Abstract
Bacteria use a number of mechanisms for coping with the toxic effects exerted by nitric oxide (NO) and its derivatives. Here we show that the flavohemoglobin encoded by the hmp gene has a vital role in an adaptive response to protect the soil bacterium Bacillus subtilis from nitrosative stress. We further show that nitrosative stress induced by the nitrosonium cation donor sodium nitroprusside (SNP) leads to deactivation of the transcriptional repressor NsrR, resulting in derepression of hmp. Nitrosative stress induces the sigma B-controlled general stress regulon. However, a sigB null mutant did not show increased sensitivity to SNP, suggesting that the sigma B-dependent stress proteins are involved in a nonspecific protection against stress whereas the Hmp flavohemoglobin plays a central role in detoxification. Mutations in the yjbIH operon, which encodes a truncated hemoglobin (YjbI) and a predicted 34-kDa cytosolic protein of unknown function (YjbH), rendered B. subtilis hypersensitive to SNP, suggesting roles in nitrosative stress management.
Nitric oxide (NO) is a free radical of major importance as a defense and signaling molecule (26). However, at high concentrations, NO is toxic to all cells. NO exerts toxic effects by binding to heme-, iron-, and copper-containing proteins. NO can be reduced or oxidized and thereby give rise to a series of compounds that are collectively referred to as reactive nitrogen species (RNS) (6, 34). Together with superoxide, NO rapidly forms the highly reactive intermediate peroxynitrite (ONOO−), which in turn can form peroxynitrous acid (ONOOH), an unstable and reactive oxidizing species. Another important RNS, the nitrosonium ion (NO+), can react with a variety of organic side groups, especially with thiols to form S-nitroso compounds (19). RNS react with many cellular components, including metals, lipids, thiols, and DNA, leading to membrane and DNA damage and to inhibition of respiration and other cellular activities. Biological and chemical processes contribute to the presence of RNS in nature. For example, through the action of NO synthases, phagocytic cells generate NO to inhibit infectious bacteria (6), and in soil NO is present as a result of microbial denitrification (3). In addition, bacteria that use nitrite as an electron acceptor can generate NO endogenously (4).
Bacteria have evolved a number of strategies for coping with the toxic effects exerted by RNS. They adapt by activating genes that encode proteins involved in detoxification, repair, and maintenance of homeostasis (reviewed in reference 30). In Escherichia coli an NO reductase, NorVW, and a flavohemoglobin play prominent roles in RNS detoxification. The latter enzyme is present in a wide variety of bacterial and eukaryotic microorganisms (30). Flavohemoglobins are oxygen-binding proteins composed of a heme-containing globin domain fused with a ferredoxin reductase-like flavin-binding domain (41). Under oxic conditions they efficiently oxidize NO to NO3−, and in the absence of oxygen they reduce NO to N2O (9, 41). Not all bacteria encode flavohemoglobins but some, for example, Mycobacterium tuberculosis, possess a compact hemoglobin, called truncated hemoglobin (trHb), that could fulfill the same function (27). In the gram-positive soil bacterium Bacillus subtilis, both a flavohemoglobin (Hmp) and a trHb (YjbI) are present. The structure of YjbI is known (10), but its physiological role remains unclear. Expression of the B. subtilis hmp gene is induced upon exposure to NO or sodium nitroprusside (SNP) (Na2[Fe(CN)5(NO+)]) (23), a nitrosating agent that can release NO+ spontaneously at physiological pH (14). However, the physiological role of Hmp in nitrosative stress management has not been established. The promoter of the hmp gene is induced during nitrate respiration by a shift to anaerobic conditions but not during fermentative growth in B. subtilis (17). Expression of hmp has been shown to be dependent on the ResD-ResE two-component system (23). A recent study has employed DNA microarrays to analyze the response of the B. subtilis transcriptome to NO (21). It was found that nitrosative stress induces expression of hmp and members of the PerR, Fur, and sigma B general stress regulons. Studies on E. coli have shown differences in transcriptome responses to NO and NO+ (33). In this study we present a genome-wide transcriptome analysis of the B. subtilis response to NO+ (SNP) and compare our data to the transcriptome analysis of the response to NO. A major difference revealed is that none of the genes that are up-regulated by SNP are members of the Fur regulon. We show that Hmp but not gene products that depend on sigma B for expression is required for an adaptive response to SNP in B. subtilis. Cells lacking trHb and YjbH, a protein of unknown function, were found to be hypersensitive to SNP indicating a role in nitrosative stress management. Moreover, we have elucidated the mechanism by which hmp transcription is regulated in response to nitrosonium stress.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The B. subtilis strains and plasmids used in this study are listed in Table 1. Oligonucleotides used as primers are listed in Table 2. E. coli strain TOP10 [mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG] was used for the propagation of plasmids. B. subtilis strains were grown at 37°C in nutrient sporulation medium with phosphate supplemented with 0.5% glucose (NSMPG) (39). Tryptose blood agar base medium (TBAB) was used for growth of bacteria on plates. Growth for RNA extraction was performed in 250-ml baffled E-flasks containing 25 ml of NSMPG at 37°C and 100 rpm. For microarray analysis and real-time PCR, B. subtilis strains were grown to exponential phase (optical density at 600 nm [OD600] = 0.6), at which point SNP, NaNO2, or NaNO3 was added to different final concentrations. Control cultures were left untreated. At 5 minutes after the addition, samples were taken out for RNA preparation. For reverse transcriptase PCR (RT-PCR), B. subtilis 1A1 cells were grown to stationary phase. L-broth or L-agar was used for growth of E. coli strains (32). The following antibiotics were used when required: chloramphenicol (5 μg ml−1), kanamycin (5 μg ml−1), tetracycline (15 μg ml−1), and a combination of erythromycin (0.5 μg ml−1) and lincomycin (12.5 μg ml−1) for B. subtilis strains and ampicillin (100 μg ml−1) and chloramphenicol (12.5 μg ml−1) for E. coli strains.
TABLE 1.
