Abstract
The affinities of the bacteriophage 434 repressor for its various binding sites depend on the type and/or concentration of monovalent cations. The ability of bacteriophage 434 repressor to govern the lysis-lysogeny decision depends on the DNA binding activities of the phage's cI repressor protein. We wished to determine whether changes in the intracellular ionic environment influence the lysis-lysogeny decision of the bacteriophage λimm434. Our findings show that the ionic composition within bacterial cells varies with the cation concentration in the growth media. When λimm434 lysogens were grown to mid-log or stationary phase and subsequently incubated in media with increasing monovalent salt concentrations, we observed a salt concentration-dependent increase in the frequency of bacteriophage spontaneous induction. We also found that the frequency of spontaneous induction varied with the type of monovalent cation in the medium. The salt-dependent increase in phage production was unaffected by a recA mutation. These findings indicate that the salt-dependent increase in phage production is not caused by activation of the SOS pathway. Instead, our evidence suggests that salt stress induces this lysogenic bacteriophage by interfering with 434 repressor-DNA interactions. We speculate that the salt-dependent increase in spontaneous induction is due to a direct effect on the repressor's affinity for DNA. Regardless of the precise mechanism, our findings demonstrate that salt stress can regulate the phage lysis-lysogeny switch.
In vitro studies show that the stability and specificity of protein-DNA complexes are remarkably dependent on the type and concentration of ions present in the solvent milieu (for a review, see reference 43). In large measure, the sensitivities of these complexes to changes in the salt concentration derive from the contributions of charge-charge interactions between the protein and DNA. In addition, the binding of cations to DNA and/or protein-DNA complexes can influence complex stability by modulating the overall structure of the protein-DNA interface (4, 5, 32). The demonstrated importance of ionic conditions for protein-DNA complex formation in vitro suggests that the intracellular ionic environment may also influence protein-DNA interactions in vivo.
We were interested in knowing whether changes in the intracellular ionic environment influence the lysis-lysogeny decision of lambdoid bacteriophages, in particular, bacteriophage 434 that infects Escherichia coli. Similar to bacteriophage λ, λimm434 is a temperate phage whose life cycle alternates between the lysogenic and lytic developmental pathways (11, 38). In a lysogen, the phage's genome is integrated into the chromosome of its host and is replicated along with the host chromosome. The lysogen is a metastable developmental state; all lysogenized phage can undergo lytic development (40). In lytic growth, phage DNA is not integrated into the chromosome; rather, its intracellular replication, assembly into phage particles, and subsequent host cell lysis result in phage production.
The switch from lysogenic to lytic growth is governed by the activities of the phage's cI repressor protein. The repressor directs the establishment and maintenance of the lysogenic state by simultaneously repressing transcription of the genes needed for lytic phage growth and activating transcription of a gene needed for lysogenization (40). Each phage genome contains two operator regions, OL and OR. Both regions contain promoters whose expression is controlled by repressor binding to multiple closely spaced binding sites. Efficient functioning of the genetic switch between lysis and lysogeny depends on the ability of the repressor to bind with a different affinity to each of these sites. Thus, inactivation of repressor DNA binding or alteration of the relative affinities for its binding sites would affect the repressor's gene-regulatory activities and lead to derepression of lytic genes and lytic growth (11, 12, 46).
Like most protein-DNA complexes, the affinity of 434 repressor for its binding sites varies with the salt concentration (2). However, the salt concentration dependence of 434 repressor's affinity for DNA also varies with the sequence of the binding site (2, 3). Changing the type of monovalent cation markedly affects the affinity of 434 repressor for OR1 but has no effect on the affinity of the repressor for OR3 (32). Since the repressor's ability to direct the lysis-lysogeny decision depends on its overall affinity for DNA and its relative affinities for OR1 and OR3, these findings suggest that the cytosolic cation type and concentration may play important roles in regulating the lysis-lysogen decisions of bacteriophage 434.
Previous studies indicated that the intracellular ionic concentration and composition within a bacterial cell vary in response to many stimuli, e.g., the external osmolarity, the external salt concentration, changes in the rate of cell growth, and the developmental stage of the cell (17-20, 30, 36, 44, 48-50). Increasing the external salt concentration causes accumulation of monovalent cations within the cell and immediate plasmolysis (cell shrinkage). These responses alter the concentrations of all cytoplasmic solutes. In addition, this treatment causes a rapid increase in negative DNA supercoiling. The increased negative supercoiling alters the activities of several osmoregulated genes (10, 22, 24, 25). These acute responses are followed by a distinct sequence of cellular events that includes active potassium influx and glutamate synthesis to restore the intracellular water balance and, finally, replacement of potassium glutamate with osmoprotectants more compatible with cell growth (see references 41 and 42 for reviews).
Our in vitro results demonstrating that changing the monovalent cation type and concentration differentially affects 434 repressor binding to its various operator sites (32, 33) suggested to us that an increased internal salt concentration might affect the interaction of the repressor with DNA, at least transiently. This hypothesis predicted that changing the external salt concentration would influence the stability of λimm434 lysogens stabilized by the DNA binding activity of 434 repressor. As an initial step in testing this idea, we determined the effect of changing the external salt type on the internal ionic composition and concentration inside cells. We then determined the effects of these changes on the spontaneous-induction frequencies of bacteriophage λimm434 lysogens. Our results show that changing the external salt type or concentration affects the internal ionic composition and consequently the spontaneous induction of λimm434 lysogens. Our data indicate that the salt-dependent changes in the spontaneous-induction frequency result from alterations in 434 repressor-DNA interactions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and phages.
The host strain for all manipulations was MG1655. Bacteriophage λimm434, a bacteriophage λ derivative that contains the immunity region of bacteriophage 434, was a gift from David Friedman (University of Michigan, Ann Arbor). A recA version of MG1655 was created by P1 transduction using a lysate derived from GW4212 (a gift from Mark Sutton, University at Buffalo, Buffalo, NY), which bears the recA938::cat allele (55). Both wild-type and recA derivatives of MG1655 were lysogenized with λimm434 as described previously (1). Where necessary, wild-type 434 repressor, a nondimerizing non-DNA binding mutant 434 repressor, or P22 repressor was overproduced from pRW220 (53, 54), p434R-E (14, 15), or pTP15 (39), respectively.
Effect of salt on spontaneous induction.
Cultures of MG1655 lysogenized with λimm434 were grown to saturation overnight in LB or M9 minimal medium at 37°C. To remove phage that were produced during the overnight growth, the stationary-phase cells were washed three times by centrifugation at 4,000 × g for 5 min and resuspended in fresh medium. Control experiments established that this procedure was sufficient to reduce the phage titer to <10 PFU/ml. We used these washed cells to examine the effect of added salt on spontaneous induction of lysogens in both stationary- and log-phase cells.
