Abstract
Plasminogen recruitment is a common strategy of pathogenic bacteria and results in a broad-spectrum surface-associated protease activity. Neisseria meningitidis has previously been shown to bind plasminogen. In this study, we show by several complementary approaches that endolase, DnaK, and peroxiredoxin, which are usually intracellular proteins, can also be located in the outer membrane and act as plasminogen receptors. Internal binding motifs, rather than C-terminal lysine residues, are responsible for plasminogen binding of the N. meningitidis receptors. Recombinant receptor proteins inhibit plasminogen association with N. meningitidis in a concentration-dependent manner. Besides binding purified plasminogen, N. meningitidis can also acquire plasminogen from human serum. Activation of N. meningitidis-associated plasminogen by urokinase results in functional activity and allows the bacteria to degrade fibrinogen. Furthermore, plasmin bound to N. meningitidis is protected against inactivation by α2-antiplasmin.
Neisseria meningitidis can be found as a commensal in the nasopharynges of 3 to 40% of healthy individuals (14, 52, 55, 59). Only in rare cases will this pathogen cross the epithelial barrier of its natural habitat to cause invasive disease. Sepsis and meningitis are the hallmark manifestations of meningococcal disease, which mainly afflicts infants and adolescents (51). Whereas the interaction of N. meningitidis with cells of the epithelial and endothelial barriers crossed during the course of disease has been the focus of several studies, little is known about the components necessary for interaction with the extracellular matrix (ECM) and distribution in tissue (19, 37, 42).
Plasminogen is the key proenzyme of the fibrinolytic system. After plasminogen activation by specific proteases, such as tissue-type plasminogen (tPA) activators and urokinase plasminogen activator (uPA), the active enzyme plasmin becomes a broad-spectrum protease that, besides its main substrate fibrin, can cleave vitronectin, fibronectin, and laminin, major components of the basal laminae and ECM (48). In addition, plasmin can activate other proteolytic enzymes, including matrix metalloproteinases and latent macrophage elastase, thus triggering further proteolytic activity, which results in the cleavage of collagen, elastin, and proteoglycans (40).
Plasminogen binding to bacterial surfaces has been linked to the invasiveness and pathogenicity of different pathogens (32, 33). For example, Borrelia burgdorferi requires plasminogen derived from human blood to disseminate in its tick vector (17). Furthermore, plasmin-coated borreliae were found to display an enhanced capability to transmigrate endothelial cell layers, and surface-bound plasmin activity could be shown to enhance spirochetemia in mice (16, 17). The plasminogen activator Pla of Yersinia pestis contributes to bacterial invasiveness by proteolytically activating plasminogen and localizing plasmin activity to basement membranes, which enhances the migration of Y. pestis through tissue (34, 57). In a similar way, streptokinase secreted by group A streptococci activates plasminogen and could be shown to be a key virulence factor in vivo (58). Binding of plasminogen to Haemophilus influenzae and Streptococcus pneumoniae has been shown to result in degradation of the ECM and enhanced penetration of the bacteria (7, 22, 63).
Many of the pathogens that recruit human plasminogen to their surfaces express a wide variety of several plasminogen binding proteins. At least two of the B. burgdorferi plasminogen binding proteins, OspA and the 70-kDa BPBP, have been shown to be exposed at the bacterial surface; two receptors, enolase and glyceraldehyde-3-phosphate dehydrogenase, have been identified for pneumococci, and eight plasminogen binding proteins have been proposed for Candida albicans (5, 6, 16-18). The interaction between plasminogen and its receptors is mediated by triple-disulfide-bonded kringle domains within the plasminogen molecule that contain lysine binding sites. Binding of plasminogen to other proteins has been reported to involve C-terminal lysine residues or internal binding motifs enriched with lysine residues (8, 23, 48, 65).
Historically, the observation that meningococcal meningitis is associated with enhanced fibrinolytic activity (9) was one argument for the hypothesis that plasminogen activation could be involved in the pathogenesis of systemic bacterial infection. Thereafter, binding of plasminogen to N. meningitidis and its subsequent conversion to enzymatically active plasmin by uPA and tPA was demonstrated (61). Binding of plasminogen can be blocked by ɛ-amino capronic acid, indicating the involvement of lysine binding domains (61). However, no neisserial plasminogen receptor has yet been defined, although Scatchard analysis has suggested the existence of at least two receptors. In this study, the identification and characterization of three plasminogen binding molecules at the surface of N. meningitidis are reported.
MATERIALS AND METHODS
Bacterial strains and media.
Amplification of various plasmids was performed in Escherichia coli strain DH5, with expression of recombinant proteins in E. coli strain M15(pREP4). The N. meningitidis strains used in this study are listed in Table 1. E. coli strains were cultured at 37°C in Luria Bertani (LB) broth or on LB agar, and N. meningitidis was cultured at 37°C and 5% CO2 either on gonococcal (GC) agar (BD Difco, Heidelberg, Germany) or in proteose peptone broth (PPM+) (BD Difco) supplemented with Poly ViteX (bioMerieux, Marcy l'Etoile, France). Bacteria were stored at −80°C in glycerol stocks. If appropriate, chloramphenicol (for N. meningitidis, 7 mg/ml; for E. coli, 30 mg/ml), spectinomycin (125 μg/ml; 75 μg/ml), kanamycin (100 μg/ml; 30 μg/ml), and/or ampicillin (for E. coli, 100 μg/ml) was added to the media.
TABLE 1.
Meningococcal strains and mutants used in this study
| Strain | Parental strain | Genotype | Serogroup | Reference |
|---|---|---|---|---|
| MC58 | Wta | B | 20 | |
| 3240 | MC58 | ΔsiaD | 31 | |
| 3697 | 3240 | ΔsiaD Δperox | This study | |
| 3077 | MC58 ΔsiaD | ΔsiaD ΔlgtA | 49 | |
| 3272 | 3240 | ΔsiaD Δpgm | 31 | |
| 3604 | 3240 | ΔsiaD ΔrfaF | 31 | |
| H44/76 | Wt | B | 28 | |
| Z2491 | Wt | A | 14 | |
| 2120 | Wt | C | 64 | |
| 171 | Wt | W-135 | 15 | |
| 172 | Wt | Y | 15 | |
| a14 | Wt | None (cnl) | 13 |
WT, wild type.
Recombinant-DNA techniques and meningococcal mutants.
The oligonucleotides used in this study were purchased from Sigma-ARK (Darmstadt, Germany). For cloning work, pBSII (Fermentas) and pUC4K (Amersham), and for expression of recombinant proteins, pQE32 and pQE60 (QIAGEN) were used. Chromosomal DNA was isolated from N. meningitidis using the Genomic-tip system (QIAGEN, Hilden, Germany). Plasmid DNA was isolated from E. coli using the Plasmid Mini Kit (QIAGEN). PCR products were purified with the QIAquick PCR Purification Kit (QIAGEN). Restriction enzymes and Taq polymerase were purchased from NEB (Frankfurt, Germany). Newly constructed plasmids were analyzed by DNA sequencing. Site-directed PCR mutagenesis was performed as previously described (27). Enolase C-terminal Lys-428, DnaK Lys-641 and Lys-642, and peroxiredoxin Lys-244 and Asn-245 were replaced by alanine. Substitutions were verified by DNA sequence analysis.
For construction of the peroxiredoxin deletion mutant, strain MC58 ΔsiaD was transformed with plasmid pMW10 containing a kanamycin resistance cassette in the open reading frame of perox. Transformation of N. meningitidis was performed as previously described (25). The mutant strain was checked for expression of peroxiredoxin and the major surface markers (Opc, Opa, and pili) in Western blots using monoclonal antibodies (MAb) SM1 (anti-class 1 pili; kindly provided by M. Virji, Bristol, United Kingdom), 1:4,000 (vol/vol); MAb B306 (anti-outer membrane protein Opc; kindly provided by M. Achtman, Berlin, Germany), 1:5,000 (vol/vol); and MAb 4B12/C11 (anti-outer membrane protein Opa; kindly provided by M. Achtman, Berlin, Germany), 1:1,000 (vol/vol).
