Abstract
PII proteins have been shown to be key players in the regulation of nitrogen fixation and ammonia assimilation in bacteria. The mode by which these proteins act as signals is by being in either a form modified by UMP or the unmodified form. The modification, as well as demodification, is catalyzed by a bifunctional enzyme encoded by the glnD gene. The regulation of this enzyme is thus of central importance. In Rhodospirillum rubrum, three PII paralogs have been identified. In this study, we have used purified GlnD and PII proteins from R. rubrum, and we show that for the uridylylation activity of R. rubrum GlnD, α-ketoglutarate is the main signal, whereas glutamine has no effect. This is in contrast to, e.g., the Escherichia coli system. Furthermore, we show that all three PII proteins are uridylylated, although the efficiency is dependent on the cation present. This difference may be of importance in understanding the effects of the PII proteins on the different target enzymes. Furthermore, we show that the deuridylylation reaction is greatly stimulated by glutamine and that Mn2+ is required.
In Escherichia coli, the pivotal role of sensing the nitrogen level within the cell is played by the bifunctional uridylyltransferase/uridylyl-removing enzyme, encoded by the glnD gene, through the interaction with glutamine as an indicator of the intracellular nitrogen status (12). The signal is then further transduced to the regulatory PII proteins by uridylylation/deuridylylation catalyzed by GlnD. PII proteins are among the most highly conserved signaling proteins in nature, with regulatory roles in both transcriptional and posttranslational processes (2, 23). In the E. coli system, GlnD catalyzes the uridylylation of PII proteins at low glutamine concentrations, whereas at high concentrations of glutamine, the removal of UMP groups from the PII proteins is stimulated (17). A number of targets for PII proteins have been identified; in some cases the modified PII interacts with its target(s), in some the unmodified form, and in some both forms (24). It seems, however, that in order to efficiently interact with a target, ATP and α-ketoglutarate must be bound to the PII protein (17, 18). When considering the multiple targets for PII proteins in their different uridylylation states, the role of GlnD in catalyzing the modification/demodification is of crucial importance.
A number of bacterial PII proteins have been shown to be substrates for GlnD, but GlnB from E. coli is the most thoroughly studied (12, 17). In this bacterium, uridylylated GlnB does not interact with NtrB, the sensor of the two-component Ntr system. NtrB then catalyzes the phosphorylation of NtrC, the response regulator, that then acts as a transcriptional activator of operons involved in ammonium assimilation and nitrogen metabolism (3, 5, 13, 29). In contrast, under high-nitrogen conditions, GlnB is deuridylylated, and this form interacts with NtrB, promoting the phosphatase activity of NtrB, leading to dephosphorylation of NtrC. Another target enzyme for uridylylated GlnB is adenylyltransferase, encoded by glnE, a bifunctional enzyme catalyzing the adenylylation/deadenylylation of glutamine synthetase (9, 11, 14, 20, 31). Under low-nitrogen conditions, adenylyltransferase catalyzes the removal of AMP groups from glutamine synthetase, leading to a more-active enzyme. On the other hand, unmodified GlnB stimulates the adenylylation activity of GlnE and consequently the inactivation of glutamine synthetase.
Rhodospirillum rubrum is a photosynthetic purple free-living nitrogen-fixing bacterium, and as with other diazotrophs, the fixed nitrogen is further assimilated via the glutamine synthetase and glutamate synthase pathway (21, 22, 30). In R. rubrum, three PII proteins have been identified, encoded by glnB, glnK, and glnJ, respectively (37). It was shown that in this bacterium, either GlnB or GlnJ is required for proper regulation of the dinitrogenase reductase ADP-ribosyl transferase (DRAT)/dinitrogenase reductase-activating glycohydrolase (DRAG) system, whose two enzymes are involved in posttranslational regulation of nitrogenase activity (26). Recently a mutation in amtB1, encoding a putative ammonia transporter, resulted in a strain impaired in DRAT/DRAG regulation (36, 40). The function of AmtB in this regulation is proposed to be to sequester unmodified GlnJ.