In experiments with stationary-phase cells, subsequent to the last wash, the cells were resuspended in a volume of culture medium equal to the starting culture volume. The suspension medium was either identical to the initial growth medium or contained various concentrations of the desired salt in the absence or presence of osmoprotectants, antibiotics, and/or IPTG (isopropyl-β-d-thiogalactopyranoside) as indicated. The culture was incubated at 37°C for 0.5 to 5 h, as desired. To examine the effect of salt on the stability of lysogens in log-phase cells, the washed cells were diluted 100-fold in medium that was identical to the initial growth medium. These resuspended cells were grown to early log phase (optical density at 600 nm, 0.25). To remove phage that were produced during growth to log phase, the cells were washed three times by centrifugation at 4,000 × g for 5 min and resuspended in fresh medium. To examine the effects of various growth media on phage production in this case, subsequent to the last wash, these log-phase cells were treated as described above.
To quantify the effect of salt shock on the amount of phage released, at the desired time, a portion of unshocked and salt-shocked cultures were centrifuged at 8,000 × g and the phage-containing supernatant was sterilized by addition of chloroform, followed by centrifugation at 8,000 × g. An aliquot of the remaining culture was plated to determine the number of CFU. The amount of phage released into the supernatant (PFU) was determined by plating them on nonlysogenic MG1655 as described previously (1). The amount of phage released per cell in culture under each condition was calculated as the PFU/CFU ratio. This was done to normalize the data across various experiments and experimental conditions. The effect of salt on phage production was evaluated as an n-fold increase determined from the number of phage produced per viable cell incubated in the presence of salt ([PFU/CFU]Salt) divided by the number of phage produced per viable cell in the absence of added salt ([PFU/CFU]No Salt). It should be noted that none of the treatments examined (salt changes, osmolyte addition, etc.), significantly affected cell viability.
Measurement of the internal ion composition.
The effect of changing the external salt type and concentration on the internal cation concentration and composition was determined by using inductively coupled plasma spectroscopy-mass spectrometry (ICP-MS). Salt-shocked cells were prepared as described above, except the cells were grown in acid-washed sterile glassware. To remove any monovalent or divalent cations associated with the external surfaces of the cells, after a 4-hour salt shock, the unshocked and shocked cells were pelleted at 4°C and washed three times with an ice-cold sterile buffer containing 50 mM ultrapure Tris (AnalR-BDH Chemicals), pH 7, 10 mM EDTA (free acid), and 100 mM glucose in acid-washed sterile tubes. Control experiments established that this procedure reduced the external salt (Na+, K+, Li+, or Mg2+) concentration to <1 part per billion. An aliquot of the washed cells was used to obtain a viable-cell count. Another aliquot of these cells was used lyophilized to calculate the dry weight. Digestion of cells to release internally retained ions was performed as described previously (31). Briefly, the washed cells were resuspended in trace metal grade 12 N nitric acid (Fisher Scientific) and incubated at 80°C for 1 h. Following digestion, the acid concentration was reduced to 2 N, and particulate matter was removed. The resulting supernatant was analyzed for the contents of K+, Na+, Li+, and Mg2+ at the Analytical and Technical Services facility of the State University of New York College of Environmental Science and Forestry. Measurements were reported as parts per million. Internal ion concentrations were calculated from these data, along with the dry weight of the cells and viable-cell counts to normalize for internal volume changes that resulted from salt shock.
Effect of varying the repressor concentrations in vivo on spontaneous induction.
To examine the effect of low-level overproduction of 434 repressor on spontaneous induction, MG1655 lysogenized with λimm434 was transformed with pRW220 (53, 54), a plasmid that directs the expression of 434 repressor from the IPTG-inducible lacUV5 promoter. In the absence of IPTG, this plasmid produces ∼10-fold more repressor than is present in a lysogen. In the presence of 1 mM IPTG, the plasmid directs the synthesis of ∼1% of the cell's protein as 434 repressor. The role of 434 repressor DNA binding activity in modulating the effect of salt shock on phage induction was evaluated by determining the amount of phage released from salt-shocked MG1655::λimm434 lysogens transformed with plasmids that direct the synthesis of ∼1% of the cell's protein as a non-DNA binding, nondimerizing mutant 434 repressor or the heterologous P22 represssor.
RESULTS
Changing the type and/or concentration of monovalent cations differentially alters the affinities of 434 repressor for its various binding sites (32, 33) in vitro. We wished to determine whether changing the salt type and concentration affected the gene-regulatory activities of bacteriophage 434 repressor in vivo. To do this, MG1655 cells (6) bearing a lysogenic bacteriophage containing the immunity region of bacteriophage 434 (λimm434) were grown to early log or stationary phase, washed with medium without added salt, and suspended in fresh medium without added salt or fresh media containing increasing concentrations of NaCl. Both cultures were incubated at 37°C for 0.5 to 5 h as desired. We obtained identical results with cells grown in LB or minimal medium using either stationary- or log-phase cells. The results obtained using stationary-phase cells grown in LB are discussed below.
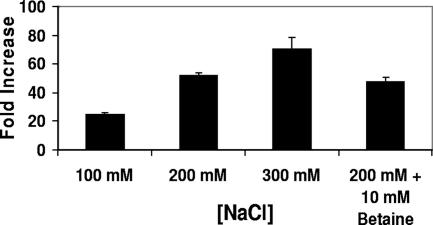
Under either growth regimen, when the washed MG1655 434 lysogens were incubated in medium without added salt, they spontaneously produced phage with a titer of ∼102/ml within 5 hours. However, incubating these stationary-phase cells in medium containing an additional 100 mM NaCl increased the amount of phage produced by the cells by ∼25-fold in this time period (Fig. 1). Growing these lysogenic cells in media containing increasing [NaCl] progressively increased the amount of bacteriophage released (Fig. 1).
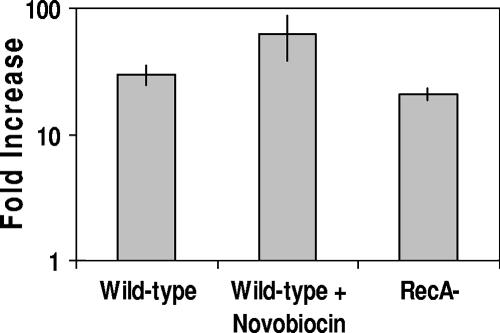
FIG. 1.
Effect of changing the external monovalent cation concentration on spontaneous induction of λimm434 434 lysogens in MG1655. Phage-containing supernatants were obtained from MG1655 cells lysogenized with λimm434 bacteriophage grown at the indicated salt concentrations in the absence or presence of the osmolyte betaine as described in Materials and Methods. The effect of salt shock (Fold Increase) is expressed as the number of phage produced per viable cell incubated in the presence of salt ([PFU/CFU]Salt) divided by the number of phage produced per viable cell in the absence of added salt ([PFU/CFU]No Salt) The error bars indicate standard deviations.