Recombinant proteins and antisera.
The protein-expressing E. coli M15(pREP4) strains were plated overnight at 37°C on LB agar. The next day, a fresh culture was inoculated in LB broth. Expression of the His-tagged fusion proteins was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (Obiogene, Heidelberg, Germany) after they reached an optical density at 600 nm (OD600) of 0.7, and growth continued at 37°C for another 5 h. Purification of the recombinant fusion proteins was performed under nondenaturating conditions according to the protocol (QIAGEN). Antisera against the purified fusion proteins enolase, DnaK, and peroxiredoxin were raised in rabbits by using purified recombinant proteins (ImmunoGlobe Antikoerpertechnik GmbH, Himmelstadt, Germany). In Western blots, the sera were used at 1:20,000 (vol/vol) dilution. Horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) (Dianova, Hamburg, Germany) was used as a secondary antibody for enzyme-linked immunosorbent assay (ELISA) at 1:2,500 (vol/vol) and for Western blotting at 1:30,000 (vol/vol).
Plasminogen overlay and plasmin activity assays.
Binding of plasminogen to specific proteins was detected by an overlay assay. Proteins were separated by SDS-PAGE and blotted on a nitrocellulose membrane (Protran BA 85; Schleicher & Schüll, Dassel, Germany). For detection of plasminogen binding under nondenaturing conditions, proteins were dotted directly on nitrocellulose. The membranes were blocked with phosphate-buffered saline-0.1% (vol/vol) Tween 20 (PBS-T) supplemented with 5% (wt/vol) nonfat dry milk overnight at 4°C. Subsequently, the membranes were incubated with Glu-plasminogen (8 μg/ml) (Haemochrom Diagnostica GmbH, Essen, Germany) in PBS-T for 1 h at room temperature (RT). After the membranes were washed, an anti-human plasminogen goat antibody (Affinity Biologicals, Ancaster, Canada) was incubated in PBS-T, 1:1,500 (vol/vol), for 1 h at RT. The second antibody, HRP-conjugated anti-goat IgG (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 1:15,000 (vol/vol) in PBS-T, was added for 1 h at RT. For detection, an enhanced-chemiluminiscence (ECL) kit (Amersham, Freiburg, Germany) was used. For N-terminal sequence analysis of proteins reactive with plasminogen, these proteins were transferred to a polyvinylidene difluoride membrane. Proteins showing reactivity were excised from a duplicate membrane and subjected to N-terminal sequencing.
Bacterium-bound plasmin activity was measured by proteolytic cleavage of the chromogenic substrate S-2251 (Haemochrom Diagnostica, Essen, Germany). Bacteria were grown on GC agar overnight. A fresh culture in PPM+ was inoculated and grown to an OD600 of 0.5. Pellets were resuspended in 100 μl of PBS-T containing 5 μg plasminogen (Haemochrom Diagnostica, Essen, Germany) and were incubated for 30 min at 37°C with shaking (200 rpm). Likewise, bacteria were incubated with heat-inactivated human serum in different concentrations diluted in 100 μl PBS-T to detect the ability of meningococci to recruit plasminogen from plasma. After three washes in PBS-T, the bacteria were resuspended in 100 μl PBS-T containing 1.7 units uPA (American Diagnostica, Pfungstadt, Germany) and incubated for 30 min at 37°C with shaking. Then, 100 μl of a 1:5 dilution of S-2251 in water was added for 1 h at 37°C with shaking. The bacteria were pelleted, and the absorbance of 150 μl supernatant was measured at 405 nm using a Titertek Multiskan. To check the influence of α2-antiplasmin on bound plasmin activity, 0.01 unit α2-antiplasmin (Calbiochem, Merck Biosciences Ltd., Darmstadt, Germany) was added to plasmin-coated bacteria, and the bacteria were incubated for another 30 min (37°C with shaking at 200 rpm). Measurement of plasmin activity was performed as described above.
Competitive inhibition of plasminogen binding.
N. meningitidis overnight cultures were suspended in PBS to an OD600 of 0.2; 25 μl (approximately 2.5 × 108 bacteria) was added to each well of a microtiter plate and incubated for 1 h at 37°C. To each well, 100 μl of 0.05% (vol/vol) glutaraldehyde solution was added for 10 min at 37°C. The plates were washed three times with PBS, and nonspecific binding sites were blocked with 150 μl of 1% bovine serum albumin (BSA) in PBS for 1 h at 37°C. Twenty-five microliters of plasminogen solution (2 mg/ml PBS) was added to each well for 1 h at 37°C. For competitive inhibition, 50-, 100-, and 200-fold molar excess of recombinant proteins was added to the plasminogen solution before incubation.
Bound plasminogen was detected by ELISA. An anti-human plasminogen-goat antibody (Affinity Biologicals, Ancaster, Canada) at 1:500 (vol/vol) in PBS-1% BSA was incubated for 1 h at 37°C. The second antibody, HRP-conjugated anti-goat IgG (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 1:7,500 (vol/vol) in PBS-1% BSA, was incubated for 45 min at 37°C. For detection, 50 μl ABTS [2,2≪-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate solution (Roche, Mannheim, Germany) was added to each well, and the absorbance at 414 nm was measured using a Titertek Multiskan after 10 min and 20 min.
Quantification of plasminogen receptors.
Meningococci were grown overnight on GC agar. The next day, a fresh culture was inoculated in PPM+ and grown to an OD600 of 0.5. Fifty microliters (1 × 107 bacteria) of a fresh culture of N. meningitidis was adjusted to an OD600 of 0.2 with PPM+, and aliquots were placed in each well of a microtiter plate and incubated for 3 h at 37°C and 5% CO2. A standard of 1 pmol, 0.3 pmol, 0.1 pmol, 30 fmol, 10 fmol, and 3 fmol of the respective protein was provided in parallel. After incubation, the wells were fixed with PBS-3.7% formaldehyde (vol/vol) for 5 min at RT. After the plates were washed with PBS, nonspecific binding sites were blocked with PBS-0.05%Tween-5% nonfat dry milk (blocking buffer) for 30 min at 37°C. The rabbit antisera against the plasminogen receptor proteins were added at 1:200 in blocking buffer for 1.5 h at 37°C. After two washes with PBS-0.05% Tween, the second antibody, HRP-conjugated goat anti-rabbit IgG (Dianova, Hamburg, Germany), was added at 1:2,500 in blocking buffer for 1 h at 37°C. Subsequently, the plates were washed three times with PBS-0.05% Tween and twice with PBS alone. Finally, 50 μl of ABTS substrate solution was added to each well, and the absorbance at 414 nm was measured using a Titertek Multiskan after 5 min, 10 min, and 15 min.
Electron microscopy.
