytic enzymes have attracted considerable interest from industry because of their biotechnological potential (51). The wide range of properties with respect to substrate specificity and enantioselectivity has opened up a broad spectrum of applications (27). Despite their industrial importance, there is very little information on the physiological functions of esterases (45). Some esterases appear to be involved in metabolic pathways that provide access to carbon sources, e.g., the acetyl and cinnamoyl esterases that take part in the degradation of hemicellulose (14, 19). Cell-degrading esterases are believed to be important virulence factors in plant pathogens (39). The inactivation of biocides may be important in certain situations for Bacillus subtilis, which has been shown to produce an esterase that hydrolyzes the phytotoxin brefeldin A (67).
Acknowledgments
We thank Raquel García-Hernández, Patrick Curtis, and Swapna Bhat for critical review of the manuscript.
This material is based on work supported by the National Science Foundation under grant 0343874 to L.J.S. A.M.-M. is a postdoctoral fellow from “Programa de becas postdoctorales en España y en el extranjero, incluidas las becas MEC/Fulbright,” Ministerio de Educación y Ciencia, España.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Akoh, C. C., G. C. Lee, Y. C. Liaw, T. H. Huang, and J. F. Shaw. 2004. GDSL family of serine esterases/lipases. Prog. Lipid Res. 43:534-552. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, D. L., B. Beames, M. D. Summers, and W. D. Park. 1988. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem. J. 252:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthonsen, H. W., A. Baptista, F. Drablos, P. Martel, S. B. Petersen, M. Sebastiao, and L. Vaz. 1995. Lipases and esterases: a review of their sequences, structure and evolution. Biotechnol. Annu. Rev. 1:315-371. [DOI] [PubMed] [Google Scholar]
- 4.Arpigny, J. L., and K. E. Jaeger. 1999. Bacterial lipolytic enzymes: classification and properties. Biochem. J. 343:177-183. [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji, S., and A. Flieger. 2004. Patatin-like proteins: a new family of lipolytic enzymes present in bacteria? Microbiology 150:522-525. [DOI] [PubMed] [Google Scholar]
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bonner, P. J., and L. J. Shimkets. 2006. Phospholipid directed motility of surface-motile bacteria. Mol. Microbiol. 61:1101-1109. [DOI] [PubMed] [Google Scholar]
- 8.Bornscheuer, U. T. 2002. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 26:73-81. [DOI] [PubMed] [Google Scholar]
- 9.Campos, J. M., J. Geisselsoder, and D. R. Zusman. 1978. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:167-178. [DOI] [PubMed] [Google Scholar]
- 10.Carinato, M. E., P. Collin-Osdoby, X. Yang, T. M. Knox, C. A. Conlin, and C. G. Miller. 1998. The apeE gene of Salmonella typhimurium encodes an outer membrane esterase not present in Escherichia coli. J. Bacteriol. 180:3517-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, K., and D. R. Zusman. 1999. AsgD, a new two-component regulator required for A-signalling and nutrient sensing during early development of Myxococcus xanthus. Mol. Microbiol. 34:268-281. [DOI] [PubMed] [Google Scholar]
- 12.Curtis, P. D., R. Geyer, D. C. White, and L. J. Shimkets. 2006. Novel lipids in Myxococcus xanthus and their role in chemotaxis. Environ. Microbiol. 8:1935-1949. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick, A., F. R. Stirling, S. L. Lindsay, and T. J. Evans. 2006. The type III pseudomonal exotoxin U activates the c-Jun NH2-terminal kinase pathway and increases human epithelial interleukin-8 production. Infect. Immun. 74:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalrymple, B. P., Y. Swadling, D. H. Cybinski, and G. P. Xue. 1996. Cloning of a gene encoding cinnamoyl ester hydrolase from the ruminal bacterium Butyrivibrio fibrisolvens E14 by a novel method. FEMS Microbiol. Lett. 143:115-120. [DOI] [PubMed] [Google Scholar]
- 15.Downard, J., and D. Toal. 1995. Branched-chain fatty acids: the case for a novel form of cell-cell signalling during Myxococcus xanthus development. Mol. Microbiol. 16:171-175. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworkin, M., and D. Kaiser (ed.). 1993. Myxobacteria II. ASM Press, Washington DC.