We analyzed the characteristics of the spontaneously released phage to determine whether salt affects lysogen stability by stimulating mutagenesis of the repressor gene and/or its binding sites. These studies showed that (i) the released phage form turbid plaques, indicating that the released phage encode a functional repressor, and (ii) the lysogens formed by the spontaneously released phage are as stable as those formed by naïve phage, and the salt inducibility profiles are identical to those of lysogens formed by phage collected by mitomycin C induction (data not shown). These findings indicate that salt stress alters the ability of 434 phage to maintain the lysogenic state directly and not indirectly by increasing the mutation frequency of the 434 repressor gene or its binding sites. These findings are consistent with the ideas that (i) an increased external [NaCl] increases the [Na+] within the cell and (ii) this increase decreases lysogen stability by disrupting 434 repressor-DNA interactions.
To begin to explore whether salt shock destabilizes 434 lysogens via a salt-dependent decrease in 434 repressor's DNA binding activity, we measured the internal concentrations of various cations in cells that were or were not subjected to salt shock. A comparison of the internal cation concentrations in unshocked cells and cells shocked with 200 mM NaCl (see Materials and Methods) showed that our salt shock procedure increased the amount of Na+ found within cells by ∼4-fold (Table 1). In contrast, salt Na+ shock had little effect on the amounts of K+ or Mg2+ found within these cells.
TABLE 1.
Effect of changing the external [NaCl] on the intracellular cation concentrationa
| Growth conditions | Ion | Concn (ppm) | Cell vol (liter) | Concn (mM) |
|---|---|---|---|---|
| LB | Na+ | 0.65 | 1 × 10−15 | 10 |
| K+ | 42.76 | 1 × 10−15 | 404 | |
| Mg++ | 34.93 | 1 × 10−15 | 290 | |
| LB + 200 mM NaCl | Na+ | 2.84 | 6.3 × 10−16 | 35 |
| K+ | 56.09 | 6.3 × 10−16 | 421 | |
| Mg++ | 29.23 | 6.3 × 10−16 | 338 |
Cells were grown and processed by ICP-MS analysis as described in Materials and Methods.
In addition to changing the concentration of NaCl in the growth medium, the presence of 200 mM salt changed the osmolarity of the medium, which in turn changed the internal cell volume and affected the precise internal salt concentration. We assumed that cells grown in LB had an internal cell volume of 1 μm3 (1 × 10−15 liter) (7). To estimate the internal concentration of Na+, we measured the change in cell volume caused by salt shock by determining the number of viable cells per gram (dry weight) for both unshocked and salt-shocked cells. As expected from the increased osmolarity of the culture medium, these measurements indicated that salt shock decreased the internal cell volume by ∼40% (Table 1). Using these values, we found that salt shock increased the internal Na+ concentration from 10 mM in unshocked cells to 35 mM in shocked cells (Table 1). This degree of increase in the internal Na+ concentration in response to an increased external [Na+] mirrors that seen by other investigators (19, 30). Most importantly, these results indicate that increasing the external salt concentration increases the intracellular [Na+].
Changing the salt concentration in the growth media could influence the ability of 434 repressor to stably maintain the lysogenic state indirectly via one or more features of the cell's response to the alteration in osmotic pressure that accompanies the change in the external salt concentration. To examine this possibility, we determined the frequency of spontaneous phage induction of lysogens grown in high-salt media in the absence or presence of the osmolyte betaine. This compound is a naturally occurring neutral osmoprotectant solute that, when present, obviates the effect of increased salt concentration on osmotic pressure but does not block the increase in the internal salt concentration (8, 47). The presence of 10 mM betaine had only a minimal effect on the salt-dependent increase in the spontaneous induction frequency, reducing the amount of phage released in response to salt shock by ≤10% (Fig. 1). Identical results were obtained at higher concentrations of betaine. This finding shows that changing the external salt concentration influences the stability of λimm434 lysogens by altering the intracellular ionic environment and not by its osmotic effects.
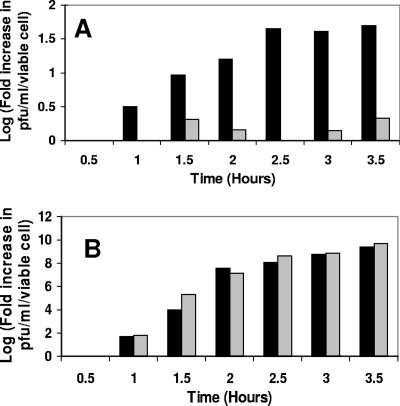
Based on the phage titer, salt shock causes ∼103 to 104/ml lysogenic cells in the culture to induce (data not shown). Hence, the salt-induced increase in phage production apparently results from a salt-dependent increase in the spontaneous-induction frequency. Alternatively, it is possible that the observed increase in the phage titer resulting from salt shock is due to a salt-dependent increase in the number of phage released by each cell. Changes in the number of phage released per cell are related to a delay in lysis, with longer times resulting in more phage released per cell. We reasoned that if salt influenced the number of phage released by each cell, this effect should be visible under any conditions that caused phage induction. Hence, to examine this idea, we measured the time course of phage production with or without salt shock in the absence or presence of the DNA-damaging agent mitomycin C. For these experiments, we determined the amount of phage released at various times after the washed cells were suspended in rich medium containing either (i) no added salt, (ii) 200 mM NaCl, (iii) mitomycin C, or (iv) mitomycin C plus 200 mM NaCl. To normalize for changes in cell numbers over the growth period and to highlight the effects of a treatment on phage growth, the results of this experiment are expressed as the number of PFU per ml per viable cell determined as described in Materials and Methods. As seen in Fig. 2A, the number of phage released from salt-shocked cells gradually increased relative to those grown in media without added salt, starting at ∼1 h after treatment. The amount of phage released from salt-shocked lysogens plateaued between 120 and 180 min after the cells were suspended in high-salt culture medium. At this time point, the salt-shocked cells had released >50-fold more phage than did cells grown in rich medium in the absence of added salt.
FIG. 2.
Time-dependent increase in phage titer as a consequence of added (A) salt and (B) salt and mitomycin C. Phage-containing supernatants were obtained from MG1655 cells lysogenized with λimm434 bacteriophage grown under the following conditions. For the experiments shown in panel A, stationary-phase cells were washed, suspended in rich medium without (gray bars) or with (black bars) 200 mM added NaCl, and grown for the indicated times as described in Materials and Methods. For the experiments shown in panel B, cells were treated exactly as for panel A, except that 3.5 μg/ml mitomycin C was added to the growth medium after the cells were washed. The data are plotted as the logarithm of the increase in PFU per viable cell from time zero to the indicated time.