Furthermore, by mutational studies it has been shown that activation in R. rubrum of NifA, a transcriptional activator of nitrogen-fixing (nif) genes, requires the uridylylated form of GlnB and that neither GlnJ-UMP nor GlnK-UMP can substitute for GlnB-UMP (38).
Recently a number of mutations in R. rubrum glnD were constructed, and it was shown that the N-terminal region of GlnD is essential for uridylylation of GlnB and GlnJ, similar to the E. coli system (34, 39). In addition, the results in this study also suggest that NifA activation, DRAT/DRAG regulation, and NtrBC regulation require different levels of GlnD activity (39).
Although the regulation of GlnD has been studied with a number of bacteria, these studies have in most cases been performed at a physiological level or by mutational approaches (6-8, 10, 28, 32, 35).
In vitro studies of the uridylylation/deuridylylation activities of GlnD using purified components have to our knowledge been reported only for GlnD, GlnB, and GlnK from E. coli (4, 12, 17). In addition, purified GlnZ, a PII protein from Azospirillum brasilense, and purified GlnB from R. rubrum have been shown to be substrates for E. coli GlnD (1, 41). The efficiency by which GlnD catalyzes uridylylation/deuridylylation of PII proteins has been shown to depend on the ligands bound to the PII protein and the divalent cation present (12). Different PII proteins also show different characteristics, e.g., using E. coli proteins, deuridylylation of GlnK-UMP was reported to be slower than that of GlnB-UMP in the presence of magnesium ions (4).
Detailed studies at the molecular level have been described only for the E. coli system, but this may not be fully applicable to other bacteria, e.g., diazotrophs. Considering the different primary targets for the three PII proteins in R. rubrum, it is of special interest to establish if the three PII proteins show different characteristics with respect to uridylylation/deuridylylation catalyzed by GlnD. Here we report our studies of the requirements for GlnD to catalyze the uridylylation of the three PII paralogs in R. rubrum with different cations. The effects of α-ketoglutarate, glutamine, and ATP have also been investigated for the uridylylation/deuridylylation activities, showing characteristics of GlnD not reported previously. All experiments were performed with purified R. rubrum GlnD and PII proteins.
MATERIALS AND METHODS
Plasmid construction.
Bacterial strains and plasmids used in this study are listed in Table 1. For cloning of glnJ and glnK into the pGEX-6P-2 and pET-15b plasmids and glnD into the pGEX-6P-3 plasmid, primers were designed with appropriate restriction sites indicated in Table 1. All genes were obtained by PCR using Pfu polymerase (Stratagene) with R. rubrum S1 DNA as a template, except for the cloning of glnB into pGEX-6P-2, where pMJET was used as a template. For cloning of E. coli glnK into pET-15b, E. coli DNA was used as a template; otherwise, the same principle as for the above constructs was applied. All PCR products were routinely subcloned into the pCR-Blunt II-TOPO vector (Invitrogen) and all constructs verified by sequencing. Standard molecular biology methods, essentially as described by Sambrook et al. (33), were used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant characteristics | Source or reference |
|---|---|---|
| E. coli One Shot TOP10 | Host for pCR-Blunt-TOPO derivatives | Invitrogen |
| E. coli BL21(DE3)/pLysS | Host for overexpression of pET-15b derivatives; Apr Cmr | Invitrogen |
| E. coli BL21 Star (DE3) | Source of E. coli GlnD activity | Invitrogen |
| E. coli RB 9040 | ΔglnD; Host for overexpression of pGEXderivatives; Tcr | 5 |
| R. rubrum S1 | Wild type | |
| Plasmids | ||
| pCR-Blunt II-TOPO | Blunt PCR cloning vector; Kmr | Invitrogen |
| pET-15b | His-tagged (N-terminal) overexpression vector; Apr | Novagen |
| pGEX-6P-2/3 | GST-tagged (N-terminal) overexpression vectors; Apr | Amersham Biosciences |
| pMJET | pET-15b derivative giving recombinant GlnB; Apr | 16 |
| pET-GlnK | pET-15b derivative with glnK cloned between NdeI and BamHI, giving recombinant GlnK; Apr | Unpublisheda |
| pET-GlnJ | pET-15b derivative with glnJ cloned between NdeI and BamHI, giving recombinant GlnJ; Apr | Unpublisheda |
| pET-E.c GlnK | pET-15b derivative with E. coli glnK cloned between NdeI and XhoI, giving recombinant E. coli GlnK; Apr | This study |
| pGEX-GlnD | pGEX-6P-3 derivative with glnD cloned between BamHI and EcoRI, giving recombinant GlnD; Apr | This study |
| pGEX-GlnB | pGEX-6P-2 derivative with glnB cloned between BamHI and EcoRI, giving recombinant GlnB; Apr | This study |
| pGEX-GlnJ | pGEX-6P-2 derivative with glnJ cloned between BamHI and EcoRI, giving recombinant GlnJ; Apr | This study |
| pGEX-GlnK | pGEX-6P-2 derivative with glnK cloned between BamHI and EcoRI, giving recombinant GlnK; Apr | This study |
| pET-GlnB39 | pET-15b derivative encoding recombinant GlnB Q39E; Apr | This study |
| pET-GlnJ39 | pET-15b derivative encoding recombinant GlnJ Q39E; Apr | This study |
| pET-GlnK39 | pET-15b derivative encoding recombinant GlnK Q39E; Apr | This study |