B. subtilis strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or referenceb |
|---|---|---|
| Strains | ||
| LAB2026 | trpC2 pheA1 Δhmp::cat Cmr | 17 |
| MH5081 | trpC2 pheA1 resDE::tet Tcr | 36 |
| 1A1 | trpC2 | BGSCc |
| LUW219 | ΔyjbIH::spc Spr | 18 |
| LUW222 | yjbIΩpMUTIN2mcs-yjbI′ Emr | This study |
| LUW229 | sigBΔ2::spc Spr | 18 |
| LUW260 | Δhmp::cat Cmr | LAB2026→1A1 |
| LUW261 | Δhmp::cat yjbIH::spc Cmr Spr | LUW219→LUW260 |
| LUW264 | ΔresDE::tet Tcr | 18 |
| LUW272 | ΔyjbH::spc Spr | This study |
| LUW340 | ΔnsrR::spc Spr | This study |
| LUW365 | ΔresDE::tet ΔnsrR::spc Tcr Spr | MH5081 → LUW340 |
| Plasmids | ||
| pCR-Blunt II-TOPO | Cloning vector; Kmr | Invitrogen |
| pCW7 | Expression vector capable of replication in E. coli and B. subtilis; Cmr | This study |
| pCW7-yjbI | yjbI under the Pspac promoter in pCW7 | This study |
| pCW7-yjbH | yjbH under the Pspac promoter in pCW7 | This study |
| pCW7-yjbIH | yjbIH under the Pspac promoter in pCW7 | This study |
| pDG1726 | Integration vector for B. subtilis; Spr Amr | 11 |
| pDG1727 | Integration vector for B. subtilis; Spr Amr | 11 |
| pDG148 | Expression vector capable of replication in E. coli and B. subtilis; Amr Kmr | 35 |
| pDG148-hmp | hmp under the Pspac promoter in pDG148 | This study |
| pHP13 | Expression vector capable of replication in E. coli and B. subtilis; Emr Cmr | 12 |
| pMUTIN2mcs | Integration vector for B. subtilis; Emr Amr | 38 |
| pΔnsrR1 | Kmr Spr | This study |
| pΔyjbIH1 | Apr Spr | 18 |
| pΔyjbH1 | Kmr Spr | This study |
| pYjbI2 | Insertional Emr plasmid carrying an internal part of yjbI | This study |
| pTOPOYjbIH | Kmr | This study |
All LUW strains are derivatives of 1A1 and thus carry the trpC2 auxotrophic marker. Amr, Cmr, Emr, Kmr, Tcr, and Spr, resistance to ampicillin, chloramphenicol, erythromycin, kanamycin, tetracycline, and spectinomycin, respectively.
Arrows indicate transformation and point from donor DNA to recipient strain.
BGSC, Bacillus Genetic Stock Center, Department of Biochemistry, Ohio State University, Columbus.
TABLE 2.
Oligonucleotide sequences
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| yjbI1 | GGCAGATGGGACAATCGTTTAACGCACC |
| yjbI4 | CCAAGCTTCTATCGCAACTTGTTGATAC |
| yjbI5 | CCGAATTCCAGCCCTACATGGTCCATTGCG |
| yjbI9 | GGGCATGCTGCTGATGAAAGTCAAGCAGTCTTA |
| yjb14 | CCGAATTCCTCCTTTCCAAGAAACGC |
| yjb16 | TTATCGCTTCCGCAAGCCTTACCGC |
| yjb17 | ATAGGCTCTCCTGCATATTTCTGAG |
| yjbH2 | TTTTTCACATGATTGATATTCATCAG |
| yjbH4 | TGATCTAGATGACAAACTATCAGCATGAGC |
| hmp1 | GGTCTAGATGTTAGATAACAAAACAATCG |
| hmp2 | GGGTCGACTCAAACGGACTGCGCCAAACTTA |
| yxgB1 | CAATTTACTTAAAAGACACAAGCC |
| lytF1 | CGAAATCATTGTGTTAATCTTCGC |
| hmpRTfw | CCACAAGCACCGCAGTATC |
| hmpRTrev | GCTTGCTCTTCAGCCTGTTC |
| nasDRTfw | TGACTTTGTGAGGCGTGTTC |
| nasDRTrev | TTGATATGCCATCGGGCTAC |
DNA techniques.
DNA manipulations and E. coli transformations were carried out as described by Sambrook et al. (32). Isolation of B. subtilis chromosomal DNA and transformation of B. subtilis strains with chromosomal or plasmid DNA were performed as described by Hoch (13). DNA sequencing was carried out on isolated plasmid DNA or PCR products using the BigDye terminator v3.1 cycle sequencing reaction kit (Applied Biosystems) and an ABI Prism 3100 DNA sequencer (PE Biosystems).
Construction of mutants.
The template used for the PCRs described below was chromosomal DNA isolated from B. subtilis strain 1A1. The yjbH mutant was constructed as follows. A fragment containing yjbIH and the region downstream of yjbH was amplified by PCR using primer pair yjbI1/yjbI9 (Table 2) and inserted into pCR-Blunt II-TOPO. The recombinant plasmid was transformed into E. coli. The resulting plasmid, pTOPOYjbIH, was isolated, cut with NruI and BglII and ligated to a 1,484 bp NruI/BamHI fragment from pDG1727 containing a spectinomycin resistance gene to give pΔyjbH1. This plasmid was used to transform B. subtilis 1A1 to spectinomycin resistance. Transformants were screened by PCR amplification to identify recombinants, which carried a deletion of the yjbH gene arising from a double-crossover event (data not shown). One obtained recombinant (LUW272) was chosen for further analysis. The yjbI and yjbH genes were inactivated as follows. To create an insertion in yjbI and a conditional yjbH mutation, a 270-bp HindIII-EcoRI fragment containing an internal part of yjbI was produced by PCR using the primer pair yjbI4/yjbI5 (Table 2) and ligated with the HindIII-EcoRI-digested integration vector pMUTIN2mcs, generating plasmid pYjbI2. Upon transformation into B. subtilis 1A1 and integration into the chromosome by recombination, pYjbI2 disrupts the yjbI gene and places yjbH under the control of the isopropyl-d-thiogalactopyranoside (IPTG)-inducible promoter Pspac. At the same time, a transcriptional fusion between the natural yjbI promoter and the lacZ gene is created. The resulting strain was designated LUW222. An nsrR (yhdE) mutant was constructed as follows. A fragment containing nsrR and part of the upstream (ygxB) and the downstream (lytF) genes was amplified by PCR using primer pair yxgB1/lytF1 (Table 2) and inserted into pCR-Blunt II-TOPO. The recombinant plasmid was transformed into E. coli. The resulting plasmid was isolated, cut with HpaI and SspI, and ligated to a 1,498-bp PvuII fragment from pDG1726 containing a spectinomycin resistance gene to give pΔnsrR1. This plasmid was used to transform B. subtilis 1A1 to spectinomycin resistance. Transformants were screened by PCR amplification to identify recombinants, which carried a deletion of the nsrR gene arising from a double-crossover event (data not shown). One obtained recombinant (LUW340) was chosen for further analysis.
Construction of plasmids.