A culture of the unencapsulated N. meningitidis strain 3240 was grown overnight and suspended to an OD600 of 1.0 in PBS, and 100 μl (1 × 109 bacteria) of this bacterial solution was centrifuged with a Cytospin centrifuge on glass slides. Subsequently, the meningococci were fixed with 3.7% formaldehyde-20% saccharose in PBS for 30 min at RT, leading to osmotic swelling of the periplasmic space. The slides were washed three times with PBS, blocked with PBS-0.5% BSA for 5 min at RT, and incubated for 1 h at RT with the rabbit antiserum diluted 1:2,000 (vol/vol) in PBS-0.5% BSA. After three washes in PBS for 5 min each time, the slides were incubated for 1.5 h at RT with an anti-rabbit IgG antibody linked to 12-nm gold particles (Dianova, Hamburg, Germany) and diluted 1:20 (vol/vol) in PBS-0.5% BSA. The slides were washed again three times for 5 min each time and fixed with 2.5% glutaraldehyde in PBS for 45 min at 4°C. The slides were then postfixed in 2% osmium tetroxide, stained with 0.5% uranyloacetate, dehydrated in graded alcohols, and finally embedded in Lowicryl K4M 812 overnight. Electron micrographs were taken with Zeiss EM900 and EM10 microscopes.
For detection of surface-bound plasminogen, meningococci were incubated with 50 μg/ml plasminogen for 30 min at 37°C with shaking and washed three times with PBS before they were centrifuged on the glass slides. The procedure was the same as described above. A goat anti-human plasminogen antibody (1:150 in PBS-0.5% BSA; Affinity Biologicals, Ancaster, Canada) was used as the first antibody, and a mouse anti-goat IgG antibody linked to 12-nm gold particles (Dianova, Hamburg, Germany) was used as a second antibody in a 1:20 dilution.
Confocal microscopy.
For confocal microscopy, meningococci that had been preincubated with plasminogen were fixed on glass slides in methanol for 5 min. The respective antisera (1:1,000) and a secondary tetramethyl rhodamine isothiocyanate-labeled anti-rabbit antibody (1:300) were used for staining of meningococcal enolase, DnaK, and peroxiredoxin. Stained specimens were mounted in Fluoprep (bioMerieux, Nürtingen, Germany) and examined using a Zeiss LSM500 confocal microscope with a minimized pinhole for layer thicknesses below 1 μm.
Flow cytometry.
For flow cytometric analyses, the IgG fraction of the antisera and corresponding preimmune sera was purified by protein A-Sepharose chromatography following standard protocols. Log-phase meningococci were incubated with shaking either with 5 μg plasminogen (in PBS-T; 30 min; 37°C), followed by anti-plasminogen antibody (2 μg/ml IgG in PBS with 0.5% BSA; 45 min; 37°C), or with the affinity-purified sera (2 μg/ml IgG in PBS with 0.5% BSA; 45 min; 37°C). Preimmune sera and isotype-matched antibodies were used as controls. After being washed twice with PBS, the corresponding second antibody, fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG, 1:150; FITC-conjugated anti-mouse IgG, 1:100 (vol/vol); or FITC-conjugated anti-goat IgG, 1:100 in 100 μl BSA-PBS, was added for another 45 min at 37°C with shaking (200 rpm). Subsequently, the bacteria were washed twice with PBS, and 200 μl 3.7% formaldehyde was added for fixation (>20 min at 4°C). Analysis was performed in a FACScalibur (Becton-Dickinson, Heidelberg, Germany) by counting 5,000 events and analyzed using CellQuest software. Subsequent evaluation of the received data was performed with WinMDI version 2.8.
Degradation of fibrinogen.
Meningococci were preincubated with plasmin and plasminogen as described above, and 1 × 108 cells were incubated for up to 2 h at 37°C with 10 μg human fibrinogen (Calbiochem, Läufelfingen, Germany) in a final volume of 100 μl of PBS-T. Degradation was followed by plasminogen in the presence of 1.7 units of uPA, and the reaction was stopped with 20 μl of SDS-containing 5× sample buffer. The bacteria were sedimented, the supernatant was boiled for 10 min, and 15-μl samples were separated by SDS-PAGE, followed by transfer of the proteins to a nitrocellulose membrane (Protran BA 85; Schleicher & Schüll, Dassel, Germany) using a wet blotting system. The membranes were blocked by incubation with PBS-T supplemented with 5% (wt/vol) nonfat dry milk overnight at 4°C prior to incubation with goat antiserum to human fibrinogen, 1:2,500 (vol/vol) (ICN, Ohio). Detection of fibrinogen peptides was carried using an HRP-conjugated anti-goat antibody, 1:7,500 (vol/vol), as described above, followed by detection with the ECL kit (Amersham, Freiburg, Germany).
Statistical analysis.
Statistical analysis was performed with Microsoft Excel. A two-tailed Student's t test was used to calculate significance values. All experiments were performed in triplicate unless otherwise stated and were repeated at least three times.
RESULTS
Identification of enolase, DnaK, and peroxiredoxin as meningococcal plasminogen binding proteins.
Plasminogen binding of N. meningitidis strain MC58 (serogroup B, ST-32 complex) was shown in flow cytometric analyses and immunogold labeling of surface-associated plasminogen after incubation of the bacteria with plasminogen and labeling with an anti-plasminogen antibody (data not shown). Both approaches clearly demonstrated that significant amounts of plasminogen bind to this strain, as has been described for other N. meningitidis isolates (61). In order to screen for meningococcal plasminogen binding proteins, plasminogen overlay assays were performed. Whole bacterial lysates of strain MC58 were separated by SDS-PAGE, blotted to a nitrocellulose membrane, and incubated with human plasminogen. After the membranes were washed, plasminogen binding was detected with a specific antibody (Fig. 1). In several independent assays, six meningococcal plasminogen binding proteins were detected. In control assays without plasminogen, no signals could be detected, even after prolonged exposure of the film (Fig. 1). The pattern of the putative plasminogen receptors was highly conserved in strains from different clonal lineages and serogroups A, B, C, W-135, and Y, as well as cnl (capsule null locus) strains (data not shown). Putative plasminogen binding proteins of N. meningitidis strain MC58 were excised from the blot, extracted, and subjected to N-terminal sequencing. Using the databases available for the complete genome sequence of MC58, three proteins were identified as DnaK, enolase, and peroxiredoxin. Three other proteins could not be identified unequivocally (approximate molecular masses, 27 kDa, 35 kDa, and 80 kDa). Enolase, DnaK, and peroxiredoxin were recombinantly expressed, blotted, and subjected to the same plasminogen overlay assay as the total cellular lysate (Fig. 1). Indeed, the recombinant proteins were identical in size to the putative plasminogen receptors (Fig. 1). Antisera against the three putative receptors used in Western blots of total bacterial lysates also detected protein bands of the same size (data not shown). Using equimolar amounts of the recombinant proteins in the overlay assays, DnaK was consistently found to bind less plasminogen than peroxiredoxin, which itself showed lower plasminogen binding capacity than enolase. Assessment of plasminogen binding with nondenatured receptor proteins was performed in a modified plasminogen overlay assay. Dilutions of recombinant proteins, buffered in PBS, were dotted directly on a membrane. Incubation with plasminogen and detection were performed as described above for the overlay assays. In contrast to different plasminogen binding capacities of denatured receptor proteins, nondenatured enolase, DnaK, and peroxiredoxin recruited approximately equal amounts of plasminogen (Fig. 2A). No binding of plasminogen to dotted albumin was detected, excluding nonspecific binding of plasminogen to dotted proteins.
FIG. 1.
Plasminogen binds to several meningococcal proteins. A representative plasminogen overlay assay is shown for strain MC58. Lane 1, silver staining of the total protein extract; lane 2, plasminogen overlay assay; lane 3, negative control without the addition of plasminogen; lane 4, recombinant meningococcal enolase; lane 5, recombinant meningococcal DnaK; lane 6, recombinant meningococcal peroxiredoxin. At least six meningococcal proteins binding to plasminogen can be identified in the overlay assay. Three of them could be identified by N-terminal sequencing as enolase, DnaK, and peroxiredoxin. The other bands have not yet been identified unequivocally.
FIG. 2.