A. Jonsson, P. Teixeira, and S. Nordlund, unpublished.
Cell growth and purification of R. rubrum proteins.
Transformation of plasmid pGEX-GlnD into E. coli strain RB 9040 was done according to the method of Sambrook et al. (33). Cells were grown at 20 to 25°C in Luria-Bertani medium supplemented with ampicillin (50 μg/ml), tetracycline (15 μg/ml), and 10 mM glutamine. Cells were grown to an optical density at 600 nm (OD600) of 1.1, followed by induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 1 h at 20 to 25°C. Cells were then harvested and frozen as pellets in liquid nitrogen. Pellets were resuspended in glutathione-S-transferase (GST)-binding buffer (pH 7.4) containing 142.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, complemented with 20 μg/ml DNase, one tablet of Complete Mini EDTA-free protease inhibitor/liter (Roche), 2 mM phenylmethylsulphonylfluoride, and 0.5 mg/ml lysozyme, followed by sonication (six times, 10 s each). The supernatant, after centrifugation at 40,000 × g for 20 min, was filtered through a 0.45-μm filter and applied to a GSTrap column (5 ml; Amersham Biosciences), equilibrated in GST-binding buffer (without additions) at a flow rate of 0.2 ml/min. The column was washed with cleavage buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM KCl, 1 mM EDTA, 1 mM dithiothreitol (DTT), and 200 mM glutamine. PreScission protease (160 U; Amersham Biosciences) was then added to the column, which was incubated at 4°C overnight, followed by 2 h at room temperature. After digestion, GlnD was eluted in cleavage buffer and assayed for GlnD activity.
All pET-15b derivatives were transformed into E. coli BL21(DE3)/pLysS. Cultures were grown in Luria-Bertani medium at 37°C to an OD600 of 0.6 and then induced with 1 mM IPTG for 1 h. Cell cultures were then harvested and frozen in liquid nitrogen as pellets. Cell pellets were resuspended in His-Binding buffer (pH 7.4) containing 500 mM NaCl, 10 mM imidazole, and 20 mM Na2PO4, complemented with 20 μg/ml DNase, 2 mM phenylmethylsulphonylfluoride, and one tablet of Complete Mini EDTA-free protease inhibitor (Roche)/liter. The solution was then frozen in liquid nitrogen and thawed three times, followed by sonication (six times, 10 s each), and then centrifuged for 20 min at 40,000 × g. The resulting supernatant was filtered through a 0.45-μm filter and applied to a HisTrap HP column (1 ml; Amersham Biosciences), equilibrated in His-binding buffer (without additions). Washing and elution of recombinant PII proteins from the column were performed with His-binding buffer containing 200 or 500 mM imidazole, respectively. To obtain untagged and unmodified PII proteins, all pGEX derivatives were transformed into E. coli strain RB 9040. The purification and growth was the same as for GlnD with the following exceptions. The cells were grown at 37°C to an OD600 of 0.6, followed by induction with 1 mM IPTG for 2 h at 20 to 25°C. The GST-binding buffer contained 140 mM NaCl and 2.7 mM KCl. The cleavage buffer contained 150 mM NaCl instead of 150 mM KCl, and a GSTrap column (1 ml; Amersham Biosciences) was used. All PII proteins purified were applied to a desalting column, PD-10 (Amersham Biosciences), and equilibrated in 50 mM HEPES (pH 8.0), 200 mM KCl, and 10% glycerol.