Plasmid pCW7 is a low-copy-number vector for overproduction of proteins in B. subtilis. It was derived from pHP13 and pDG148. It carries the pTA1060 origin of replication (37), the pTA1060 rep60 gene, the cat gene from pHP13, and the spac-I promoter and the lacI gene from pDG148. Details of the construction will be described elsewhere. Plasmids for autologous expression of yjbI and yjbH were derived from pCW7. Plasmid pCW7-yjbIH was constructed by amplifying yjbIH by using primers yjbI1 and yjbI9 (Table 2). The PCR product was cloned into pCW7 at XbaI and SalI restriction sites. Plasmid pCW7-yjbH was constructed by amplifying yjbH by using primers yjbH2 and yjbH4 (Table 2). In the primary annotation of the yjbH region, no apparent ribosome-binding site precedes the ATG start codon (16). By examination of the sequence upstream of the suggested translational start site of yjbH, the choice of start codon was refined. An alternative start codon (TTG) present upstream of the ATG codon is preceded by a putative ribosome-binding site (GGAGG). This alternative start codon would specify YjbH containing an additional 24 amino acid residues compared to that previously predicted. The yjbI translation termination codon overlaps the putative yjbH translation initiation codon by two bases. Such an overlap can lead to translational coupling. The alternative start codon was used in the yjbH expression plasmid. The yjbH PCR product was cut with XbaI and EcoRI and cloned into pCW7-yjbIH that had been cut with the same restriction enzymes. Plasmid pCW7-yjbI was constructed by deletion of a large part (a 753-bp SphI fragment) of yjbH in plasmid pCW7-yjbIH. Plasmid pDG148-hmp was constructed using primers hmp1 and hmp2 (Table 2). The amplified DNA fragment was cut with XbaI and SalI and cloned into pDG148 that had been cut with the same restriction enzymes. The template used for all the PCRs described above was chromosomal DNA isolated from B. subtilis strain 1A1.
Microarray analysis.
The procedures used for microarray analysis, cDNA labeling, slide hybridization, data collection, and normalization have been described previously (18). Growth was performed as described above, and in exponential phase (OD600 = 0.6), SNP was added to a final concentration of 0.5 mM. RNA isolated from control cells and SNP-treated cells was used to prepare cDNA labeled with Cy3-dCTP and Cy5-dCTP, respectively.
RNA isolation and cDNA synthesis for real-time PCR and RT-PCR.
Growth was performed as described above, and total RNA was isolated as described previously (18) and was purified further using the QIAGEN RNeasy Mini Protocol for RNA Cleanup with on-column DNase digestion with the QIAGEN RNase-free DNase set. RNA was quantified spectrophotometrically. Two micrograms of total RNA was used for cDNA synthesis with random hexamer primers and Superscript III RT from Invitrogen according to the manufacturer's protocol. A control without RT was also prepared to make sure that no chromosomal DNA contamination gave false-positive results in the PCR analyses.
RT-PCR.
Growth, RNA isolation, and cDNA synthesis were performed as described above. PCR was performed using three different primer pairs: yjbI4/yjbI5, yjb16/yjb17, and yjbI4/yjb17 (Table 2). Each primer pair was used with three different templates: B. subtilis 1A1 chromosomal DNA, 1A1 cDNA prepared as described above, and control without RT. As an additional negative control, a fourth reaction was performed without template.
Real-time PCR.
RNA isolation and cDNA synthesis were performed as described above. cDNA and no-RT controls were diluted fivefold in 10 mM Tris-HCl (pH 8.0) before use in real-time PCR analysis. Primers used for real-time PCR (hmpRTfw, hmpRTrev, nasDRTfw, and nasDRTrev) are listed in Table 2. Real-time PCR was performed in a Rotor-Gene 2072 real-time cycler (Corbett Research). Each 20-μl reaction mixture contained 2.75 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 1:20,000 (vol/vol) SYBR green (Sigma), 1 unit Platinum Taq polymerase (Invitrogen), 1× Platinum Taq PCR buffer, 250 nM of each primer, and 2 μl of the fivefold-diluted cDNA or no-RT control. To controls without template, 2 μl 10 mM Tris-HCl (pH 8.0) was added. A minimum of two reactions were performed for each biological replicate. Three biological replicates per strain were run with each primer pair. The relative transcript abundance was determined using the comparative quantitation feature of the software Rotor-Gene version 4.6, where the take-off point of each individual reaction is calculated from the second derivative of the raw data. This take-off point is then used, together with the reaction efficiency, to calculate the relative concentration of the samples compared to a selected control sample, which is set to 1. For each primer pair, one cDNA preparation from 1A1 cells not treated with SNP was chosen as the default control sample. This sample was included in all runs. The control sample was, just like the other samples, always run in duplicate, and the lowest value of the two was set to 1. For graphs, all data were normalized so that the average of all 1A1 cDNA preparations from cells not subjected to SNP treatment was set to 1. A two-way Student t test assuming equal variances was used to compare data.
Determination of cell survival after exposure to SNP.
B. subtilis strains were grown to exponential phase (OD600 = 0.6), at which point a sublethal amount of SNP (0.5 mM) was added. Control cultures were left untreated. Growth was continued for 30 min, after which two 1 ml-samples of the culture were withdrawn and treated with a toxic amount of SNP (10 mM) or a corresponding volume of water for 30 min at room temperature (20 to 25°C). Viable counts were performed, and survival rates were calculated as the CFU after treatment with a toxic dose of SNP divided by the CFU in the same culture treated with water. Each assay was repeated at least three times.
Miscellaneous methods.
Protein concentrations were estimated using the bicinchoninic acid method (Pierce) with bovine serum albumin as the standard. Polyclonal antisera against YjbI and YjbH were obtained by immunizing rabbits with purified YjbI and YjbH produced in E. coli. For immunoblot analysis, cell extracts were incubated for 10 min at 95°C in the presence of 0.4% (wt/vol) sodium dodecyl sulfate, 2.9 mM 2-mercaptoethanol, 5% (vol/vol) glycerol, and 12 mM Tris buffer (pH 6.8), and the proteins were then fractionated by Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to Hybond P membranes (GE Healthcare). Immunodetection was carried out by chemiluminescence using the ECL system (Pierce; Super Signal). Primary antisera were used at a 1,000-fold dilution, and secondary antibodies (donkey anti-rabbit immunoglobulin G conjugated to horseradish peroxidase [Amersham Biosciences]) were used at a 5,000-fold dilution.
RESULTS AND DISCUSSION
Bacillus subtilis exhibits an adaptive response to SNP.
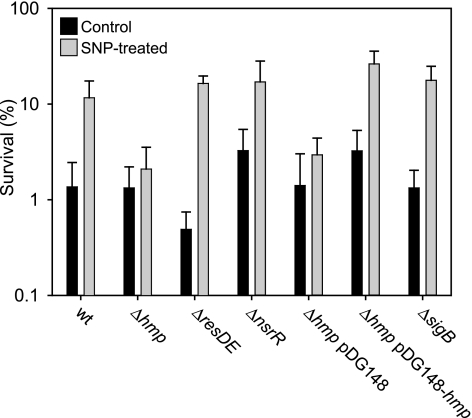
To investigate how B. subtilis responds to nitrosative stress we exposed cells to the nitrosating agent SNP (Na2[Fe(CN)5(NO+)]). When wild-type B. subtilis cells were exposed to a high dose of SNP (10 mM), only 1% were found to survive. However, if the cells were first induced with a sublethal dose of SNP (0.5 mM), their ability to survive the subsequent challenge with a high dose of SNP increased 10-fold (Fig. 1), showing that there is an adaptive response to SNP in B. subtilis. Fluorescence microscopic visualization by live-dead staining (LIVE/DEAD BacLight bacterial viability kit; Molecular Probes) gave essentially the same result (data not shown).