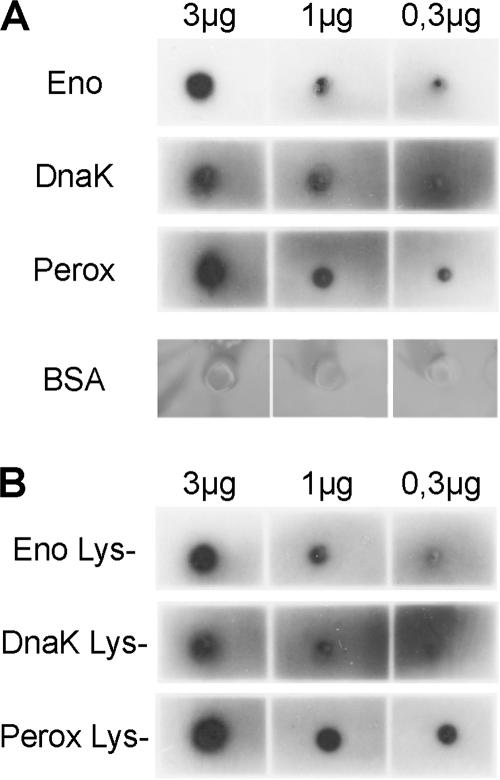
Binding of plasminogen does not depend on C-terminal lysine residues. The recombinant proteins His-6-enolase (Eno), His-6-peroxiredoxin (Perox), and His-6-DnaK (DnaK) (A) and the respective Lys-negative variants (B) were dotted directly on a membrane to assess plasminogen binding of the native proteins by overlay assays. Three, 1, and 0.3 μg of each protein were applied for the dot blot. In this native binding assay, no difference in binding capacity could be detected between the recombinant receptors (A) and their C-terminal lysine-depleted variants (B), indicating that internal binding motifs are responsible for plasminogen recognition. No significant signal could be detected for albumin (A).
Soluble receptors competitively inhibit binding of plasminogen to meningococci.
To provide experimental evidence for the binding specificities of the identified putative meningococcal plasminogen binding proteins with plasminogen, recombinant enolase, DnaK, and peroxiredoxin were used as competitors in an N. meningitidis plasminogen binding assay. Binding of plasminogen to meningococci was performed in the presence of different concentrations of soluble recombinant receptor molecules, and the amounts of surface-bound plasminogen were determined by ELISA (Fig. 3). Plasminogen binding to N. meningitidis was inhibited by all three competitors. In contrast, addition of albumin, a serum protein with a broad but nonspecific binding capacity for other proteins, resulted in only a small reduction of the plasminogen binding capacity compared to the experiments performed without the addition of an inhibitor (Fig. 3). Inhibition of plasminogen binding was dependent on the concentrations of the soluble receptors enolase, DnaK, and peroxiredoxin. A 100-fold molar excess of the receptors resulted in an inhibition of 70% or more (P < 0.01). Inhibition efficiencies were comparable for the three assayed receptors. To unequivocally confirm the roles of the three identified meningococcal plasminogen binding proteins in plasminogen acquisition by N. meningitidis, construction of deletion mutants was attempted. However, several attempts did not lead to successful construction of mutants that were deficient for enolase or DnaK. As meningococci contain only one copy of the genes encoding enolase and DnaK, these proteins are likely to be essential for bacterial viability, as has been shown previously for other pathogens, including S. pneumoniae (8). In contrast, a deletion mutant for peroxiredoxin was constructed by insertion of an antibiotic resistance cassette. Plasminogen binding of this mutant was quantified by measuring the bound plasmin activity after activation of plasminogen with uPA. Plasmin activity was determined via cleavage of the plasmin-specific chromogenic substrate S-2251. In an independent approach, plasminogen binding of MC58 Δperox was quantified in an ELISA and compared to that of the parental strain. However, no difference in plasminogen binding or plasmin activity could be detected between the Δperox mutant and its parental strain, suggesting that peroxiredoxin alone is not essential to acquire bound plasmin activity at the meningococcal surface (data not shown).
FIG. 3.
Soluble recombinant receptor proteins competitively inhibit plasminogen binding to N. meningitides. Microtiter wells were coated with 4 × 106 bacteria (strain MC58 ΔsiaD), and plasminogen (11 pM) was added to individual wells with increasing amounts of the recombinant receptor proteins enolase (E), DnaK (D), and peroxiredoxin (P) for 30 min at 37°C. The recombinant proteins were used in 50-fold (550 pM), 100-fold (1.1 nM), and 200-fold (2.2 nM) molar excess over plasminogen. Plasminogen binding to bacteria without inhibitor was set to 100%. Albumin (B), which displays nonspecific binding capacities to a large number of proteins, was used as a negative control. *, P < 0.001. The error bars indicate standard deviations.
Plasminogen-receptor interaction depends on sequence and structural motifs.
C-terminal lysine residues have been described as major binding sites for plasminogen. However, recent results indicate that in some bacterial plasminogen receptors, C-terminal lysine residues are not involved in complex formation. In contrast, internal lysine-rich domains are critical for the interaction between plasminogen and these receptors (7, 8, 23). Meningococcal enolase and DnaK contain lysine residues at the C termini of the proteins, whereas peroxiredoxin contains a lysine residue in its penultimate C-terminal position. To assess the contribution of the C-terminal lysine residues to plasminogen binding, the C-terminal lysine residue of enolase was replaced by alanine, the two C-terminal lysine residues of DnaK were replaced by two alanines, and the C-terminal Lys-Asn dipeptide of peroxiredoxin was replaced by two alanines. The plasminogen binding capacities of wild-type and mutant proteins were assessed in plasminogen overlay assays after SDS electrophoresis. Under these conditions, the binding capacities of the mutant DnaK and peroxiredoxin were decreased. In contrast, no effect could be observed for enolase (data not shown). To investigate the role of C-terminal lysine residues for in vivo relevant binding of the native proteins to plasminogen, dot blots were performed with nondenatured wild-type and mutated recombinant receptors (Fig. 2B). In contrast to the overlay assay, binding of plasminogen to the lysine-depleted plasminogen receptors was not affected under these conditions. All mutated receptors bound plasminogen as well as the wild-type proteins did. This indicates that internal binding motifs, rather than the C-terminal lysine residues, are important for plasminogen recruitment by the meningococcal receptor proteins.
Plasminogen binding proteins are expressed at the meningococcal surface.