Figure 2B shows that the amount of phage produced in mitomycin C-induced cells and the rate of phage production under these conditions were unaffected by salt shock. This finding suggests that salt has no influence on the amount of phage produced by an induced lysogen. Importantly, the rate at which phage were produced by salt-shocked cells was identical to the rate at which mitomycin C-induced cells produced phage (compare Fig. 2A and B). These data indicate that the salt-induced increase in phage production resulted from a salt-dependent increase in the spontaneous-induction frequency and that salt and mitomycin C induced lysogens via the same pathway, i.e., by decreasing repressor activity. Together, these findings indicate that salt shock destabilizes 434 lysogens by decreasing 434 repressor's DNA binding activity.
The data mentioned above are consistent with the idea that changing the internal salt concentrations alters repressor-DNA interactions. However, it is formally possible that the increased phage titer observed under high-salt conditions reflects salt dependence of the adsorption of spontaneously released phage to dead cells or live cells that remain uninduced. According to this idea, many spontaneously released phage are adsorbed to the dead or remaining live cells under low-salt conditions, whereas under higher-salt conditions, this adsorption is inhibited, leading to an apparent increase in the phage titer under higher-salt conditions. While this idea in incompatible with other results (see below), we performed two experiments to directly test this alternative.
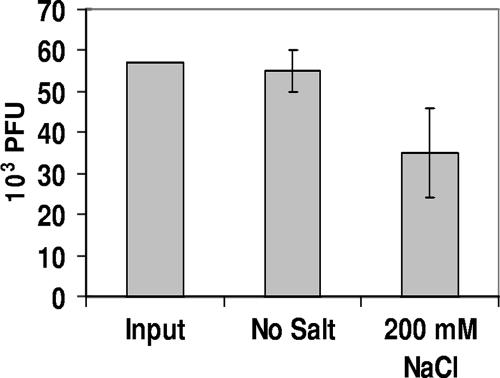
In the first experiment, MG1655::λimm434 lysogens were grown to either stationary or mid-log phase. After these cultures were washed to remove spontaneously induced phage, a known amount (5.5 × 104 PFU/ml) of λimm434 phage was incubated for 20 min with 1 × 109/ml washed cells in LB medium containing no added salt or 200 mM NaCl. Since these cells were immune to λimm434, phage could adsorb to the cells but could not complete a productive infection. Control experiments showed that during the time of incubation, no phage were released from the washed cells (data not shown). Subsequent to incubation, the cells were pelleted by centrifugation, and the amount of phage remaining in the supernatant was assayed. The results presented in Fig. 3 show that adding MG1655::λimm434 lysogens does not reduce the amount of phage found in the supernatant. Importantly, the amount of phage recovered was essentially unaffected by the presence of added salt.
FIG. 3.
Effect of salt on the adsorption of λimm434 bacteriophage to MG1655::λimm434 lysogens. Phage (λimm434 at 5 × 104 PFU/ml) were incubated at 37°C for 20 min with 1 × 109 CFU of stationary-phase MG1655::λimm434 lysogens in LB without or with 200 mM NaCl. Subsequent to removal of the cells by centrifugation, the amount of phage remaining in the supernatant was determined as described in Materials and Methods. The error bars indicate standard deviations.
In another experiment, we tested whether salt influences how many phage remain bound to cells after our normal experimental treatment. For these experiments, λimm434::MG1655 lysogens were grown to early log or stationary phase, washed with medium without added salt, and suspended in fresh medium containing no added salt or fresh medium containing increasing concentrations of NaCl as described above. After 4 h of incubation, the cells were collected by centrifugation and resuspended in medium containing 0.5 M NaCl and incubated for 5 min at room temperature, and the amount of phage released into the supernatant by this treatment was determined after the cells were removed by centrifugation. Our results showed that no additional phage were recovered from cells initially grown in the absence or presence of added salt. Identical results were obtained when the cell membranes were disrupted by vortexing them with chloroform. Taken together, the results of these experiments indicate that the increased phage titer observed under high-salt conditions was not due to differential salt-dependent adsorption of spontaneously released phage to the remaining cells or cellular debris.
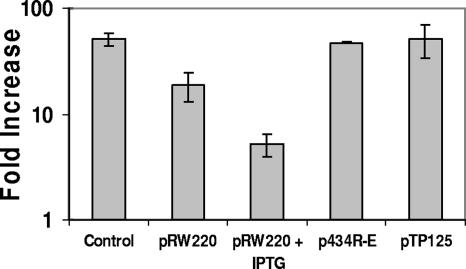
The suggestion that salt shock increases the spontaneous-induction frequency of 434 lysogens by decreasing 434 repressor's DNA binding activity predicts that overproduction of 434 repressor should overcome the deleterious effect of salt shock on lysogen stability. To test this prediction, we transformed our MG1655::λimm434 lysogen strain with pRW220, a plasmid that directs the expression of the wild-type 434 repressor gene under the control of the IPTG-inducible lacUV5 promoter. We compared the salt shock-dependent increases in phage production in the absence and presence of IPTG. As described in Materials and Methods, pRW220 directs the synthesis of a variable amount of 434 repressor, depending on the amount of IPTG added to the cells. As seen previously (Fig. 2), when the washed MG1655 434 lysogens were incubated in medium with 200 mM NaCl added, they spontaneously produced ∼50-fold more phage than did cells grown in medium without added salt (Fig. 4). In contrast, 434 lysogens bearing pRW220 grown in the absence of IPTG, washed and treated as described above, produced only 18-fold more phage under high-salt conditions than under lower-salt conditions. Hence, the presence of “excess” 434 repressor in the lysogen mitigates the effect of salt shock on lysogen stability. Consistent with this finding, λimm434 lysogens bearing pRW220 grown in the presence of IPTG produced only ∼5-fold more phage under high-salt conditions than under lower-salt conditions.
FIG. 4.
Effect of phage repressor overproduction on the salt-dependent increase in spontaneous-induction frequency. MG1655 cells lysogenized with λimm434 bacteriophage were transformed with either a control plasmid (pUC18 [35]) or one that directs the synthesis of 434 repressor (pRW220 [54]); a nondimerizing, non-DNA binding 434 mutant repressor (p434R-E [14, 15]); or a P22 repressor (pT15 pTP15 [39]) under the control of the lacUV5 promoter. Phage-containing supernatants were obtained from λimm434::MG1655 lysogens grown in the absence or presence of IPTG in medium without or with 200 mM of added NaCl, as described in Materials and Methods. The effect of salt shock (Fold Increase) is expressed as the number of phage produced per viable cell incubated in the presence of salt divided by the number of phage produced per viable cell in the absence of added salt. Note the logarithmic scale of the y axis. The error bars indicate standard deviations.