FIG. 1.
Analysis of the adaptive response to SNP in B. subtilis. Cultures were grown aerobically and treated with a sublethal concentration (0.5 mM) of SNP or left untreated (for details, see Materials and Methods). The cultures were then challenged with 10 mM SNP for 30 min, and dilutions were plated on TBAB medium. Viable counts were performed, and survival rates were calculated as the CFU after treatment with a toxic dose of SNP divided by the CFU in the same culture treated with water. Strains 1A1 (wild type [wt]), LUW260 (Δhmp), LUW264 (ΔresDE), LUW340 (ΔnsrR), LUW260/pDG148, LUW260/pDG148-hmp, and LUW229 (ΔsigB) were used. The results shown are the means from at least three independent experiments. Error bars indicate the standard deviation.
To exclude the possibility that cyanide liberated from SNP is responsible for the growth inhibition, B. subtilis cells were exposed to 10 mM K3[Fe(CN)6]. The survival of cells treated with K3[Fe(CN)6] was not affected relative to that of control cultures (data not shown).
Transcriptome analysis of the response to SNP.
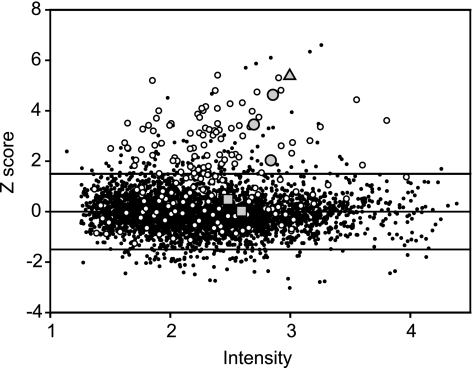
To investigate the cellular response to SNP at the transcriptional level, B. subtilis cells were grown aerobically to exponential phase and were then left untreated or exposed to 0.5 mM SNP. This amount of SNP did not affect growth. Cells were harvested 5 min after SNP addition to capture the earliest transcriptional responses elicited by the nitrosative stress. The results obtained from five pairs of cultures (control and SNP treated) showed that 181 genes were significantly up-regulated and that 89 were repressed in cells exposed to SNP (Fig. 2). Primarily, SNP affected the transcription of genes assigned to the following categories, based on annotations in the Comprehensive Microbial Resource database (29): transport and binding proteins, energy metabolism, amino acid biosynthesis, and protein synthesis. This reflects the wide variety of targets for stress elicited by SNP. Of the SNP-induced genes, more than half could be assigned to the σB-controlled general stress regulon (Fig. 2; see Fig. S1A in the supplemental material). Genes belonging to the ResDE (42) and Rex (18) regulons were also up-regulated in response to SNP (see Fig. S1B in the supplemental material). The ResDE and the Rex regulons are induced by decreases in oxygen availability or changes in the redox status of the cells. NO is known to rapidly inhibit respiration by binding to heme-copper terminal oxidases, leading to changes in redox balance (20).
FIG. 2.
Nitrosative stress-regulated transcription in B. subtilis. Changes in gene expression levels as determined by microarray analysis of mRNA levels in exponentially aerobically growing 1A1 (wild-type) cells treated with 0.5 mM SNP for 5 min are shown. The signal intensity represents the base 10 logarithm of the square root of the product of Cy3 and Cy5. The intensity-dependent Z score measures the number of standard deviations by which a particular data point differs from the local mean. The horizontal lines mark the Z-score threshold value (1.5 or −1.5) that is considered significant. Data points above or below these lines represent genes that are significantly up- or down-regulated, respectively, in SNP-treated cells. Open circles, transcripts reported to depend on σB; gray circles outlined in black, nasDEF; triangle, hmp; squares, yjbIH. For the transcriptome data set, see supplemental data 1 in the supplemental material.
The adaptive response to SNP requires the flavohemoglobin encoded by hmp.
In our microarray data set, hmp, encoding a flavohemoglobin, is among the most highly up-regulated (∼100-fold) genes in response to SNP (Fig. 2). The up-regulation of hmp expression in response to SNP treatment was confirmed using quantitative real-time PCR (qRT-PCR), showing an approximately 50-fold increase in the amount of hmp transcript after SNP induction (Fig. 3A). Flavohemoglobins have been implicated in NO detoxification and protection against nitrosative stress in several bacteria and unicellular fungi (8, 41). To investigate the possible role of Hmp in the adaptive response to SNP, an hmp null mutant was analyzed. In the mutant, the adaptive response to SNP was completely abolished: no difference in survival between induced and uninduced cells was observed (Fig. 1).
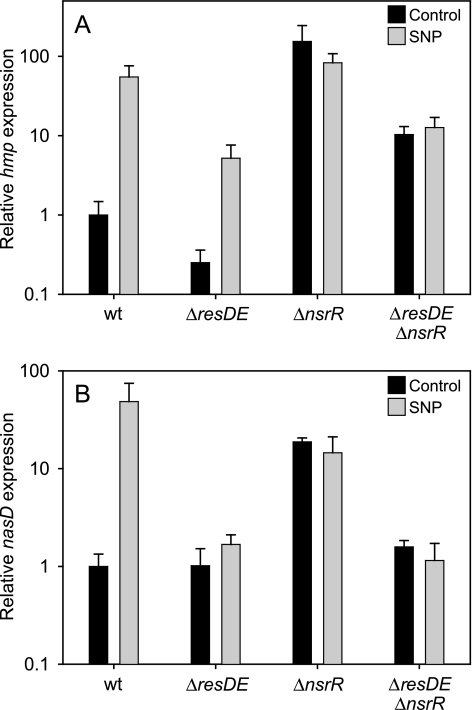
FIG. 3.
Changes in hmp (A) and nasD (B) expression in response to SNP. cDNA was prepared from cultures grown aerobically and treated with 0.5 mM SNP or grown without SNP treatment, and the relative hmp and nasD expression was determined using qRT-PCR. Strains 1A1 (wild type [wt]), LUW264 (ΔresDE), LUW340 (ΔnsrR), and LUW365 (ΔnsrR ΔresDE) were used.
NsrR (YhdE) regulates hmp and nasD in response to nitrite and nitrosative stress.