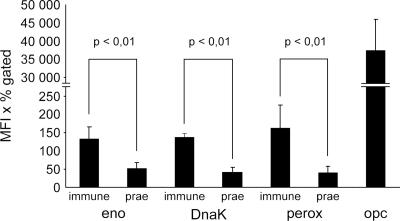
The bacterial plasminogen receptors that have been characterized so far immobilize plasminogen at the bacterial surface and endow bacteria with a surface-associated proteolytic activity. To characterize the surface accessibilities of the putative meningococcal plasminogen receptors, antisera were raised against recombinantly expressed meningococcal enolase, peroxiredoxin, and DnaK. The specificities of the antisera were confirmed in immunoblots using recombinant proteins and total bacterial lysates. Each antiserum identified a unique band in a total-protein lysate of N. meningitidis that was identical in size to the bands detected in plasminogen overlay assays. In contrast, no signals could be detected for N. meningitidis with the respective preimmune sera in ELISA or by fluorescence microscopy (data not shown). In crude outer membrane preparations of N. meningitidis, all three putative plasminogen receptors were clearly detectable using the antisera (data not shown), although the majority of the proteins were found in total cellular lysates, as would be expected from proteins primarily present in the cytosol. To further corroborate the surface accessibility of peroxiredoxin, DnaK, and enolase, flow cytometric analyses were performed using the purified IgG fraction of the rabbit antisera, followed by FITC-labeled anti-rabbit IgG for detection. The corresponding IgG fractions from preimmune sera were used as negative controls, and detection of Opc, a highly abundant outer membrane protein, was used as a positive control. For all three putative receptors, low but significant levels of surface-exposed protein could be detected (Fig. 4). In contrast, on the Δperox deletion mutant, only signals near background levels were detectable. In the control experiments, large amounts of Opc were detected on strain MC58 (Fig. 4), whereas no Opc could be detected on an Opc deletion mutant (data not shown). Transmission electron microscopy with immunogold labeling was used to visualize the surface association of the putative plasminogen receptors (Fig. 5). All three proteins could be detected on the surfaces of intact meningococci. In contrast, no labeling was detected using the preimmune sera from the same animals used for antibody production instead of the antisera. Coaccessibility of bound plasminogen and receptor molecules was demonstrated by confocal microscopy. On the surfaces of meningococci preincubated with plasminogen, each of the three receptors and plasminogen were labeled by immune staining and detected by confocal microscopy (Fig. 6). The resolution of confocal microscopy did not allow detection of colocalization of plasminogen and receptor molecules on a molecular scale but gave evidence for the presence of enolase, DnaK, and peroxiredoxin at least in the vicinity of plasminogen on the outer membranes of meningococci. The surface accessibility of meningococcal enolase, peroxiredoxin, and DnaK was further investigated by ELISA. Using a molar standard of recombinant proteins in the ELISA, the number of surface-displayed receptor molecules per meningococcal cell was estimated to be 9,560 ± 2,322 molecules of peroxiredoxin, 502 ± 139 of enolase, and 199 ± 31 of DnaK, confirming the results of fluorescence-activated cell sorter analyses. Thus, with several independent experimental approaches, the surface accessibility of the meningococcal plasminogen receptors was demonstrated.
FIG. 4.
Flow cytometric analysis shows that meningococcal enolase (eno), DnaK, and peroxiredoxin (perox) are present at the bacterial surface. Bacteria were labeled with the IgG fraction of the rabbit antisera raised against the three proteins. IgG fractions from preimmune sera were used as negative controls. Significantly higher signals were detected with IgG fractions from sera than with IgG fractions from preimmune sera (P < 0.01). The values are mean fluorescence intensity times the percentage of labeled bacteria from three independent experiments ± the standard deviation. Signals measured on Δperox show background levels detected by the anti-peroxiredoxin IgG fraction. Opc served as a control for an abundant meningococcal surface protein. Significantly more peroxiredoxin than enolase or DnaK was detected (P < 0.01).
FIG. 5.
Transmission electron micrographs of N. meningitidis strain MC58 ΔsiaD demonstrate that enolase (A), DnaK (B), and peroxiredoxin (C) are present at the surface of N. meningitidis. Magnification of single gold particles (right) clearly shows association with the outer membrane (OM), which has been dissociated from the inner membrane by osmotic swelling. Enolase, DnaK, and peroxiredoxin were detected with rabbit antisera and a gold-labeled anti-rabbit mouse monoclonal antibody. No signals were detected with the preimmune sera. The arrows indicate gold particles in the lower magnification.
FIG. 6.
Enolase (A), DnaK (B), and peroxiredoxin (C) were detected on the surfaces of meningococci preincubated with plasminogen. Receptor molecules were detected by the respective rabbit antisera and a tetramethyl rhodamine isothiocyanate-labeled anti-rabbit antibody (magenta). Plasminogen was detected with a goat anti-plasminogen antibody (1:1,000) and a FITC-labeled rabbit-anti-goat antibody (1:500) (blue). The arrows indicate overlapping of receptor and plasminogen staining (light magenta).
Capsule and LPS influence binding of plasminogen to N. meningitidis.
Outer membrane-associated proteins of N. meningitidis are shielded from recognition by host factors via a polysaccharide capsule. In addition, the outer membrane of N. meningitidis contains large amounts of negatively charged lipopolysaccharide (LPS), which can also affect the interaction of meningococci with the host. To test whether capsule expression prevents plasminogen binding, wild-type serogroup B strain MC58 was compared with an isogenic capsule deletion mutant, MC58 ΔsiaD, in plasminogen binding assays. The binding capacity of the unencapsulated strain was enhanced to about 140% of that of the wild-type isolate (plasminogen binding of strain MC58 ΔsiaD, 146 ± 21% compared to strain MC58; P < 0.001). This indicates that although expression of the capsule leads to slightly reduced binding, it does not prevent plasminogen from coming into contact with the meningococcal receptors. To examine the influence of the LPS on plasminogen binding, different meningococcal mutants expressing truncated LPS carbohydrate moieties were used in plasminogen binding and subsequent plasmin activity assays. Interestingly, truncation of the alpha chain alone by inactivation of the gene lgtA or pgm did not impair binding of plasminogen. In contrast, truncation of all three sugar chains in the ΔrfaF mutant significantly enhanced binding of plasminogen and plasmin activity in unencapsulated MC58 mutants (plasminogen binding of MC58 ΔsiaD ΔrfaF, 202% compared to MC58 ΔsiaD; P < 0.001). Similar results were obtained for encapsulated strains, although the net effect of LPS was lower in the presence of the meningococcal capsule (plasminogen binding of MC58 ΔrfaF, 123% compared to MC58; P = 0.01). Therefore, only substantial truncations of meningococcal LPS can increase plasminogen binding, and this effect occurs mainly in unencapsulated variants of N. meningitidis.
Plasminogen is recruited to the N. meningitidis surface from human serum and is protected against inactivation after conversion to plasmin.
To assess the functional relevance of the meningococcal in vitro capacity for plasminogen binding, the recruitment of plasminogen from human serum was investigated. Meningococci acquired comparable quantities of surface-bound plasmin activity after pretreatment with purified plasminogen or after incubation with human serum and consecutive activation with uPA (Fig. 7A). Therefore, plasminogen can be recruited to the meningococcal surface from serum. Identical results were obtained with human saliva, which also contains considerable amounts of plasminogen. These data indicate that no inhibition of plasminogen binding to N. meningitidis and subsequent activation by uPA occurs in human serum. To confirm the biological activity of bacterium-bound plasmin, degradation of fibrinogen was examined after pretreatment of plasminogen-coated unencapsulated meningococci with urokinase. Degradation was illustrated in an immunoblot using anti-fibrinogen antibodies (Fig. 7B). After 30 min, the amount of detectable intact fibrinogen was markedly decreased. After 120 min, only traces of fibrinogen had sustained plasmin-mediated degradation. In control experiments, no degradation was detected after the incubation of fibrinogen with bacteria in the absence of plasminogen. In addition, in control experiments in which the N. meningitidis-associated plasminogen was not activated by uPA, no fibrinogen degradation could be observed. Identical results were obtained with the encapsulated strain MC58. In vivo, plasmin activity is subtly controlled by plasmin antagonists like α2-antiplasmin. However, whereas free plasmin is rapidly inactivated by α2-antiplasmin, plasmin activity resulting from receptor-bound plasminogen is protected against inactivation. To test, whether binding of plasminogen to N. meningitidis also results in protection of activated plasmin against α2-antiplasmin, plasmin-coated bacteria were incubated with α2-antiplasmin. Whereas the activity of free plasmin was completely abolished by α2-antiplasmin in control experiments, a large percentage of N. meningitidis-associated plasmin activity was protected against inactivation (Fig. 7C). Degradation of fibrinogen by N. meningitidis-associated plasmin was not inhibited in the presence of α2-antiplasmin, whereas degradation of fibrinogen by free plasmin was completely abolished in the presence of α2-antiplasmin (data not shown). Therefore, plasminogen recruitment can equip N. meningitidis with proteolytic activity that is no longer controlled by serum antagonists.