To determine whether the lysogen-stabilizing effect of “excess” 434 repressor production in salt-shocked cells was related to 434 repressor's DNA binding activity at 434 OR and OL, we measured the effect of salt shock on phage production in cells bearing plasmids that produce either a nondimerizing, non-DNA binding 434 repressor mutant (p434R-E) (14, 15) or the heterologous phage P22 repressor (pTP15) (39), a protein that does not bind 434 operators. We chose to use a nondimerizing 434 mutant to prevent complications from dominant-negative effects on repressor function. As shown in Fig. 4, neither the non-DNA binding 434 repressor mutant nor the P22 repressor had any effect on the salt shock-induced increase in phage production. Together, the findings shown in Fig. 4 indicate that salt shock increases the spontaneous-induction frequency of λimm434 lysogens by decreasing the DNA binding activity of 434 repressor.
We envision three potential mechanisms by which salt-dependent changes in the internal ionic environment of the cell may affect repressor activity. First, the increase in the internal salt concentration may lower the intracellular concentration of repressor by an indirect effect on the repressor-DNA affinity, such as altering the level of supercoiling inside the cell (9). Alternatively, while changes in the internal salt concentration are not thought to induce the SOS response (9, 23), it is formally possible that salt destabilizes 434 repressor lysogens by activating RecA to stimulate repressor autocleavage (13). Finally, an increase in the internal salt concentration may directly decrease the repressor's DNA affinity by a mechanism similar to the way in which salt influences the repressor's DNA affinity in vitro. It is also possible that salt may affect lysogen stability through more than one of these strategies. We directly examined each of these alternatives.
Immediately upon salt shock, E. coli cells respond by increasing the degree of negative DNA supercoiling (9). Given that 434 repressor prefers to bind overwound DNA sites (26, 27) and that an increase in negative supercoiling would oppose this overwinding, one possible mechanism by which salt stress may destabilize repressor-DNA interactions and cause an increase in the spontaneous-induction frequency is by affecting the level of DNA supercoiling.
Salt-dependent changes in the cellular supercoil density in E. coli are regulated, in part, by alterations in the relative opposing activities of gyrase (topoisomerase II) and topoisomerase I (9). Using sublethal concentrations of the antibiotic novobiocin, we tested the idea that salt increases the spontaneous-induction frequency of 434 lysogens by altering the level of DNA supercoiling. Novobiocin inhibits gyrase (34) and, at the concentrations used, obviates much of the increase in negative supercoiling induced by salt (9). Figure 5 shows that the increases in the spontaneous-induction frequencies in cells that had been treated with 0.2 M NaCl alone and with 0.2 M NaCl plus 80 μg/ml novobiocin were identical. This finding indicates that the salt-induced increase in the frequency of spontaneous induction is not due to a change in negative supercoiling in vivo.
FIG. 5.
Roles of DNA supercoiling and RecA activity in the salt-dependent increase in spontaneous induction of λimm434 prophage. Phage-containing supernatants were obtained from wild-type or recA mutant MG1655 cells lysogenized with λimm434 bacteriophage grown for 5 h without or with 200 mM of added NaCl. For experiments done in the presence of novobiocin, cells were grown in the presence of 80 μg/ml novobiocin as described in Materials and Methods. The effect of salt shock on phage production is expressed as the number of phage produced per viable cell incubated in the presence of salt divided by the number of phage produced per viable cell in the absence of added salt. Note the logarithmic scale of the y axis. The error bars indicate standard deviations.
The time course of salt-induced lysogen induction is identical to the kinetics of mitomycin C induction of 434 lysogens (Fig. 2). This finding is consistent with the idea that salt destabilizes 434 repressor lysogens by activating RecA to stimulate repressor autocleavage. To test whether salt destabilizes λimm434 lysogens by activating RecA, we measured the frequency of salt-dependent induction of λimm434::MG1655 recA mutant lysogens. Consistent with previous observations (29), under both low- and high-salt conditions, the overall frequency of spontaneous lysogen induction in recA mutant strains was decreased relative to recA+ strains (data not shown). However, the number of phage released from cells grown under high-salt conditions was 20-fold higher than for cells grown in isotonic medium. Thus, recA mutation does not prevent salt induction of λimm434 prophage (Fig. 5). This finding shows that the salt-dependent increase in the frequency of spontaneous induction does not depend on RecA-mediated repressor inactivation.
Our previous findings showed that in vitro, the affinity of 434 repressor for its specific binding sites depends not only on the concentration, but also the type, of monovalent cation (32). These results also indicated that changing the type of divalent cation had little effect on repressor-DNA complex stability. Hence, if salt shock affects spontaneous phage induction by directly altering the affinity of the repressor for its DNA sites, we reasoned that the frequency of spontaneous induction would vary with the monovalent, but not the divalent, cation type present in the medium.
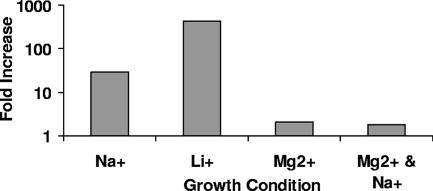
Figure 6 shows that changing the monovalent cation added to the growth media from 200 mM Na+ to 200 mM Li+ increased the spontaneous-induction frequency of λimm434 lysogens by nearly 500-fold. ICP analysis of the cation content of the Li+-shocked cells showed that this growth regimen increased the internal [Li+] by 70-fold without significantly affecting the concentration of K+, Na+, or Mg2+ (Table 2). The spontaneous-induction frequency of cells shocked with medium containing 60 mM Mg2+, a concentration that provides nearly the same osmotic effect as does 200 mM of a monovalent salt, was essentially identical to that of cells grown in medium without added salt (Fig. 6). Consistent with the idea that the internal concentration of cations modulates the spontaneous-induction frequency, treatment with 60 mM Mg2+ did not alter the internal concentration of cations (Table 2). Hence, the effects of changing the cation type on spontaneous induction of lysogenic λimm434 bacteriophage mirror those seen on 434 repressor's affinity for its naturally occurring binding sites. These findings indicate that salt shock causes induction of λimm434 bacteriophage by directly interfering with 434 repressor-DNA interactions.
FIG. 6.
Effect of salt type on the spontaneous-induction frequency of MG1655 cells lysogenized with λimm434 bacteriophage. Phage-containing supernatants were obtained from MG1655 cells lysogenized with λimm434 bacteriophage and grown for 5 h in the absence of added salt or the presence of either 200 mM of the indicated monovalent salt or 60 mM of the indicated divalent salt. Induction is shown as the number of phage produced per viable cell incubated in the presence of salt divided by the number of phage produced per viable cell in the absence of added salt. Note the logarithmic scale of the y axis.