Induction of hmp has been suggested to require the two-component system ResDE (23), and several members of the ResDE regulon were induced by SNP (see Fig. S1B in the supplemental material). However, in a resDE null mutant, no decrease in induced survival was observed (Fig. 1). A likely explanation for this was provided by microarray analysis (see supplemental data 3 in the supplemental material) and qRT-PCR experiments, which showed that hmp is up-regulated in the resDE mutant after SNP induction, although to a lesser extent than in the wild type (20-fold in the resDE mutant, compared to 50-fold in the wild type as determined using qRT-PCR) (Fig. 3A). Apparently, the induced hmp expression observed in the resDE mutant was sufficient to develop an adaptive resistance to SNP under the conditions tested.
Recent studies show that NsrR, a member of the Rrf2 family of transcription factors, acts as a regulator in response to nitrosative stress in E. coli and Nitrosomonas europaea (1, 2). Putative NsrR-binding sites are present in front of two genes in the B. subtilis genome: hmp and nasD. The nasDEF operon encodes an NADH-dependent nitrite reductase (NasDE) that catalyzes the reduction of nitrite to ammonium and NasF, an enzyme involved in the synthesis of siroheme, a cofactor of NasDE (25). The nasDEF operon is a member of the ResDE regulon (42). In our microarray data set, nasD, nasE, and nasF are highly up-regulated in response to SNP (50-, 20-, and 6-fold, respectively) (Fig. 2; see supplemental data 1 in the supplemental material). This up-regulation was confirmed for the first gene in the operon, nasD, using qRT-PCR, showing an approximately 50-fold increase in the amount of nasD transcript after SNP induction (Fig. 3B). No significant up-regulation of nasD in response to SNP was seen in a resDE mutant when analyzed by microarray analysis (see supplemental data 3 in the supplemental material) and qRT-PCR (Fig. 3B). In an nsrR (previously yhdE) null mutant strain, hmp and nasD transcript levels similar to those present after SNP induction in wild-type cells were observed both before and after SNP induction (Fig. 3A and B), indicating that NsrR works as a repressor of these genes. In an nsrR resDE double mutant, hmp transcription levels before and after SNP induction were similar to those of the resDE mutant after SNP induction (Fig. 3A), while nasD expression stayed at the same level as before SNP induction in the wild type and as both before and after SNP induction in the resDE mutant (Fig. 3B). These results suggest that ResDE is essential for the up-regulation of nasD but not hmp expression, while NsrR acts as a repressor of both hmp and nasD in the absence of nitrosative stress. Thus, even though hmp and nasDEF are controlled by the same regulators, hmp, unlike nasDEF, can be expressed under conditions that do not activate ResD.
During the course of this work, Nakano et al. identified B. subtilis NsrR as a negative regulator of hmp and nasDEF by using lacZ fusion experiments (24). However, in contradiction to our data, it was suggested that an nsrR mutation does not cause derepression of nasD under aerobic conditions.
Expression of hmp is not enough to yield an increased resistance to SNP.
The deregulated expression of hmp in the nsrR mutant resulted in a small increase in SNP tolerance under noninducing conditions (Fig. 1). When the nsrR mutant was induced with a sublethal concentration of SNP, the cells exhibited a more than 10-fold-higher survival (Fig. 1). These results were confirmed when the hmp null mutant was complemented with a plasmid bearing the hmp gene under the control of the IPTG-inducible Pspac promoter. Expression of hmp from the plasmid resulted in a marginal increase in cell survival under noninducing conditions compared with that of the isogenic strain carrying only the vector without the hmp insert. When the complemented hmp mutant was first induced with a sublethal level of SNP, a more than 10-fold-higher survival was observed (Fig. 1). This indicates that Hmp alone is not sufficient to provide a large increase in resistance to SNP, but an additional factor, induced by SNP, needs to be present. Alternatively, in the absence of nitrosative stress, Hmp is inactive due to superoxide-generated self-destruction. It has been shown that E. coli Hmp is irreversibly inactivated in the presence of O2 and NADH due to superoxide formed during turnover. However, in the presence of NO, superoxide production is abolished (40). Thus, we speculate that a small amount of NO induces hmp expression and protects the flavohemoglobin from self-destruction.
Nitrite induces hmp and nasD expression.
Nakano et al. (24) suggested that it is NO and not nitrite that is sensed by NsrR, and this has also been implied for NsrR in Neisseria gonorrhoeae (28). However, in N. europaea (1, 2), it has been suggested that nitrite, not NO, controls the activity of NsrR. In our experiments, induction with nitrite yielded hmp and nasD expression at approximately the same level as after SNP induction. However, the concentration of nitrite required to reach this expression level was 40-fold higher than the SNP concentration used (data not shown). Control experiments with 20 mM NaNO3 gave no induction of hmp or nasD (data not shown). Nitrite is a source of NO in vivo, and it is possible that the small amount of NO formed from nitrite interacts with NsrR to cause derepression of hmp and nasD. However, treatment with 20 mM nitrite did not give an increased resistance to SNP (data not shown). We speculate that the amount of NO needed to activate NsrR is smaller than that required to protect Hmp from superoxide-generated self-destruction.
The yjbIH operon confers an apparent constitutive protection against SNP.
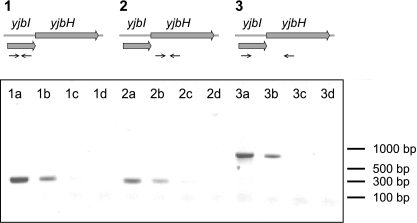
trHbs have been reported to protect bacterial cells from nitrosative stress. For example, in M. tuberculosis a trHb (trHbN) catalyzes the oxidation of NO into nitrate (27). B. subtilis possesses a trHb encoded by the yjbI gene. The chromosomal context suggests that the yjbI gene is part of a yjbIH operon. To verify cotranscription of yjbI and yjbH, total RNA was isolated and used to perform RT-PCR with a pair of primers that allows coamplification of both genes. A single product of the expected size was detected, indicating that yjbI and yjbH are cotranscribed in vivo (Fig. 4). To investigate the roles of yjbI and yjbH in nitrosative stress, we compared SNP resistance in the wild type and in yjbI, yjbH, and yjbIH mutant strains. When the B. subtilis cells lacking yjbI, yjbH, or both yjbI and yjbH were exposed to a toxic dose of SNP, an approximately 100-fold decrease in cell survival compared to that of the wild-type strain was observed (Fig. 5). Expression of yjbIH from a low-copy-number plasmid in cells lacking yjbIH resulted in wild-type survival levels (Fig. 5). In contrast, expression of only yjbI in cells lacking yjbIH did not restore the survival levels (Fig. 5).
FIG. 4.
Organization of the yjbIH locus in Bacillus subtilis. RT-PCR analysis of yjbI and yjbH is shown Amplification reactions were carried out using pairs of oligonucleotide primers as schematically illustrated by arrows below the map of the yjbIH locus. The DNA products were analyzed by agarose gel electrophoresis. For the samples loaded in lanes 1a, 2a, and 3a, chromosomal DNA was used as the template; in lanes 1b, 2b, and 3b, cDNA was used as the template; in lanes 1c, 2c, and 3c, RNA was used as the template (RT was absent in the cDNA synthesis reaction); and in lanes 1d, 2d, and 3d, no template was present.