Site-directed mutagenesis of GlnB, GlnJ, and GlnK.
Upper and lower primers were designed for generating Q39E variants of all PII proteins by standard PCR-mediated site-directed mutagenesis by using Pfu polymerase (Stratagene). The primers contained a 1-bp mismatch, converting the codon for glutamine 39 to that for glutamate. Templates used were pET-GlnK, pET-GlnJ, and pMJET. and the plasmids produced were named pET-GlnK39, pET-GlnJ39, and pET-GlnB39, respectively.
Uridylylation of GlnB, GlnK, and GlnJ.
Uridylylation of all PII proteins was carried out at 30°C in a reaction mixture (100 μl) containing 0.5 μM purified PII protein, 0.13 μM GlnD, 50 mM HEPES (pH 7.6), 100 mM KCl, 2 mM ATP, 1 mM DTT, 0.3 mg/ml bovine serum albumin, 0.5 mM UTP supplemented with ≈0.5 μCi [α-32P]UTP, 25 mM MgCl2, and 250 μM α-ketoglutarate or 60 μM when 3 mM MnCl2 was used in the assay. In the experiments with extracts of wild-type R. rubrum, 15 μM R. rubrum GlnB or E. coli GlnK together with extracts corresponding to 120 μg of protein were also added to the reaction mixture. Samples were taken from the reaction mixtures after different time intervals and mixed with sodium dodecyl sulfate (SDS) cocktail (130 mM TRIS, pH 6.8, 4.2% SDS, 20% [vol/vol] glycerol, 10% 2-mercaptoethanol, and 0.003% bromphenol blue), followed by boiling for 2 min, and then run on an 18% SDS-polyacrylamide gel. After staining with Coomassie Brilliant blue followed by destaining, gels were dried and subjected to autoradiography on a phosphorimager for 1 h and overnight. Incorporation of [α-32P]UMP into the proteins was then quantified and visualized using the Image Quant program.
Deuridylylation of GlnB and GlnJ.
To determine deuridylylation of modified PII proteins, the reaction mixtures were the same as for uridylylation except that 500 μM α-ketoglutarate (or omitted when indicated), 10 mM glutamine (or omitted when indicated), 0.4 μM uridylylated PII protein and 1.3 μM GlnD were used. UTP and [α-32P]UTP were omitted. Samples were taken from the reaction mixtures after different time intervals and analyzed as in the uridylylation assay except that [32P]UMP remaining was quantified.
Purification of uridylylated PII proteins.
Either His-GlnB or His-GlnJ were incubated overnight at 30°C with 0.13 μM GlnD together with the same effectors and substrates as mentioned above for uridylylation, in a total reaction volume of 5 ml. The samples were then applied to PD-10 columns equilibrated in 50 mM HEPES (pH 8.0), 200 mM KCl and 10% glycerol and purified using 1 ml HisTrap HP columns precharged with Ni2+ ions according to the same procedures as for purification of unmodified his-tagged PII proteins. Uridylylation efficiency was close to 100% as analyzed by 18% SDS-polyacrylamide gel electrophoresis (PAGE).
Purification of E. coli GlnD activity.
As a source for producing E. coli GlnD activity, E. coli BL21 Star (DE3) cells (without any plasmid) were used. Purification was essentially the same as for the His-tagged PII proteins with the exception that cell breakage was done in a Ribi fractionator. E. coli GlnD activity was recovered by elution with 200 mM imidazole followed by desalting using a PD-10 column (Amersham Biosciences) into buffer containing 50 mM HEPES (pH 7.6). Fifty micrograms of protein was used in the assays.
Preparation of wild-type R. rubrum extracts.