FIG. 7.
(A) N. meningitidis can recruit plasminogen activity directly from human serum. MC58 ΔsiaD was incubated with either purified plasminogen (PLG), 30% human serum (HS) (41) diluted in PBS, or 100% human serum washed and activated with uPA. Plasmin activity was measured at 405 nm by degradation of the chromogenic substrate S-2251. The activity detected after incubation with purified plasminogen was set to 100%. (B) N. meningitidis-associated plasminogen can cleave fibrinogen after activation by uPA. Purified fibrinogen was incubated with either N. meningitidis alone (lane 1), plasmin (lanes 2 and 3), plasminogen-coated meningococci (lane 4), or plasminogen-coated meningococci that were subsequently activated with uPA (lanes 5 to 7). Fibrinogen degradation was detected in an immunoblot at different time points (indicated in minutes at the bottom of each lane). (C) N. meningitidis-associated plasmin activity is protected against inactivation by α2-antiplasmin. Plasmin activity was measured by S-2251 degradation in the absence or presence of α2-antiplasmin. Lane 1, plasmin; lane 2, plasmin plus α2-antiplasmin (activity in relation to lane 1); lane 3, N. meningitidis-associated plasmin; lane 4, N. meningitidis-associated plasmin plus α2-antiplasmin (activity in relation to lane 3), *, P < 0,001. The error bars indicate standard deviations.
DISCUSSION
Many pathogenic bacteria have been shown to recruit host plasminogen to their surfaces, thereby acquiring a broad-spectrum proteolytic activity. Known plasminogen receptors comprise proteins as heterogeneous as fimbriae (47), glycolytic enzymes (5, 6, 46), and adhesion proteins (4). In this report, three proteins of N. meningitidis have been shown to act as plasminogen receptors. Although at least two of them (enolase and DnaK) are considered to be primarily cytoplasmic, the availability of enolase, DnaK, and peroxiredoxin at the surface of N. meningitidis has been documented by flow cytometry, immune electron microscopy, confocal microscopy, and ELISA. Enolase, a 46-kDa glycolytic enzyme, is highly conserved in prokaryotes and eukaryotes. Even though no signal peptide for secretion or localization to the outer membrane has yet been identified, enolases of bacteria and eukaryotic cells have been shown to be localized in the outer membrane (11, 21, 43, 56, 60). Indeed, the recruitment of plasminogen to cellular surfaces by enolase seems to be a conserved pattern in different cells, including bacteria, fungi, and eukaryotic cells (5, 36, 38, 41, 46). A recent proteomic analysis identified enolase and DnaK as components of outer membrane vesicles derived from serogroup B N. meningitidis (24). DnaK is a protein of 67 kDa that belongs to the heat shock protein 70 family (10). The members of this family function as chaperones involved in the folding and transport of proteins within the cell. DnaK has been shown to be present at the cellular surfaces of H. influenzae after heat shock and confers binding to sulfated glycolipids on this pathogen (26). In Listeria monocytogenes, DnaK is also associated with the bacterial surface and has been implicated in plasminogen recruitment (54). Peroxiredoxin, a small protein of 24 kDa, is characterized by domains that are common in reducing enzymes and electron transporters (Pfam domains: glutaredoxin and AhpC-TSA). A peroxiredoxin homologue in Escherichia coli has been suggested to be localized at the cell envelope (3, 44). Screening for potential vaccine candidates against Bacillus anthracis revealed peroxiredoxin as a surface-associated protein (1).
The presence of primarily cytoplasmic proteins that lack known secretion signals at bacterial surfaces seems to be common in different species. The prerequisites for secretion of these proteins are not yet understood. In S. pneumoniae, it has been suggested that enolase released by frequently occurring autolysis of the bacteria can be recruited to the surfaces of intact bacteria (5). However, no similar mechanism could be detected for any of the meningococcal plasminogen receptors described in this study, although autolysis occurs at high levels in the naturally competent species N. meningitidis (data not shown). A recent genetic approach in C. albicans indicated that in this pathogenic yeast, sequence motifs within the first 169 amino acids of enolase are sufficient for secretion of the protein (36). Furthermore, no proteolytic cleavage seems to be involved in the secretion process, except perhaps the removal of the N-terminal methionine (36, 54). Given the high degree of conservation of molecules like enolase and DnaK in the protein sequence, it is intriguing to speculate that there might be a yet-unknown signal for protein export that has been conserved over a long period in evolution (45).
The results presented strongly suggest the existence of internal plasminogen binding sites, as all three receptors showed interaction with plasminogen independent of the C-terminal lysine residues in their native form. Similar findings have been documented for pneumococcal enolase, which also has a 9-amino-acid internal plasminogen binding motif (FYDKERKVY) (8). Sequence homology searches of the meningococcal receptors showed only weak similarities to the pneumococcal plasminogen binding peptide, although several lysine-rich motifs could be found. Alternatively, plasminogen can bind to arginine and histidine residues, as shown for a group A streptococcal M-like surface protein (50) and a PAM-related protein (53). This also might explain plasminogen binding independent of C-terminal lysine residues. Flow cytometric analyses have shown that all three receptors are present at the meningococcal surface, but at low levels. However, it is well known from other bacterial pathogens that plasminogen receptors can be present at the cell surface at very low levels, as plasminogen is highly abundant in many body fluids (serum concentrations, ∼1.6 to 2 μmol/liter, roughly corresponding to 100 to 200 mg/liter) (2, 12, 35, 39). The presence of pneumococcal enolase, which has been shown to act as the major plasminogen receptor of S. pneumoniae, at the surfaces of these bacteria has been studied in detail (29). Comparing these data, N. meningitidis plasminogen receptors are present at the meningococcal surface in at least the same quantity as pneumococcal enolase (29). The overall number of enolase, DnaK, and peroxiredoxin molecules was calculated in a quantitative ELISA to be approximately 10,000. Although several aspects, including polymerization of the proteins in vivo, could not be considered in this estimation, the calculated number of receptors is roughly in agreement with the data of Ullberg et al., who calculated between 13,000 and 26,000 receptor molecules per meningococcus (61), especially as the overlay assay suggests the presence of further plasminogen binding proteins in N. meningitidis. As attempts to identify these factors directly from the overlay assay have failed so far, other approaches will be necessary for their identification.
The contribution of the single identified meningococcal receptor to plasminogen recruitment by this pathogen is determined by different variables. Availability at the cell surface, the number of exposed molecules, and the number of binding epitopes per molecule are the crucial factors, whereas due to the high concentration of plasminogen in human serum, the affinity of the receptors is likely to be of minor importance. In this context, it is noteworthy that the meningococcal capsule, although diminishing surface plasminogen, prevents neither plasminogen recruitment nor surface-associated plasmin activity in N. meningitidis. Identical results have been obtained for pneumococci (5), indicating sufficient access of plasminogen to the receptor molecules through the capsular polysaccharide. In contrast to gram-positive S. pneumoniae, meningococci possess an outer membrane that contains large amounts of LPS. Meningococcal LPS has been shown to inhibit receptor-mediated interaction of meningococci with eukaryotic cells (31). In addition, it has been demonstrated that a lack of repetitive O antigen is essential for plasminogen activation by Y. pestis and Salmonella enterica serovar Typhimurium (30). Similarly, in N. meningitidis, plasminogen binding can be increased by modification of the LPS carbohydrate moiety. However, only substantial truncations lead to a significant increase of plasminogen binding, whereas alterations in the alpha chain of meningococcal LPS, which can occur naturally due to phase variation, do not seem to influence plasminogen recruitment or activation.