- 18.Faber, K. 1997. Biotransformations of non-natural compounds: state of the art and future development. Pure Appl. Chem. 69:1613-1633. [Google Scholar]
- 19.Ferreira, L. M., T. M. Wood, G. Williamson, C. Faulds, G. P. Hazlewood, G. W. Black, and H. J. Gilbert. 1993. A modular esterase from Pseudomonas fluorescens subsp. cellulosa contains a non-catalytic cellulose-binding domain. Biochem. J. 294:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman, B. S., W. C. Nierman, D. Kaiser, S. C. Slater, A. S. Durkin, J. Eisen, C. M. Ronning, W. B. Barbazuk, M. Blanchard, C. Field, C. Halling, G. Hinkle, O. Iartchuk, H. S. Kim, C. Mackenzie, R. Madupu, N. Miller, A. Shvartsbeyn, S. A. Sullivan, M. Vaudin, R. Wiegand, and H. B. Kaplan. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. USA 103:15200-15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribbon, E. M., W. J. Cunliffe, and K. T. Holland. 1993. Interaction of Propionibacterium acnes with skin lipids in vitro. J. Gen. Microbiol. 139:1745-1751. [DOI] [PubMed] [Google Scholar]
- 22.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 23.Hirschberg, H. J., J. W. Simons, N. Dekker, and M. R. Egmond. 2001. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. Eur. J. Biochem. 268:5037-5044. [DOI] [PubMed] [Google Scholar]
- 24.Inouye, M., S. Inouye, and D. R. Zusman. 1979. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev. Biol. 68:579-591. [DOI] [PubMed] [Google Scholar]
- 25.Inouye, S., M. Y. Hsu, S. Eagle, and M. Inouye. 1989. Reverse transcriptase associated with the biosynthesis of the branched RNA-linked msDNA in Myxococcus xanthus. Cell 56:709-717. [DOI] [PubMed] [Google Scholar]
- 26.Jaeger, K. E., S. Ransac, B. W. Dijkstra, C. Colson, M. van Heuvel, and O. Misset. 1994. Bacterial lipases. FEMS Microbiol. Rev. 15:29-63. [DOI] [PubMed] [Google Scholar]
- 27.Jaeger, K. E., and M. T. Reetz. 1998. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 16:396-403. [DOI] [PubMed] [Google Scholar]
- 28.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 97:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser, D. 2003. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 1:45-54. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser, D., C. Manoil, and M. Dworkin. 1979. Myxobacteria: cell interactions, genetics, and development. Annu. Rev. Microbiol. 33:595-639. [DOI] [PubMed] [Google Scholar]
- 31.Kashefi, K., and P. L. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 32.Kearns, D. B., A. Venot, P. J. Bonner, B. Stevens, G. J. Boons, and L. J. Shimkets. 2001. Identification of a developmental chemoattractant in Myxococcus xanthus through metabolic engineering. Proc. Natl. Acad. Sci. USA 98:13990-13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalameyzer, V., I. Fischer, U. T. Bornscheuer, and J. Altenbuchner. 1999. Screening, nucleotide sequence, and biochemical characterization of an esterase from Pseudomonas fluorescens with high activity towards lactones. Appl. Environ. Microbiol. 65:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 35.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 36.Kühlwein, H., and H. Reichenbach. 1968. Schwarmentwicklung und Morphogenese bei Myxobakterien: Archangium-Myxococcus-Chondrococcus-Chondromyces, signature C 893, p. 335-359. In G. Wolf (ed.) Göttingen encyclopaedia cinematographica, Institut für den wissenschaftlichen Film, Göttingen, Germany.
- 37.Lau, J., S. Frykman, R. Regentin, S. Ou, H. Tsuruta, and P. Licari. 2002. Optimizing the heterologous production of epothilone D in Myxococcus xanthus. Biotechnol. Bioeng. 78:280-288. [DOI] [PubMed] [Google Scholar]
- 38.McBride, M. J., and D. R. Zusman. 1996. Behavioral analysis of single cells of Myxococcus xanthus in response to prey cells of Escherichia coli. FEMS Microbiol. Lett. 137:227-231. [DOI] [PubMed] [Google Scholar]
- 39.McQueen, D. A., and J. L. Schottel. 1987. Purification and characterization of a novel extracellular esterase from pathogenic Streptomyces scabies that is inducible by zinc. J. Bacteriol. 169:1967-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Dorado, J., S. Inouye, and M. Inouye. 1991. A gene encoding a protein serine/threonine kinase is required for normal development of Myxococcus xanthus, a gram-negative bacterium. Cell 67:995-1006. [DOI] [PubMed] [Google Scholar]
- 41.Nardini, M., and B. W. Dijkstra. 1999. Alpha/beta hydrolase fold enzymes: the family keeps growing. Curr. Opin. Struct. Biol. 9:732-737. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor, K. A., and D. R. Zusman. 1991. Analysis of Myxococcus xanthus cell types by two-dimensional polyacrylamide gel electrophoresis. J. Bacteriol. 173:3334-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor, K. A., and D. R. Zusman. 1991. Behavior of peripheral rods and their role in the life cycle of Myxococcus xanthus. J. Bacteriol. 173:3342-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor, K. A., and D. R. Zusman. 1991. Development in Myxococcus xanthus involves differentiation into two cell types, peripheral rods and spores. J. Bacteriol. 173:3318-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuda, H. 1991. Esterases, p. 563-577. In S. A. Kuby (ed.), A study of enzymes. CRC Press, Boca Raton, FL.