R. rubrum strain S1 was grown in the minimal medium described by Ormerod, with the omission of glutamate (27). For growth under nitrogen-fixing conditions, referred to as N−, cultures were gassed with a mixture of 95% N2/5% CO2. Ammonium-grown cells, N+, were supplemented with 28 mM NH4Cl. After harvesting (OD600 of ≈1.5), the cells were frozen as pellets in liquid nitrogen. The pellets were thawed in buffer containing 100 mM HEPES (pH 7.6), 1 mM MnCl2, 1 mM DTT, and one tablet of Complete Mini EDTA-free protease inhibitor/liter (Roche). Cell breakage was performed in a Ribi cell fractionator followed by centrifugation at 2,500 × g to remove cell debris and unbroken cells. The extracts were then applied to PD-10 columns equilibrated in buffer containing 100 mM HEPES (pH 7.6), 1 mM DTT, and one tablet of Complete Mini EDTA-free protease inhibitor/liter.
Electrophoresis and Western blotting.
Samples for SDS-PAGE (18%) followed by Western blotting were mainly the same as for the autoradiographic procedures with some exceptions. After electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane and further incubated with antibodies against R. rubrum GlnB or GlnJ. The ECL (Amersham Biosciences) was used for detection. Samples for Western blotting were loaded after at least 3 weeks to avoid interference of radioactivity from the labeled [α-32P]UMP-PII proteins.
RESULTS AND DISCUSSION
Overexpression and purification of GlnD, GlnB, GlnK, and GlnJ from R. rubrum.
R. rubrum GlnD is a 936-amino-acid protein with a molecular mass of about 105 kDa, as deduced from the gene sequence (http://genome.ornl.gov/microbial/rrub/). The glnD gene was cloned and overexpressed as a fusion protein with GST in an E. coli ΔglnD strain (RB 9040), purified, and verified by matrix-assisted laser desorption ionization-time-of-flight analysis. The final product had a molecular mass of around 100 kDa as resolved by 8% SDS-PAGE (data not shown). Growing the cells at 20 to 22°C gave the best yield of purified GlnD (0.46 mg/liter culture). When overexpressed with a six-His tag at the N-terminal end instead of the GST tag, most of the GlnD fraction formed inclusion bodies. Attempts to dissolve the inclusion bodies were unsuccessful.
Sequence analysis showed that in all three R. rubrum PII paralogs, there is a tyrosine at position 51, the residue being uridylylated in all bacterial PII proteins studied. The molecular masses of the purified PII proteins were estimated on 18% SDS-PAGE gels to be 12.4 kDa and 14.4 kDa when containing a six-His tag, all in good agreement with the expected molecular masses. Since we could not detect any difference in the uridylylation of untagged PII proteins and the His-tagged version in our assays (data not shown), we used the His-tagged proteins in all experiments (except in the experiment shown in Fig. 9) for convenience.
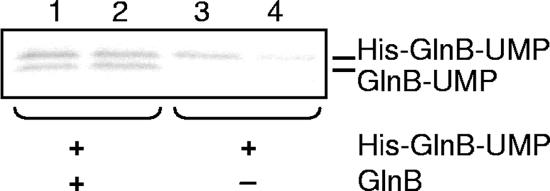
FIG. 9.
Effect of GlnB on deuridylylation. Reaction mixtures contained 1.3 μM GlnD, 3 mM MnCl2, 500 μM α-ketoglutarate, 2 mM ATP, 10 mM glutamine, and 0.5 mM UTP supplemented with [α-32P]UTP. Lanes 1 and 2 also contained 0.4 μM GlnB and 0.4 μM His-GlnB-UMP, while only His-GlnB-UMP was added in lanes 3 and 4. Samples were withdrawn from the reaction mixtures after 5 min (lanes 1 and 3) or after 60 min (lanes 2 and 4). Note that both experiments were performed under deuridylylation conditions (1.3 μM GlnD and 10 mM glutamine) but with 0.5 mM UTP supplemented with [α-32P]UTP also included in the assay. Samples were subjected to SDS-PAGE and visualized by autoradiography.
Uridylylation—the effect of divalent cations.