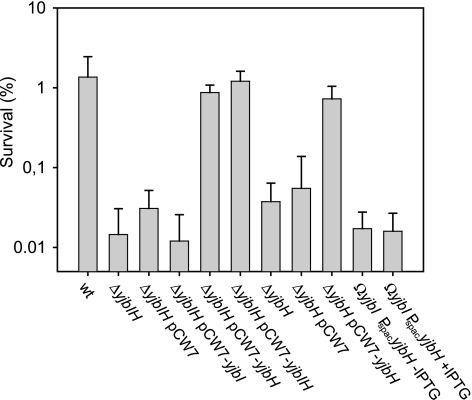
FIG. 5.
Effects of nitrosative stress on the viability of different B. subtilis strains. Aerobically growing cultures were challenged with 10 mM SNP for 30 min and then plated on TBAB medium. CFU are plotted as a percentage of the values for untreated cultures. The results are the means from at least three independent experiments. Error bars indicate the standard deviation. Strains 1A1 (wild type [wt]), LUW219 (ΔyjbIH), LUW219/pCW7, LUW219/pCW7-yjbI, LUW219/pCW7-yjbH, LUW219/pCW7-yjbIH, LUW272 (ΔyjbH), LUW272/pCW7, LUW272/pCW7-yjbH, LUW222 (yjbI insertion) without IPTG, and LUW222 with IPTG were used.
As expected, expression of yjbH from a low-copy-number plasmid in cells lacking yjbH resulted in wild-type survival levels (Fig. 5). However, expression of only yjbH from a plasmid in cells lacking yjbIH also resulted in wild-type survival levels (Fig. 5). This finding is contradicted by data obtained with the yjbI insertion mutant LUW222, where the yjbI gene was inactivated by insertion of the suicide plasmid pYjbI2. pYjbI2 disrupts the yjbI gene and places yjbH under the control of the IPTG-inducible Pspac promoter. When the yjbI mutant LUW222 was grown in the presence of 1 mM IPTG, survival levels were very similar to those observed in the absence of IPTG, suggesting that the tolerance to SNP could not be restored by expression of yjbH (Fig. 5).
Immunoblot analysis confirmed that YjbH antigen is present in cell extracts of LUW222 grown in the presence of IPTG (data not shown). However, the level of YjbH antigen in cells complemented with yjbH on a plasmid was at least 20-fold higher. Thus, complementation in LUW219/pCW7-yjbH could be due to the nonphysiological concentration of YjbH.
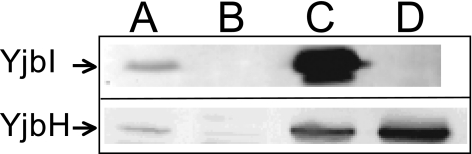
The steady-state levels of YjbI and YjbH in strains containing yjbIH or yjbI on a plasmid as determined using immunoblotting show that the levels of YjbI and YjbH increased more than 10-fold compared to those in the wild type (Fig. 6). The increased levels of YjbI and YjbH do not appear to promote tolerance to SNP above the levels observed for the wild-type cells, indicating that the amount of these proteins is not limiting in wild-type cells or that they are not directly involved in the SNP detoxification mechanism.
FIG. 6.
Overproduction of YjbI and YjbH in B. subtilis cells. Immunoblot analysis of cell extracts (10 μg total protein loaded in each lane) from different B. subtilis strains, using YjbI or YjbH antiserum, is shown. The blots were overexposed to demonstrate the presence of small amounts of YjbI and YjbH antigen. Lanes: A, 1A1/pCW7; B, LUW219 (ΔyjbIH)/pCW7; C, LUW219/pCW7-yjbIH; D, LUW219/pCW7-yjbH.
Deletion of yjbI and/or yjbH did not affect the adaptive response to SNP (Fig. 7), and the expression levels of yjbI, measured with microarray analysis (Fig. 2) and qRT-PCR (data not shown), remained constant after SNP induction. The expression of yjbI was not affected by mutations in nsrR or resDE (data not shown).
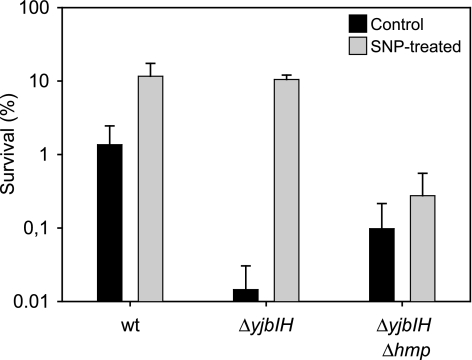
FIG. 7.
The induced resistance to SNP is not affected by YjbI or YjbH. Cultures were grown aerobically and treated with a sublethal concentration (0.5 mM) of SNP or left untreated. The cultures were then challenged with 10 mM SNP for 30 min and plated on TBAB medium. Viable counts were performed, and survival rates were calculated as the CFU after treatment with a toxic dose of SNP divided by the CFU in the same culture treated with water. The results shown are the means from at least three independent experiments. Error bars indicate the standard deviation. Strains 1A1 (wild type [wt]), LUW219 (ΔyjbIH), and LUW261 (ΔyjbIH Δhmp) were used.
Deletion of hmp in the yjbIH mutant significantly reduced the induced tolerance to SNP (Fig. 7). Taken together, the results indicate that deletion of either yjbI or yjbH affects the uninduced tolerance to SNP. We have previously shown that a yjbIH mutation affects growth and oxygen consumption (18). It is possible that the deletion of yjbI or yjbH affects the cells in a more global way that makes them hypersensitive to nitrosative stress.
The sigB general stress response is not required for the adaptive response to SNP.
To further examine the induction of the general stress response by SNP, a transcriptome analysis of the response to SNP in a sigB null mutant was performed using four pairs of cultures. As expected, genes belonging to the sigB regulon that were induced by SNP in the wild type were not induced in the sigB mutant. However, interestingly, a group of genes previously reported to be sigB dependent (yvyD, yxjG, ywfH, ywhH, ynfC, and gabP) (31) were up-regulated also in the sigB mutant (see Fig. S1C in the supplemental material). These genes may have been incorrectly annotated to the sigB regulon, or they may have additional mechanisms for induction as indicated by the presence of sequences that are similar to those found at the −10 and −35 regions of sigma A promoters upstream of yxjG, ywfH, and ynfC. Another group of genes (yonA, yjlB, yisZ, yufS, yddC, and ytkC) that were previously not assigned to the sigB regulon were affected by the sigB mutation (see Fig. S1A in the supplemental material). These genes are, at least under the conditions tested, either directly or indirectly dependent on sigB for induction by SNP.