- 46.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, et al. 1992. The alpha/beta hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 47.Pandey, A., S. Benjamin, C. R. Soccol, P. Nigam, N. Krieger, and V. T. Soccol. 1999. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 29:119-131. [PubMed] [Google Scholar]
- 48.Racusen, D. 1984. Lipid acyl hydrolase of patatin. Can. J. Bot. 62:1640-1644. [Google Scholar]
- 49.Racusen, D., and M. Foote. 1980. A major soluble glycoprotein of potato tubers. J. Food Biochem. 4:43-52. [Google Scholar]
- 50.Redondo, O., A. Herrero, J. F. Bello, M. G. Roig, M. V. Calvo, F. J. Plou, and F. J. Burguillo. 1995. Comparative kinetic study of lipases A and B from Candida rugosa in the hydrolysis of lipid p-nitrophenyl esters in mixed micelles with Triton X-100. Biochim. Biophys. Acta 1243:15-24. [DOI] [PubMed] [Google Scholar]
- 51.Riedel, K., D. Talker-Huiber, M. Givskov, H. Schwab, and L. Eberl. 2003. Identification and characterization of a GDSL esterase gene located proximal to the swr quorum-sensing system of Serratia liquefaciens MG1. Appl. Environ. Microbiol. 69:3901-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosahl, S., J. Schell, and L. Willmitzer. 1987. Expression of a tuber-specific storage protein in transgenic tobacco plants: demonstration of an esterase activity. EMBO J. 6:1155-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rydel, T. J., J. M. Williams, E. Krieger, F. Moshiri, W. C. Stallings, S. M. Brown, J. C. Pershing, J. P. Purcell, and M. F. Alibhai. 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 42:6696-6708. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 55.Schaffer, A. A., L. Aravind, T. L. Madden, S. Shavirin, J. L. Spouge, Y. I. Wolf, E. V. Koonin, and S. F. Altschul. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29:2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrag, J. D., and M. Cygler. 1997. Lipases and alpha/beta hydrolase fold. Methods Enzymol. 284:85-107. [DOI] [PubMed] [Google Scholar]
- 57.Shaw, E., L. A. McCue, C. E. Lawrence, and J. S. Dordick. 2002. Identification of a novel class in the alpha/beta hydrolase fold superfamily: the N-myc differentiation-related proteins. Proteins 47:163-168. [DOI] [PubMed] [Google Scholar]
- 58.Shi, W., and D. R. Zusman. 1993. Fatal attraction. Nature 366:414-415. [DOI] [PubMed] [Google Scholar]
- 59.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 60.Shimkets, L. J. 1990. Social and developmental biology of the myxobacteria. Microbiol. Rev. 54:473-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorhaug, T. 1974. Glycerol ester hydrolase, lipase, of Myxococcus xanthus FB. Can. J. Microbiol. 20:611-615. [PubMed] [Google Scholar]
- 62.Strickland, J. A., G. L. Orr, and T. A. Walsh. 1995. Inhibition of Diabrotica larval growth by patatin, the lipid acyl hydrolase from potato tubers. Plant Physiol. 109:667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan, E. R., J. G. Leahy, and R. R. Colwell. 1999. Cloning and sequence analysis of the lipase and lipase chaperone-encoding genes from Acinetobacter calcoaceticus RAG-1, and redefinition of a proteobacterial lipase family and an analogous lipase chaperone family. Gene 230:277-286. [DOI] [PubMed] [Google Scholar]
- 64.Ueki, T., S. Inouye, and M. Inouye. 1996. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183:153-157. [DOI] [PubMed] [Google Scholar]
- 65.Verger, R. 1997. Interfacial activation of lipases: facts and artifacts. Trends Biotechnol. 15:32-38. [Google Scholar]
- 66.Voigt, C. A., W. Schafer, and S. Salomon. 2005. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 42:364-375. [DOI] [PubMed] [Google Scholar]
- 67.Wei, Y., L. Swenson, R. E. Kneusel, U. Matern, and Z. S. Derewenda. 1996. Crystallization of a novel esterase which inactivates the macrolide toxin brefeldin A. Acta Crystallogr. Sect. D 52:1194-1195. [DOI] [PubMed] [Google Scholar]
- 68.Wireman, J. 1979. Developmental induction of Myxococcus xanthus myxospores. J. Bacteriol. 140:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wireman, J. W., and M. Dworkin. 1975. Morphogenesis and developmental interactions in myxobacteria. Science 189:516-523. [DOI] [PubMed] [Google Scholar]
- 70.Yang, Z., Y. Geng, D. Xu, H. B. Kaplan, and W. Shi. 1998. A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Mol. Microbiol. 30:1123-1130. [DOI] [PubMed] [Google Scholar]
- 71.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 72.Youderian, P., N. Burke, D. J. White, and P. L. Hartzell. 2003. Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol. Microbiol. 49:555-570. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, H., N. N. Rao, T. Shiba, and A. Kornberg. 2005. Inorganic polyphosphate in the social life of Myxococcus xanthus: motility, development, and predation. Proc. Natl. Acad. Sci. USA 102:13416-13420. [DOI] [PMC free article] [PubMed] [Google Scholar]