TABLE 2.
Effect of varying the external cation type and concentration on the intracellular cation concentrationa
| Growth conditions | Ion | Concn (ppm) | Cell vol (liter) | Concn (mM) |
|---|---|---|---|---|
| LB | Na+ | 0.65 | 1 × 10−15 | 10 |
| K+ | 42.76 | 1 × 10−15 | 404 | |
| Li+ | 5.9 × 10−4 | 1 × 10−15 | 0.02 | |
| Mg2+ | 34.93 | 1 × 10−15 | 290 | |
| LB + 200 mM LiCl | Na+ | 0.66 | 8.5 × 10−16 | 12 |
| K+ | 34.8 | 8.5 × 10−16 | 387 | |
| Li+ | 0.028 | 8.5 × 10−16 | 1.4 | |
| Mg2+ | 21.9 | 8.5 × 10−16 | 316 | |
| LB + 60 mM MgCl2 | Na+ | 1.3 | 7.4 × 10−16 | 14 |
| K+ | 40.2 | 7.4 × 10−16 | 434 | |
| Li+ | 3.46 × 10−4 | 7.4 × 10−16 | 0.012 | |
| Mg2+ | 34.17 | 7.4 × 10−16 | 330 | |
| LB + 200 mM NaCl and 60 mM MgCl2 | Na+ | 0.77 | 1 × 10−15 | 11 |
| K+ | 43.3 | 1 × 10−15 | 405 | |
| Li+ | 8.7 × 10−4 | 1 × 10−15 | 0.03 | |
| Mg2+ | 29.23 | 1 × 10−15 | 338 |
Cells were grown and processed by ICP-MS analysis as described in Materials and Methods.
DISCUSSION
The stability of a bacteriophage lysogen is determined by the host cell physiology. A lambdoid prophage is thought to “choose” to grow lytically when the viability of its host cell is jeopardized. The switch from lysogenic to lytic growth requires the inactivation of the repressor's DNA binding function. The best-studied mechanism of repressor inactivation involves RecA-stimulated repressor autoproteolysis (12, 37, 46), which occurs during the host's response to DNA damage. Since the form of RecA that stimulates repressor autocleavage arises only as a consequence of DNA damage (21), this lysogenic induction mechanism is specific to this particular potentially lethal host stress. However, bacteria are subjected to many other types of stress, many of which could be lethal to the host and might be expected to cause the phage to enter the lytic pathway. Our data show that increasing the extracellular salt concentration decreases the stability of a lysogenic 434 bacteriophage. These data also indicate that the effect of changing the salt concentration on lysogen stability is mediated neither by the osmotic stress response nor by RecA. Instead, our findings suggest that changes in the in vivo concentration of monovalent cations directly affect lysogen stability by altering the repressor's occupancy of DNA. Thus, our findings describe a mechanism by which changes in the internal ionic environment of the host cell can affect the lysis-lysogeny decisions of a lambdoid bacteriophage.
Our data indicate that the increase in the intracellular monovalent cation concentration influences the repressor's DNA occupancy. A key question we wished to answer was how an increased internal salt concentration influences 434 repressor's DNA occupancy. We gained some insight into possible answers to this question by considering the effect of changing the external salt concentration on the internal environment. Immediately after the external salt concentration was increased, bacterial cells underwent plasmolysis. This response increased the concentrations of all cytoplasmic solutes. Also, exposing cells to a high external salt concentration caused a rapid increase in negative DNA supercoiling. After 10 to 15 min, the cells began to accumulate monovalent cations (M+) in order to reestablish the internal osmotic pressure.
Based on these observations, three possible mechanisms can be envisioned to explain the effect of the intracellular salt concentration on 434 repressor-DNA complex stability. According to one idea, the change in DNA supercoiling may alter the strength of the protein-DNA contacts. Consistent with this idea, we showed previously that the affinity of 434 repressor for its DNA sites depends on the linking number and twist of its DNA site (26). Both these parameters would be affected by changes in DNA supercoiling. Similarly, a salt-dependent change in another cellular homeostatic response might impact the repressor-DNA complex stability in some as-yet-uncharacterized fashion. This suggestion is consistent with the finding that the artificially induced overproduction of DsrA and/or RcsA increased the spontaneous-induction frequencies of several lambdoid bacteriophages in RecA− hosts (45). Both dsrA and rcsA gene products are involved in regulating E. coli's long-term response to growth under elevated-salt conditions (16, 51, 52). While each of these alternatives provides a potential explanation for the general effects of salt on 434 repressor-DNA complex stability, several lines of evidence suggest that these cellular responses do not contribute to the observed increase in the frequency of spontaneous induction in response to an elevated external salt concentration. First, the spontaneous-induction frequency of λimm434 lysogens is unaffected by growth in novobiocin, an antibiotic that is known to affect the level of DNA supercoiling (Fig. 5). Second, the product of the rpoS gene mediates E. coli's medium- and long-term responses to salt/osmotic shock. If an as-yet-uncharacterized cellular homeostatic response to salt stress impacts the repressor-DNA complex stability, we would anticipate that lysogens formed in strains lacking rpoS would be more resistant to salt shock than λimm434 lysogens formed in wild-type strains. However, the effect of increased salt concentration on the frequency of spontaneous induction of λimm434 prophages in strains bearing deletions of the rpoS gene is identical to that seen in wild-type strains (data not shown). Finally, neither a change in supercoiling nor alteration in cellular levels of DsrA and/or RcsA can account for the observed dependence of the spontaneous-induction frequency on the monovalent cation type (Fig. 6). Moreover, based on our previous measurements (2, 32) of the effect of changing the [Na+] on 434 repressor affinity for DNA, the fourfold increase in the internal Na+ content in response to a 200 mM NaCl stress would be predicted to decrease the affinity of 434 repressor for sites in OR five- to eightfold. Such a decrease in DNA affinity would, at least transiently, substantially decrease 434 repressor occupancy of its binding sites in the lysogenic phage, leading to lysogen induction. Hence, we assert that the increased intracellular cation concentration caused by an increased external salt concentration directly competes with the formation of specific repressor-DNA contacts (32).
