Abstract
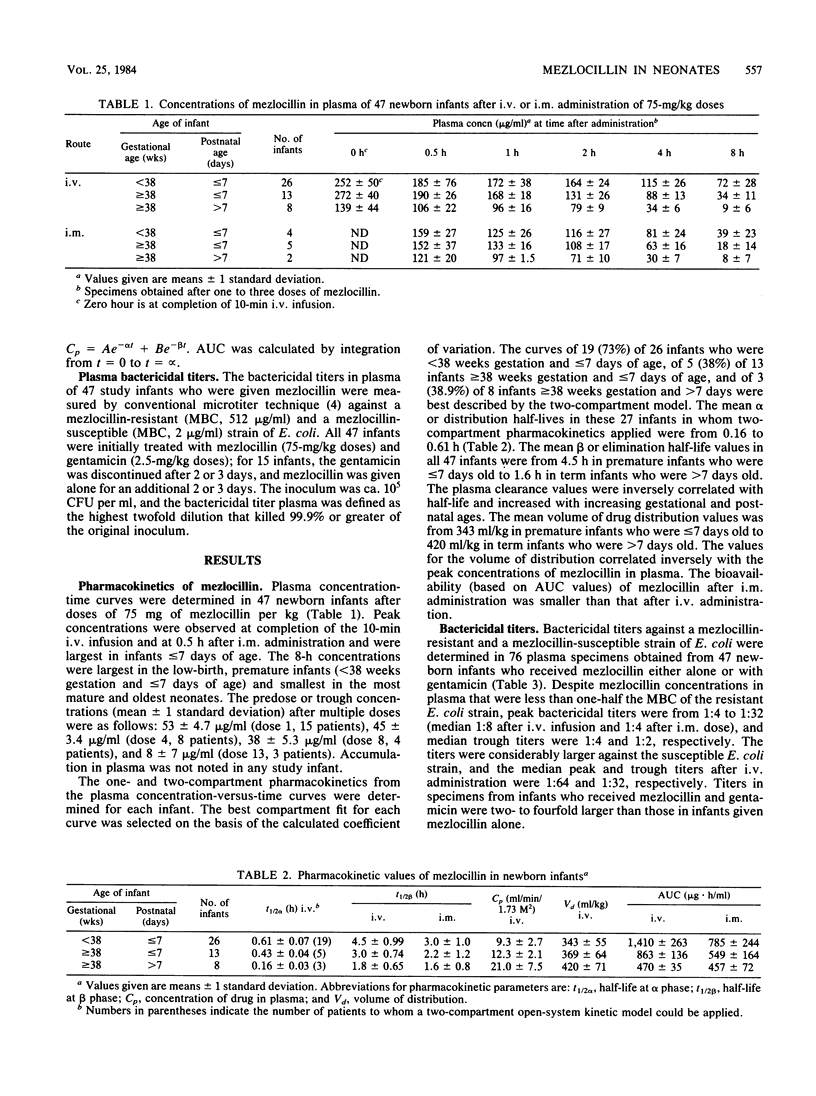
The pharmacokinetic properties of mezlocillin were evaluated in newborn infants. Mean peak and trough concentrations of drug in plasma, after 75 mg of mezlocillin per kg given intravenously, were 252 and 72 micrograms/ml, respectively, in infants who were less than 38 weeks gestation and less than or equal to 7 days old, compared with 139 and 9 micrograms/ml, respectively, in infants greater than or equal to 38 weeks gestation and greater than 7 days old. The mean elimination half-life values were from 4.5 h in preterm infants who were less than or equal to 7 days old to 1.8 h in term infants greater than or equal to 7 days old. Median peak and trough bactericidal titers of drug in plasma from neonates treated with mezlocillin were 1:8 and 1:4, respectively, against a resistant (minimal bactericidal concentration, 512 micrograms/ml) Escherichia coli strain and 1:64 and 1:32, respectively, against a susceptible (minimal bactericidal concentration, 2 micrograms/ml) E. coli strain. We propose a dosage schedule of 75 mg of mezlocillin per kg administered every 12 h to preterm (gestational age less than 38 weeks) infants less than or equal to 7 days old, every 8 h to preterm infants greater than 7 days old or term infants less than or equal to 7 days old, and every 6 h to term infants greater than 7 days old.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenbaum M. C. A method for testing for synergy with any number of agents. J Infect Dis. 1978 Feb;137(2):122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- Chiu T., Garrison R. D., Fakhreddine F., Ayoub E. M. Mezlocillin in neonatal infections: evaluation of efficacy and toxicity. J Antimicrob Chemother. 1982 Jan;9 (Suppl A):251–255. doi: 10.1093/jac/9.suppl_a.251. [DOI] [PubMed] [Google Scholar]
- Dutcher B. S., Reynard A. M., Beck M. E., Cunningham R. K. Potentiation of antibiotic bactericidal activity by normal human serum. Antimicrob Agents Chemother. 1978 May;13(5):820–826. doi: 10.1128/aac.13.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marymont J. H., Jr, Wentz R. M. Serial dilution antibiotic sensitivity testing with the microtitrator system. Am J Clin Pathol. 1966 May;45(5):548–551. doi: 10.1093/ajcp/45.5.548. [DOI] [PubMed] [Google Scholar]
- Nelson J. D., Kusmiesz H., Shelton S., Woodman E. Clinical pharmacology and efficacy of ticarcillin in infants and children. Pediatrics. 1978 Jun;61(6):858–863. [PubMed] [Google Scholar]
- Odio C., Thomas M. L., McCracken G. H., Jr Pharmacokinetics and bacteriological efficacy of mezlocillin in experimental Escherichia coli and Listeria monocytogenes meningitis. Antimicrob Agents Chemother. 1984 Apr;25(4):427–432. doi: 10.1128/aac.25.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Boccazzi A., McCracken G. H., Jr Pharmacokinetics and bacteriological effect of ceftazidime in experimental Streptococcus pneumoniae, Haemophilus influenzae, and Escherichia coli meningitis. Antimicrob Agents Chemother. 1983 Feb;23(2):213–217. doi: 10.1128/aac.23.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriological efficacy of moxalactam (LY127935), netilmicin, and ampicillin in experimental gram-negative enteric bacillary meningitis. Antimicrob Agents Chemother. 1980 Mar;17(3):406–411. doi: 10.1128/aac.17.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]