In E. coli, ammonium assimilation and thus nitrogen metabolism are controlled by the concentrations of α-ketoglutarate and glutamine (25). Both have effects on the uridylylation/deuridylylation reactions catalyzed by GlnD (12). Mutational studies performed with E. coli GlnD have shown that both activities of GlnD reside within the same domain (25). α-Ketoglutarate is believed to exert its effect by binding to the PII protein, whereas the effect of glutamine is caused by interaction with GlnD (12). An increase in the cellular concentration of α-ketoglutarate reflects a decrease in the availability of fixed nitrogen, and under these conditions, three α-ketoglutarate molecules are bound to the PII protein, enhancing the rate of uridylylation. On the other hand, glutamine acts as an inhibitor of the uridylylation reaction and stimulates deuridylylation (12, 18).
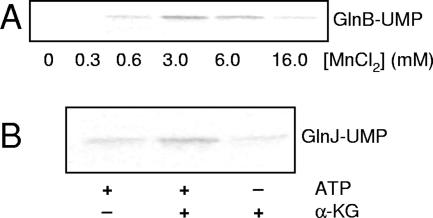
To determine the role of α-ketoglutarate, divalent cations, and glutamine in the regulation of PII uridylylation in the R. rubrum system, we examined the effect(s) in vitro using purified R. rubrum GlnD and PII proteins. To study these effect(s), we developed an assay to analyze incorporation of [α-32P]UMP into the PII proteins. Figure 1 shows a representative uridylylation experiment with GlnB in the presence of 3 mM MnCl2 and 60 μM α-ketoglutarate at 0, 2, 5, 10, and 20 min, followed by Western blotting, showing a shift over time in the migration of GlnB. The same results were obtained with GlnJ and GlnK. The highest degree of uridylylation was obtained at 3 mM MnCl2; above and below this concentration, labeling was less (Fig. 2A). No labeling of GlnB or GlnK could be detected when either ATP, α-ketoglutarate, or the divalent cations were omitted from the reaction mixture (data not shown). Surprisingly, GlnJ was uridylylated even when ATP or α-ketoglutarate was omitted from the reaction mixture but not to the same extent as with both present (Fig. 2B). However, when both ATP and α-ketoglutarate were omitted from the reaction mixture, no labeling of GlnJ was obtained (data not shown).
FIG. 1.
Uridylylation of R. rubrum GlnB. GlnB (0.5 μM) was incubated in a uridylylation reaction mixture with 0.13 μM R. rubrum GlnD, 3 mM MnCl2, 60 μM α-ketoglutarate, 2 mM ATP, and 0.5 mM UTP, supplemented with [α-32P]UTP. Samples were withdrawn from the reaction mixtures after 0, 2, 5, 10, and 20 min of incubation and stopped by addition of SDS cocktail. The samples were subjected to SDS-PAGE and visualized by autoradiography in panel A or Western blotting in panel B, immunoblotted with antibodies against R. rubrum GlnB. In panel B, the lower band corresponds to unmodified GlnB and the upper to the modified form of GlnB.
FIG. 2.
Effect of MnCl2, ATP, and α-ketoglutarate (α-KG) on uridylylation. (A) Uridylylation of GlnB (0.5 μM) with 0, 0.3, 0.6, 3.0, 6.0, or 16 mM MnCl2 together with 2 mM ATP, 60 μM α-ketoglutarate, 0.13 μM GlnD, and 0.5 mM UTP supplemented with [α-32P]UTP. (B) Effect of the absence of ATP or α-ketoglutarate on uridylylation of GlnJ (0.5 μM). The assays also contained 3 mM MnCl2, 0.13 μM GlnD, and 0.5 mM UTP supplemented with [α-32P]UTP. Uridylylation was assayed by incorporation of [α-32P]UMP as described in Materials and Methods. Samples were withdrawn from the reaction mixtures after 20 min of incubation and stopped by addition of SDS cocktail.