Several previous studies have also examined the effect of changing the internal ion concentration on the actions of specific gene-regulatory proteins in E. coli (8, 28, 44, 56). For example, the activities of the CRP-cyclic AMP (cAMP)-regulated proP P1 and other CRP-dependent promoters are remarkably dependent on the salt concentration both in vivo and in vitro. Similar to our findings with 434 repressor, the observed effect of changing the internal salt concentration on CRP-cAMP-mediated gene expression can be directly attributed to salt-dependent decreases in the occupancy of CRP binding sites. Together with our observations reported here, this finding indicates that the internal salt concentration may play a general role in modulating differential gene expression in E. coli. Consistent with this assertion, we found that, similar to λimm434 lysogens, increasing the external salt concentration increased the spontaneous-induction frequencies of both bacteriophage λimmP22 and λ lysogens (J. Benjamin and G. B. Koudelka, unpublished observations).
In contrast to this suggestion, Richey et al. reported that changing the internal salt concentration did not affect the ability of λ cI or lac repressors to regulate transcription (44). However, these authors assessed the effect of varying the internal salt concentration on gene expression after the bacterial cells were adapted to the elevated salt concentration. Our studies and those reported elsewhere (28, 56) assessed the effect of salt shock on gene expression during the acute adaptation phase that immediately follows the increase in the external salt concentration. Thus, we suggest that changing the external salt concentration transiently affects the actions of specific gene-regulatory proteins. This suggestion is consistent with the observed time-limited influence of salt shock on CRP-cAMP-mediated gene expression and cps gene expression (51, 52), as well as the limited effect of an increased salt concentration on the spontaneous-induction frequencies of λimm434 lysogens. Evidence suggests that the time limitation on the effect of an elevated internal salt concentration on gene expression likely results from the replacement of salt by neutral osmoprotective solutes ∼60 min after salt shock.
A lysogenic phage will switch from lysogenic to lytic growth when it perceives that the long-term survivability of its host is in doubt. Until recently, the only “distress” signal known to affect this switch was active RecA protein, which inactivates the repressor as a consequence of the host response to DNA damage. Our work clearly shows that the phage lysis-lysogeny switch is capable of responding to additional signal inputs, in this case, either a direct effect of the transient rise in the intracellular monovalent cation concentration on protein-DNA interactions or an immediate cellular consequence of this change in the intracellular ionic environment. Regardless of the precise mechanism, it is clear that this signal is perceived by both the host and the prophage. Since bacterial cells have evolved specific mechanisms to coordinate responses to numerous environmental stresses, we anticipate that bacteriophages will have similarly evolved to read these signals to ensure their own viability.
Acknowledgments
This work was supported by a grant from the National Science Foundation (MCB-0239000).
We thank members of the laboratory for critical comments on the work and the manuscript. We acknowledge the contributions of Jeffrey Benjamin at the early stages of this work. We also thank Lynn Thomason, Don Court, and Amos Oppenheim for helpful discussions and communication of results in advance of publication.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Arber, W., L. Enquist, B. Hohn, N. E. Murray, and K. Murray. 1983. In R. W. Hendrix, J. W. Roberts, F. W. Stahl, and R. A. Weisberg (ed.), Lambda II, p. 433-466. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 2.Bell, A. C., and G. B. Koudelka. 1993. Operator sequence context influences amino acid-base-pair interactions in 434 repressor-operator complexes. J. Mol. Biol. 234:542-553. [DOI] [PubMed] [Google Scholar]
- 3.Bell, A. C., and G. B. Koudelka. 1995. How 434 repressor discriminates between OR1 and OR3. The influence of contacted and noncontacted base pairs. J. Biol. Chem. 270:1205-1212. [DOI] [PubMed] [Google Scholar]
- 4.Bergqvist, S., R. O'Brien, and J. E. Ladbury. 2001. Site-specific cation binding mediates TATA binding protein-DNA interaction from a hyperthermophilic archaeon. Biochemistry 40:2419-2425. [DOI] [PubMed] [Google Scholar]
- 5.Bergqvist, S., M. A. Williams, R. O'Brien, and J. E. Ladbury. 2003. Halophilic adaptation of protein-DNA interactions. Biochem. Soc. Trans. 31:677-680. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Broda, P. 1968. Ribonucleic acid synthesis and glutamate excretion in Escherichia coli. J. Bacteriol. 96:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayley, S., B. A. Lewis, H. J. Guttman, and M. T. Record. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 222:281-300. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, K. J., V. Badarinarayana, D. W. Selinger, D. Janse, and G. M. Church. 2003. A microarray-based antibiotic screen identifies a regulatory role for supercoiling in the osmotic stress response of Escherichia coli. Genome Res. 13:206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conter, A., C. Menchon, and C. Gutierrez. 1997. Role of DNA supercoiling and rpoS sigma factor in the osmotic and growth phase-dependent induction of the gene osmE of Escherichia coli K12. J. Mol. Biol. 273:75-83. [DOI] [PubMed] [Google Scholar]
- 11.Daniels, D. L., J. L. Schroeder, W. Szybalski, F. Sanger, A. R. Coulson, G. F. Hong, D. F. Hill, G. F. Petersen, and F. R. Blattner. 1983. In R. W. Hendrix, J. W. Roberts, F. W. Stahl, and R. A. Weisberg (ed.), Lambda II, p. 519-676. Cold Spring Harbor Laboratory, Cold Spring Harbor.
- 12.DeAnda, J., A. R. Poteete, and R. T. Sauer. 1983. P22 c2 repressor-domain structure and function. J. Biol. Chem. 258:10536-10542. [PubMed] [Google Scholar]
- 13.DiCapua, E., R. W. Ruigrok, and P. A. Timmins. 1990. Activation of RecA protein: the salt-induced structural transition. J. Struct. Biol. 104:91-96. [DOI] [PubMed] [Google Scholar]
- 14.Donner, A. L., P. A. Carlson, and G. B. Koudelka. 1997. Dimerization specificity of P22 and 434 repressors is determined by multiple polypeptide segments. J. Bacteriol. 179:1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donner, A. L., and G. B. Koudelka. 1998. Carboxyl-teminal domain dimer interface mutant 434 repressors have altered dimerization and DNA binding specificities. J. Mol. Biol. 283:931-946. [DOI] [PubMed] [Google Scholar]
- 16.Ebel, W., and J. E. Trempy. 1999. Escherichia coli RcsA, a positive activator of colanic acid capsular polysaccharide synthesis, functions to activate its own expression. J. Bacteriol. 181:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein, W. 2003. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75:293-320. [DOI] [PubMed] [Google Scholar]
- 18.Epstein, W., and B. S. Kim. 1971. Potassium transport loci in Escherichia coli K-12. J. Bacteriol. 108:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein, W., and S. G. Schultz. 1965. Cation transport in Escherichia coli. V. Regulation of cation content. J. Gen. Physiol. 49:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein, W., and S. G. Schultz. 1966. Cation transport in Escherichia coli. VI. K exchange. J. Gen. Physiol. 49:469-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. ASM Press, Washington, DC.