Invasive meningococcal disease is initiated by a translocation of virulent N. meningitidis beyond the epithelial barrier of the nasopharynx, which is the initial locus of colonization. The mucosal cells of the nasopharynx are covered by a layer of mucus (ECM) composed of a heterogeneous mixture of glycoproteins (mucins, collagens, elastin, fibronectin, and laminin) and proteoglycans (chondroitin sulfate proteoglycan and heparin sulfate proteoglycan). Some of these ECM proteins also constitute the basal laminae and subepithelial tissue, which support all epithelial and endothelial borders. The degradation of ECM components and the activation of collagenases by plasmin can support colonization of the oropharynx and may support meningococci in colonizing the deep crypts of the tonsils. A similar strategy has been suggested for C. albicans, which also colonizes the mucosal epithelia (18). In addition, N. meningitidis has been shown to interact with components of the ECM, thus promoting integrin-mediated adhesion and invasion (62). Therefore, surface recruitment of plasmin by N. meningitidis might contribute not only to cell surface accession and the establishment of intimate adhesion by degrading several of the ECM components but also to a release of these components from the ECM network, which can then lead to invasion of the bacteria. The proteolytic inactivation of complement factors and antibodies is another possible mechanism by which surface-bound plasmin may support bacterial survival on mucosal surfaces and in the blood, especially as outer membrane vesicles, which can be shed in high numbers by N. meningitidis during infection, contain at least two of the identified plasminogen receptors (24). To further determine the effects of surface-bound plasmin on meningococcal colonization and invasion, more complex cell culture models will be required. Investigation of such models will provide new knowledge about the ways in which N. meningitidis colonizes and invades its human host.
Acknowledgments
This work would not have been possible without the technical assistance of Nina Trzeciak, Andrea Brandt, and Nicole Rexhaeuser. Electron microscopy was performed with the help of Daniela Bunsen and Elisabeth Meyer-Natus at the chair for electron microscopy (University of Wuerzburg; head, Georg Krohne). Antibodies were kindly provided by M. Achtman (MPI, Berlin, Germany) and M. Virji (University of Bristol, United Kingdom). We are grateful to Rita Getzlaff (GBF, Helmhotz-Zentrum für Infektionsforschung, Braunschweig, Germany) for N-terminal peptide analysis, to Jens Waschke (Institute for Anatomy and Cell Biology II, University of Würzburg) for support in performing confocal microscopy, and to Ulrich Vogel and Corinna Schmitt for helpful discussions and critical reading of the manuscript.
The study was financially supported by the Deutsche Forschungsgemeinschaft (SFB479, project B2, to M.F. and O.K.).
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Ariel, N., A. Zvi, K. S. Makarova, T. Chitlaru, E. Elhanany, B. Velan, S. Cohen, A. M. Friedlander, and A. Shafferman. 2003. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect. Immun. 71:4563-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow, G. H., L. Summaria, and K. C. Robbins. 1969. Molecular weight studies on human plasminogen and plasmin at the microgram level. J. Biol. Chem. 244:1138-1141. [PubMed] [Google Scholar]
- 3.Bayer, M. E., M. H. Bayer, C. A. Lunn, and V. Pigiet. 1987. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J. Bacteriol. 169:2659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berge, A., and U. Sjobring. 1993. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 268:25417-25424. [PubMed] [Google Scholar]
- 5.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. Alpha-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, S., M. Rohde, and S. Hammerschmidt. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 72:2416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann, S., M. Rohde, K. T. Preissner, and S. Hammerschmidt. 2005. The nine residue plasminogen-binding motif of the pneumococcal enolase is the major cofactor of plasmin-mediated degradation of extracellular matrix, dissolution of fibrin and transmigration. Thromb. Haemost. 94:304-311. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann, S., D. Wild, O. Diekmann, R. Frank, D. Bracht, G. S. Chhatwal, and S. Hammerschmidt. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49:411-423. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg, P., G. B. Joo, B. Brusletto, and P. Kierulf. 1990. Plasminogen activator inhibitor 1 and 2, alpha-2-antiplasmin, plasminogen, and endotoxin levels in systemic meningococcal disease. Thromb. Res. 57:271-278. [DOI] [PubMed] [Google Scholar]
- 10.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 11.Carneiro, C. R., E. Postol, R. Nomizo, L. F. Reis, and R. R. Brentani. 2004. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 6:604-608. [DOI] [PubMed] [Google Scholar]
- 12.Cederholm-Williams, S. A. 1981. Concentration of plasminogen and antiplasmin in plasma and serum. J. Clin. Pathol. 34:979-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claus, H., M. C. Maiden, R. Maag, M. Frosch, and U. Vogel. 2002. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148:1813-1819. [DOI] [PubMed] [Google Scholar]
- 14.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263-1271. [DOI] [PubMed] [Google Scholar]
- 15.Claus, H., U. Vogel, M. Muhlenhoff, R. Gerardy-Schahn, and M. Frosch. 1997. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol. Gen. Genet. 257:28-34. [DOI] [PubMed] [Google Scholar]
- 16.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 17.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe, J. D., I. K. Sievwright, G. C. Auld, N. R. Moore, N. A. Gow, and N. A. Booth. 2003. Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol. Microbiol. 47:1637-1651. [DOI] [PubMed] [Google Scholar]
- 19.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 20.Dunn, K. L., M. Virji, and E. R. Moxon. 1995. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb. Pathog. 18:81-96. [DOI] [PubMed] [Google Scholar]
- 21.Ebanks, R. O., M. Goguen, S. McKinnon, D. M. Pinto, and N. W. Ross. 2005. Identification of the major outer membrane proteins of Aeromonas salmonicida. Dis. Aquat. Organ. 68:29-38. [DOI] [PubMed] [Google Scholar]
- 22.Eberhard, T., G. Kronvall, and M. Ullberg. 1999. Surface bound plasmin promotes migration of Streptococcus pneumoniae through reconstituted basement membranes. Microb. Pathog. 26:175-181. [DOI] [PubMed] [Google Scholar]
- 23.Ehinger, S., W. D. Schubert, S. Bergmann, S. Hammerschmidt, and D. W. Heinz. 2004. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: crystal structure and evaluation of plasmin(ogen)-binding sites. J. Mol. Biol. 343:997-1005. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari, G., I. Garaguso, J. Adu-Bobie, F. Doro, A. R. Taddei, A. Biolchi, B. Brunelli, M. M. Giuliani, M. Pizza, N. Norais, and G. Grandi. 2006. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856-1866. [DOI] [PubMed] [Google Scholar]
- 25.Frosch, M., E. Schultz, E. Glenn-Calvo, and T. F. Meyer. 1990. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol. Microbiol. 4:1215-1218. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann, E., C. A. Lingwood, and J. Reidl. 2001. Heat-inducible surface stress protein (Hsp70) mediates sulfatide recognition of the respiratory pathogen Haemophilus influenzae. Infect. Immun. 69:3438-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolberg, J., A. Aase, S. Bergmann, T. K. Herstad, G. Rodal, R. Frank, M. Rohde, and S. Hammerschmidt. 2006. Streptococcus pneumoniae enolase is important for plasminogen binding despite low abundance of enolase protein on the bacterial cell surface. Microbiology 152:1307-1317. [DOI] [PubMed] [Google Scholar]