To test whether the σB regulon affects the sensitivity of B. subtilis to nitrosative stress, the sigB null mutant was exposed to SNP. The sigB mutant showed no increased sensitivity to SNP, nor did the mutation affect the induced resistance (Fig. 1), suggesting that the proteins encoded by the σB-dependent genes are involved in a nonspecific protection against stress. To exclude the possibility that Hmp might have masked any effect of the sigB mutation, a sigB hmp double mutant was tested for SNP tolerance. No difference in survival between the sigB hmp mutant and the hmp mutant was observed (data not shown).
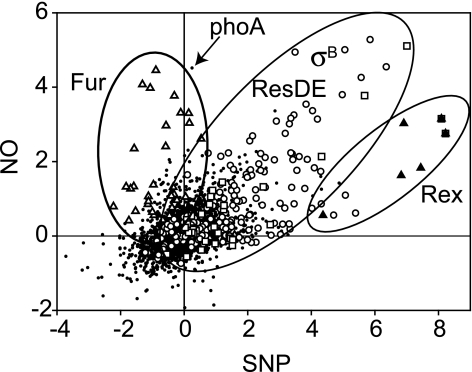
Comparison of the SNP and NO stimulons in B. subtilis.
Recently, the genome-wide response of B. subtilis to NO gas under aerobic growth conditions was reported (21). NO activates the sigB stress response via the energy-signaling pathway, in contrast to SNP, which activates it via the environmental pathway, underscoring the different actions of NO and SNP (21). To analyze the spectrum of responses of B. subtilis to different types of nitrosative stress under aerobic conditions, we compared the SNP and NO stimulons in a scatter plot (Fig. 8). Most genes showed similar expression patterns in response to SNP and NO. However, a notable difference is the apparent lack of derepression by SNP of genes controlled by the ferric uptake regulator (Fur). This could reflect the chemical differences between SNP and NO. The former is a nitrosonium donor that efficiently could form S-nitrosothiol by covalent addition to cysteine residues. The S-N bonds could subsequently be cleaved, resulting in release of NO and the formation of S-S bonds. Inactivation of E. coli Fur by NO has been shown to involve the formation of a dinitrosyl-iron complex (5). The apparent lack of inactivation of B. subtilis Fur by SNP could reflect kinetic differences, could be due to differences in the intracellular concentration of NO, or could depend on the difference in medium composition between the two experiments. For the SNP analysis, growth was performed in an iron- and phosphate-rich medium. This is also the likely reason why phoA is highly induced by NO but not by SNP, since phosphate causes repression of phoA expression (Fig. 8). Similar differences have been seen in the response of the E. coli transcriptome to various sources of NO or NO+ (7, 15, 22).
FIG. 8.
Graphical comparison of the SNP and NO stimulons of B. subtilis. The fold changes (log2) of gene expression levels of the two data sets are plotted. The data for the NO stimulon are those reported for B. subtilis cells treated with 50 μM NO for 5 min (21). The data for the SNP stimulon were obtained in this work (Fig. 2). Members of the Fur (open triangles), σB (open circles), ResDE (open squares), and Rex (closed triangles) regulons are highlighted. The signal corresponding to the phoA transcript is indicated with an arrow.
Conclusions.
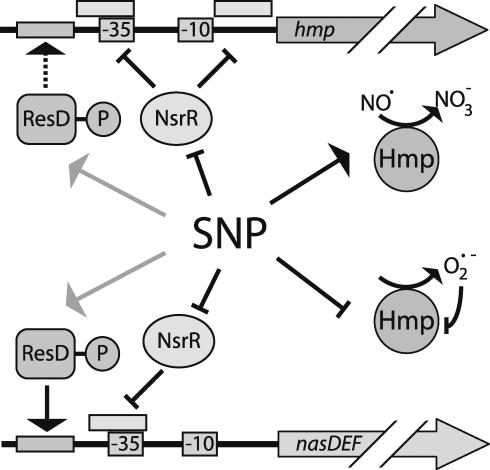
In this work we demonstrated that the flavohemoglobin encoded by the hmp gene has a vital role in an adaptive response to protect B. subtilis from nitrosative stress. Expression of hmp is regulated by the transcriptional repressor NsrR (Fig. 9). Upon exposure to SNP, it is likely that nitrosylation of NsrR results in derepression of hmp. NsrR also regulates expression of the nitrite reductase encoded by the nasDE genes. The fact that the protonated form of nitrite, nitrous acid, can spontaneously yield N2O3 (19), a powerful nitrosating agent, provides a rationale for the coregulation of hmp and nasDEF by the transcriptional repressor NsrR and the activator ResD. We also investigated the role of the trHb YjbI in RNS detoxification. Several studies have indicated a role for trHbs in the detoxification of RNS. We show that the yjbI gene is cotranscribed with yjbH, a gene that encodes a 34-kDa cytosolic protein belonging to COG2761 in the database of clusters of orthologous groups of proteins (COGs). This COG contains DsbA-like proteins that may be involved in disulfide bond formation or other redox-based processes. Expression of yjbIH is independent of NsrR and SNP. Cells lacking yjbIH are hypersensitive to nitrosative stress, possibly due to secondary effects caused by the lack of YjbIH. As in previous studies, we found that nitrosative stress induces the sigma B-controlled general stress regulon. However, a sigB null mutant did not show increased sensitivity to SNP, suggesting that the sigma B-dependent stress proteins are involved in a nonspecific protection against stress whereas Hmp is crucial for SNP detoxification.
FIG. 9.
Model of hmp and nasDEF regulation in response to nitrosative stress. During nitrosative stress inflicted by SNP, nitrosylation of NsrR results in derepression of hmp and nasDEF (consistent with the model previously suggested by Nakano et al. [24]). Under oxic conditions Hmp detoxifies NO to NO3−. However, when Hmp is present in the absence of NO, superoxide that inactivates the enzyme is produced. ResD, presumably in its phosphorylated form, is required for expression of nasDEF and enhances transcription from the hmp promoter (indicated by the dashed arrow). The mechanism of activation of ResD by SNP is not known (indicated by the gray arrows).
Supplementary Material
Acknowledgments
We thank M. Nakano for the gift of B. subtilis BSM29, X.-D. Su for the YjbH antiserum, and K. Flärdh and L. Hederstedt for critical comments on the manuscript.
This work was supported by grants from Carl Tryggers Stiftelse and Crafoordska Stiftelsen. P.K. was supported by a fellowship from the Research School of Pharmaceutical Sciences (FLÄK).