- 22.Graeme-Cook, K. A., G. May, E. Bremer, and C. F. Higgins. 1989. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol. Microbiol. 3:1287-1294. [DOI] [PubMed] [Google Scholar]
- 23.Hengge-Aronis, R. 1996. Back to log phase: σS as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, C. F., C. J. Dorman, D. A. Stirling, L. Waddell, I. R. Booth, G. May, and E. Bremer. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569-584. [DOI] [PubMed] [Google Scholar]
- 25.Jordi, B. J., and C. F. Higgins. 2000. The downstream regulatory element of the proU operon of Salmonella typhimurium inhibits open complex formation by RNA polymerase at a distance. J. Biol. Chem. 275:12123-12128. [DOI] [PubMed] [Google Scholar]
- 26.Koudelka, G. B. 1998. Recognition of DNA structure by 434 repressor. Nucleic Acids Res. 26:669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koudelka, G. B., and P. Carlson. 1992. DNA twisting and the effects of non-contacted bases on affinity of 434 operator for 434 repressor. Nature 355:89-91. [DOI] [PubMed] [Google Scholar]
- 28.Landis, L., J. Xu, and R. C. Johnson. 1999. The cAMP receptor protein CRP can function as an osmoregulator of transcription in Escherichia coli. Genes Dev. 13:3081-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livny, J., and D. I. Friedman. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol. Microbiol. 51:1691-1704. [DOI] [PubMed] [Google Scholar]
- 30.Lo, C. J., M. C. Leake, and R. M. Berry. 2006. Fluorescence measurement of intracellular sodium concentration in single Escherichia coli cells. Biophys. J. 90:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, J. F., U. A. Ochsner, M. G. Klotz, V. K. Nanayakkara, M. L. Howell, Z. Johnson, J. E. Posey, M. L. Vasil, J. J. Monaco, and D. J. Hassett. 1999. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J. Bacteriol. 181:3730-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauro, S. A., and G. B. Koudelka. 2004. Monovalent cations regulate DNA sequence recognition by 434 repressor. J. Mol. Biol. 340:445-457. [DOI] [PubMed] [Google Scholar]
- 33.Mauro, S. A., D. Pawlowski, and G. B. Koudelka. 2004. The role of the minor groove substituents in indirect readout of DNA sequence by 434 repressor. J. Biol. Chem. 278:12955-12960. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell, A. 1997. DNA gyrase as a drug target. Trends Microbiol. 5:102-109. [DOI] [PubMed] [Google Scholar]
- 35.Messing, J. 1983. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Methods Enzymol. 101:20-79. [DOI] [PubMed] [Google Scholar]
- 36.Nagata, S., K. Adachi, K. Shirai, and H. Sano. 1995. 23Na NMR spectroscopy of free Na+ in the halotolerant bacterium Brevibacterium sp. and Escherichia coli. Microbiology 141:729-736. [DOI] [PubMed] [Google Scholar]
- 37.Phizicky, E. M., and J. W. Roberts. 1980. Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22. J. Mol. Biol. 139:319-328. [DOI] [PubMed] [Google Scholar]
- 38.Pirrotta, V. 1979. Operators and promoters in the OR region of phage 434. Nucleic Acids Res. 6:1495-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poteete, A. R., and T. M. Roberts. 1981. Construction of plasmids that produce phage P22 repressor. Gene 13:153-161. [DOI] [PubMed] [Google Scholar]
- 40.Ptashne, M. 1986. A genetic switch. Blackwell Press, Palo Alto, CA.
- 41.Record, M. T., E. S. Courtenay, D. S. Cayley, and H. J. Guttman. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem. Sci. 23:143-148. [DOI] [PubMed] [Google Scholar]
- 42.Record, M. T., E. S. Courtenay, S. Cayley, and H. J. Guttman. 1998. Biophysical compensation mechanisms buffering E. coli protein-nucleic acid interactions against changing environments. Trends Biochem. Sci. 23:190-194. [DOI] [PubMed] [Google Scholar]
- 43.Record, M. T., W. T. Zhang, and C. F. Anderson. 1998. Analysis of effects of salts and uncharged solutes on protein and nucleic acid equilibria and processes: a practical guide to recognizing and interpreting polyelectrolyte effects, Hofmeister effects, and osmotic effects of salts. Adv. Protein Chem. 51:281-353. [DOI] [PubMed] [Google Scholar]
- 44.Richey, B., D. S. Cayley, M. C. Mossing, C. Kolka, C. F. Anderson, T. C. Farrar, and M. T. Record. 1987. Variability of the intracellular ionic environment of Escherichia coli—differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J. Biol. Chem. 262:7157-7164. [PubMed] [Google Scholar]
- 45.Rozanov, D. V., R. D'Ari, and S. P. Sineoky. 1998. RecA-independent pathways of lambdoid prophage induction in Escherichia coli. J. Bacteriol. 180:6306-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauer, R. T., H. C. Nelson, K. Hehir, M. H. Hecht, F. S. Gimble, J. DeAnda, and A. R. Poteete. 1983. The lambda and P22 phage repressors. J. Biomol. Struct. Dyn. 1:1011-1022. [DOI] [PubMed] [Google Scholar]
- 47.Schlax, P. J., M. W. Capp, and M. T. Record. 1995. Inhibition of transcription initiation by lac repressor. J. Mol. Biol. 245:331-350. [DOI] [PubMed] [Google Scholar]
- 48.Schultz, S. G., W. Epstein, and D. A. Goldstein. 1962. Cation transport in Escherichia coli. III. Potassium fluxes in the steady state. J. Gen. Physiol. 46:343-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz, S. G., W. Epstein, and A. K. Solomon. 1963. Cation transport in Escherichia coli. IV. Kinetics of net K uptake. J. Gen. Physiol. 47:329-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz, S. G., N. L. Wilson, and W. Epstein. 1962. Cation transport in Escherichia coli. II. Intracellular chloride concentration. J. Gen. Physiol. 46:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sledjeski, D., and S. Gottesman. 1995. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sledjeski, D. D., and S. Gottesman. 1996. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 178:1204-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wharton, R. P. 1986. Determinants of 434 repressor binding specificity. Ph.D. thesis. Harvard University, Cambridge, MA.
- 54.Wharton, R. P., E. L. Brown, and M. Ptashne. 1985. Substituting an α-helix switches the sequence specific DNA interactions of a repressor. Cell 38:361-369. [DOI] [PubMed] [Google Scholar]
- 55.Winans, S. C., S. J. Elledge, J. H. Krueger, and G. C. Walker. 1985. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J. Bacteriol. 161:1219-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, J., and R. C. Johnson. 1997. Cyclic AMP receptor protein functions as a repressor of the osmotically inducible promoter proP P1 in Escherichia coli. J. Bacteriol. 179:2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]