- 30.Kukkonen, M., M. Suomalainen, P. Kyllonen, K. Lahteenmaki, H. Lang, R. Virkola, I. M. Helander, O. Holst, and T. K. Korhonen. 2004. Lack of O-antigen is essential for plasminogen activation by Yersinia pestis and Salmonella enterica. Mol. Microbiol. 51:215-225. [DOI] [PubMed] [Google Scholar]
- 31.Kurzai, O., C. Schmitt, H. Claus, U. Vogel, M. Frosch, and A. Kolb-Maurer. 2005. Carbohydrate composition of meningococcal lipopolysaccharide modulates the interaction of Neisseria meningitidis with human dendritic cells. Cell Microbiol. 7:1319-1334. [DOI] [PubMed] [Google Scholar]
- 32.Lahteenmaki, K., S. Edelman, and T. K. Korhonen. 2005. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 13:79-85. [DOI] [PubMed] [Google Scholar]
- 33.Lahteenmaki, K., M. Kukkonen, and T. K. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 504:69-72. [DOI] [PubMed] [Google Scholar]
- 34.Lahteenmaki, K., P. Kuusela, and T. K. Korhonen. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25:531-552. [DOI] [PubMed] [Google Scholar]
- 35.Leipnitz, G., C. Miyashita, M. Heiden, G. von Blohn, M. Kohler, and E. Wenzel. 1988. Reference values and variability of plasminogen in healthy blood donors and its relation to parameters of the fibrinolytic system. Haemostasis 18(Suppl. 1):61-68. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Villar, E., L. Monteoliva, M. R. Larsen, E. Sachon, M. Shabaz, M. Pardo, J. Pla, C. Gil, P. Roepstorff, and C. Nombela. 2006. Genetic and proteomic evidences support the localization of yeast enolase in the cell surface. Proteomics 6(Suppl. 1):S107-S118. [DOI] [PubMed] [Google Scholar]
- 37.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 38.Miles, L. A., C. M. Dahlberg, J. Plescia, J. Felez, K. Kato, and E. F. Plow. 1991. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 30:1682-1691. [DOI] [PubMed] [Google Scholar]
- 39.Miyashita, C., E. Wenzel, and M. Heiden. 1988. Plasminogen: a brief introduction into its biochemistry and function. Haemostasis 18(Suppl. 1):7-13. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, G., H. Stanton, S. Cowell, G. Butler, V. Knauper, S. Atkinson, and J. Gavrilovic. 1999. Mechanisms for pro matrix metalloproteinase activation. Apmis 107:38-44. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima, K., M. Hamanoue, N. Takemoto, T. Hattori, K. Kato, and S. Kohsaka. 1994. Plasminogen binds specifically to alpha-enolase on rat neuronal plasma membrane. J. Neurochem. 63:2048-2057. [DOI] [PubMed] [Google Scholar]
- 42.Nassif, X., S. Bourdoulous, E. Eugene, and P. O. Couraud. 2002. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 10:227-232. [DOI] [PubMed] [Google Scholar]
- 43.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6:2121-2134. [DOI] [PubMed] [Google Scholar]
- 44.Nygren, H., B. Rozell, A. Holmgren, and H. A. Hansson. 1981. Immunoelectron microscopic localization of glutaredoxin and thioredoxin in Escherichia coli cells. FEBS Lett. 133:145-150. [DOI] [PubMed] [Google Scholar]
- 45.Pancholi, V. 2001. Multifunctional alpha-enolase: its role in diseases. Cell Mol. Life Sci. 58:902-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pancholi, V., and V. A. Fischetti. 1997. A novel plasminogen/plasmin binding protein on the surface of group A streptococci. Adv. Exp. Med. Biol. 418:597-599. [DOI] [PubMed] [Google Scholar]
- 47.Parkkinen, J., and T. K. Korhonen. 1989. Binding of plasminogen to Escherichia coli adhesion proteins. FEBS Lett. 250:437-440. [DOI] [PubMed] [Google Scholar]
- 48.Plow, E. F., T. Herren, A. Redlitz, L. A. Miles, and J. L. Hoover-Plow. 1995. The cell biology of the plasminogen system. FASEB J. 9:939-945. [DOI] [PubMed] [Google Scholar]
- 49.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853-50862. [DOI] [PubMed] [Google Scholar]
- 50.Rios-Steiner, J. L., M. Schenone, I. Mochalkin, A. Tulinsky, and F. J. Castellino. 2001. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A streptococcal surface protein. J. Mol. Biol. 308:705-719. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 52.Salmaso, S., P. Mastrantonio, G. Scuderi, M. E. Congiu, T. Stroffolini, M. G. Pompa, and S. Squarcione. 1997. Pattern of bacterial meningitis in Italy, 1994. Eur. J. Epidemiol. 13:317-321. [DOI] [PubMed] [Google Scholar]
- 53.Sanderson-Smith, M. L., M. Dowton, M. Ranson, and M. J. Walker. 2007. The PAM related protein Prp binds plasminogen via arginine and histidine residues. J. Bacteriol. 189:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaumburg, J., O. Diekmann, P. Hagendorff, S. Bergmann, M. Rohde, S. Hammerschmidt, L. Jansch, J. Wehland, and U. Karst. 2004. The cell wall subproteome of Listeria monocytogenes. Proteomics 4:2991-3006. [DOI] [PubMed] [Google Scholar]
- 55.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins, et al. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 56.Severin, A., E. Nickbarg, J. Wooters, S. A. Quazi, Y. V. Matsuka, E. Murphy, I. K. Moutsatsos, R. J. Zagursky, and S. B. Olmsted. 2007. Proteomic analysis and identification of Streptococcus pyogenes surface-associated proteins. J. Bacteriol. 189:1514-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodeinde, O. A., Y. V. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 58.Sun, H., U. Ringdahl, J. W. Homeister, W. P. Fay, N. C. Engleberg, A. Y. Yang, L. S. Rozek, X. Wang, U. Sjobring, and D. Ginsburg. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305:1283-1286. [DOI] [PubMed] [Google Scholar]
- 59.Syrogiannopoulos, G. A., C. J. Mitselos, and N. G. Beratis. 1995. Childhood bacterial meningitis in Southwestern Greece: a population-based study. Clin. Infect. Dis. 21:1471-1473. [DOI] [PubMed] [Google Scholar]
- 60.Taverna, F., A. Negri, R. Piccinini, A. Zecconi, S. Nonnis, S. Ronchi, and G. Tedeschi. 2007. Characterization of cell wall associated proteins of a Staphylococcus aureus isolated from bovine mastitis case by a proteomic approach. Vet. Microbiol. 119:240-247. [DOI] [PubMed] [Google Scholar]
- 61.Ullberg, M., P. Kuusela, B. E. Kristiansen, and G. Kronvall. 1992. Binding of plasminogen to Neisseria meningitidis and Neisseria gonorrhoeae and formation of surface-associated plasmin. J. Infect. Dis. 166:1329-1334. [DOI] [PubMed] [Google Scholar]
- 62.Unkmeir, A., K. Latsch, G. Dietrich, E. Wintermeyer, B. Schinke, S. Schwender, K. S. Kim, M. Eigenthaler, and M. Frosch. 2002. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 46:933-946. [DOI] [PubMed] [Google Scholar]
- 63.Virkola, R., K. Lahteenmaki, T. Eberhard, P. Kuusela, L. van Alphen, M. Ullberg, and T. K. Korhonen. 1996. Interaction of Haemophilus influenzae with the mammalian extracellular matrix. J. Infect. Dis. 173:1137-1147. [DOI] [PubMed] [Google Scholar]
- 64.Vogel, U., G. Morelli, K. Zurth, H. Claus, E. Kriener, M. Achtman, and M. Frosch. 1998. Necessity of molecular techniques to distinguish between Neisseria meningitidis strains isolated from patients with meningococcal disease and from their healthy contacts. J. Clin. Microbiol. 36:2465-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wistedt, A. C., H. Kotarsky, D. Marti, U. Ringdahl, F. J. Castellino, J. Schaller, and U. Sjobring. 1998. Kringle 2 mediates high affinity binding of plasminogen to an internal sequence in streptococcal surface protein PAM. J. Biol. Chem. 273:24420-24424. [DOI] [PubMed] [Google Scholar]