Footnotes
Published ahead of print on 9 February 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Beaumont, H. J., S. I. Lens, W. N. Reijnders, H. V. Westerhoff, and R. J. van Spanning. 2004. Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol. Microbiol. 54:148-158. [DOI] [PubMed] [Google Scholar]
- 2.Bodenmiller, D. M., and S. Spiro. 2006. The yjeB (nsrR) gene of Escherichia coli encodes a nitric oxide-sensitive transcriptional regulator. J. Bacteriol. 188:874-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, P. S., Z. Naal, C. Moore, E. Casado-Rivera, H. D. Abruna, J. D. Helmann, and J. P. Shapleigh. 2006. Assessing the impact of denitrifier-produced nitric oxide on other bacteria. Appl. Environ. Microbiol. 72:2200-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corker, H., and R. K. Poole. 2003. Nitric oxide formation by Escherichia coli. Dependence on nitrite reductase, the NO-sensing regulator Fnr, and flavohemoglobin Hmp. J. Biol. Chem. 278:31584-31592. [DOI] [PubMed] [Google Scholar]
- 5.D'Autreaux, B., D. Touati, B. Bersch, J. M. Latour, and I. Michaud-Soret. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl. Acad. Sci. USA 99:16619-16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang, F. C. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820-832. [DOI] [PubMed] [Google Scholar]
- 7.Flatley, J., J. Barrett, S. T. Pullan, M. N. Hughes, J. Green, and R. K. Poole. 2005. Transcriptional responses of Escherichia coli to S-nitrosoglutathione under defined chemostat conditions reveal major changes in methionine biosynthesis. J. Biol. Chem. 280:10065-10072. [DOI] [PubMed] [Google Scholar]
- 8.Gardner, P. R., A. M. Gardner, W. T. Brashear, T. Suzuki, A. N. Hvitved, K. D. Setchell, and J. S. Olson. 2006. Hemoglobins dioxygenate nitric oxide with high fidelity. J. Inorg. Biochem. 100:542-550. [DOI] [PubMed] [Google Scholar]
- 9.Gardner, P. R., A. M. Gardner, L. A. Martin, and A. L. Salzman. 1998. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. USA 95:10378-10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giangiacomo, L., A. Ilari, A. Boffi, V. Morea, and E. Chiancone. 2005. The truncated oxygen-avid hemoglobin from Bacillus subtilis: X-ray structure and ligand binding properties. J. Biol. Chem. 280:9192-9202. [DOI] [PubMed] [Google Scholar]
- 11.Guerout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57-61. [DOI] [PubMed] [Google Scholar]
- 12.Haima, P., S. Bron, and G. Venema. 1987. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol. Gen. Genet. 209:335-342. [DOI] [PubMed] [Google Scholar]
- 13.Hoch, J. A. 1991. Genetic analysis in Bacillus subtilis. Methods Enzymol. 204:305-320. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, M. N. 1999. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim. Biophys. Acta 1411:263-272. [DOI] [PubMed] [Google Scholar]
- 15.Justino, M. C., J. B. Vicente, M. Teixeira, and L. M. Saraiva. 2005. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem. 280:2636-2643. [DOI] [PubMed] [Google Scholar]
- 16.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 17.Lacelle, M., M. Kumano, K. Kurita, K. Yamane, P. Zuber, and M. Nakano. 1996. Oxygen-controlled regulation of the flavohemoglobin gene in Bacillus subtilis. J. Bacteriol. 178:3803-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson, J. T., A. Rogstam, and C. von Wachenfeldt. 2005. Coordinated patterns of cytochrome bd and lactate dehydrogenase expression in Bacillus subtilis. Microbiology 151:3323-3335. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg, J. O., E. Weitzberg, J. A. Cole, and N. Benjamin. 2004. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2:593-602. [DOI] [PubMed] [Google Scholar]
- 20.Mason, M. G., P. Nicholls, M. T. Wilson, and C. E. Cooper. 2006. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 103:708-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore, C. M., M. M. Nakano, T. Wang, R. W. Ye, and J. D. Helmann. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J. Bacteriol. 186:4655-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay, P., M. Zheng, L. A. Bedzyk, R. A. LaRossa, and G. Storz. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. USA 101:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano, M. M. 2002. Induction of ResDE-dependent gene expression in Bacillus subtilis in response to nitric oxide and nitrosative stress. J. Bacteriol. 184:1783-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano, M. M., H. Geng, S. Nakano, and K. Kobayashi. 2006. The nitric oxide-responsive regulator NsrR controls ResDE-dependent gene expression. J. Bacteriol. 188:5878-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano, M. M., and P. Zuber. 1998. Anaerobic growth of a “strict aerobe” (Bacillus subtilis). Annu. Rev. Microbiol. 52:165-190. [DOI] [PubMed] [Google Scholar]
- 26.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouellet, H., Y. Ouellet, C. Richard, M. Labarre, B. Wittenberg, J. Wittenberg, and M. Guertin. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. USA 99:5902-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overton, T. W., R. Whitehead, Y. Li, L. A. Snyder, N. J. Saunders, H. Smith, and J. A. Cole. 2006. Coordinated regulation of the Neisseria gonorrhoeae truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J. Biol. Chem. 281:33115-33126. [DOI] [PubMed] [Google Scholar]
- 29.Peterson, J. D., L. A. Umayam, T. Dickinson, E. K. Hickey, and O. White. 2001. The comprehensive microbial resource. Nucleic Acids Res. 29:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole, R. K. 2005. Nitric oxide and nitrosative stress tolerance in bacteria. Biochem. Soc. Trans. 33:176-180. [DOI] [PubMed] [Google Scholar]
- 31.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Spiro, S. 2006. Nitric oxide-sensing mechanisms in Escherichia coli. Biochem. Soc. Trans. 34:200-202. [DOI] [PubMed] [Google Scholar]
- 34.Stamler, J. S., D. J. Singel, and J. Loscalzo. 1992. Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898-1902. [DOI] [PubMed] [Google Scholar]
- 35.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697-704. [DOI] [PubMed] [Google Scholar]
- 36.Sun, G., E. Sharkova, R. Chesnut, S. Birkey, M. F. Duggan, A. Sorokin, P. Pujic, S. D. Ehrlich, and F. M. Hulett. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uozumi, T., A. Ozaki, T. Beppu, and K. Arima. 1980. New cryptic plasmid of Bacillus subtilis and restriction analysis of other plasmids found by general screening. J. Bacteriol. 142:315-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 39.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, G., H. Corker, Y. Orii, and R. K. Poole. 2004. Escherichia coli Hmp, an “oxygen-binding flavohaemoprotein,” produces superoxide anion and self-destructs. Arch. Microbiol. 182:193-203. [DOI] [PubMed] [Google Scholar]
- 41.Wu, G., L. M. Wainwright, and R. K. Poole. 2003. Microbial globins. Adv. Microb. Physiol. 47:255-310. [DOI] [PubMed] [Google Scholar]
- 42.Ye, R. W., W. Tao, L. Bedzyk, T. Young, M. Chen, and L. Li. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.