In an E. coli GlnB variant in which glutamine 39 was changed to glutamate, the uridylylation efficiency was lower (15). It was suggested that this effect was due to an inability of the variant to correctly bind α-ketoglutarate or ATP or effects on the interaction between GlnD and the mutated PII proteins. In order to further study the effect of α-ketoglutarate on the R. rubrum proteins, we generated Q39E variants of all three paralogs. When assaying uridylylation with the Q39E variant of GlnB or GlnK, no incorporation of [α-32P]UMP at any concentration of α-ketoglutarate used was observed. The GlnJ Q39E variant was labeled, however, but to a much lesser extent than native GlnJ (data not shown). Taken together, this indicates that the effect of α-ketoglutarate on R. rubrum GlnJ is different from that on GlnB or GlnK, since in the absence of either α-ketoglutarate or ATP, the native form of GlnJ was still uridylylated and the effect of the Q39E mutation on GlnJ was less than on the other paralogs.
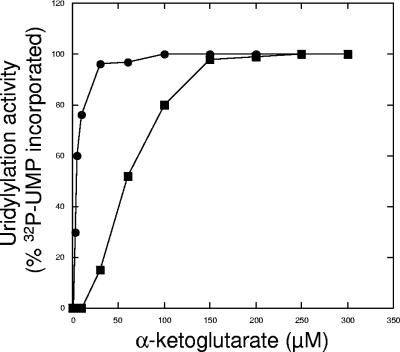
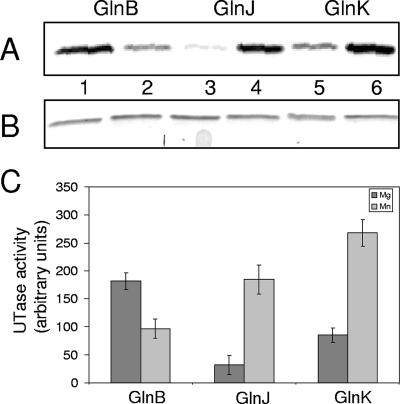
A previous report showed that Mg2+ and Mn2+ clearly have different effects on the rate at which E. coli GlnD catalyzes uridylylation (12). We investigated the influence of Mg2+ and Mn2+ in the R. rubrum in vitro system. Initial titration experiments with these cations using excess amounts of GlnD (threefold) and limiting concentrations of GlnB showed that GlnB was totally labeled after 15 min of incubation with excess concentrations of ATP and α-ketoglutarate. An additional 45 min of incubation did not increase the amount of labeling, and Western blotting also showed that all GlnB was modified (data not shown). When titrating the α-ketoglutarate concentration for uridylylation of GlnB in the presence of either Mg2+ or Mn2+, maximal modification was obtained with 250 μM and 60 μM α-ketoglutarate, respectively, as shown in Fig. 3. Similar values for α-ketoglutarate were also obtained for GlnJ and GlnK. Using these concentrations of α-ketoglutarate, we compared the degree of uridylylation of the PII proteins with either Mg2+ or Mn2+ in the assay. Results from a typical experiment are shown in Fig. 4A for all three R. rubrum PII paralogs. Figure 4B shows that the same amounts of PII proteins were loaded in the lanes. It should be noted that the SDS-PAGE system used in this experiment does not allow separation of modified and unmodified PII. Figure 4C depicts results of quantification of the labeled PII proteins. For every set of quantification experiments performed, the combination of GlnK with Mn2+ always gave the highest degree of labeling. The results also show that both divalent cations can stimulate uridylylation, with more labeling of GlnB with Mg2+ than with Mn2+ and the opposite for GlnJ and GlnK. The combination yielding the lowest level of labeled PII protein was GlnJ with Mg2+.
FIG. 3.
The effect of α-ketoglutarate on uridylylation of GlnB with either MgCl2 or MnCl2. The assay contained 0.5 μM GlnB, 2 mM ATP, 0.13 μM GlnD, and 0.5 mM UTP supplemented with [α-32P]UTP. Twenty-five millimolar MgCl2 (▪) or 3 mM MnCl2 (•). Samples were withdrawn from the reaction mixtures after 20 min of incubation and stopped by addition of SDS cocktail. The incorporation of [α-32P]UMP at 300 μM α-ketoglutarate was set to 100% for both MgCl2 and MnCl2. No more labeling was detected at α-ketoglutarate concentrations higher than 300 μM. The samples were subjected to SDS-PAGE and visualized by autoradiography. The amount of labeled, uridylylated GlnB was quantified using the Image Quant program. The data shown are representative of at least three independent experiments for both MgCl2 and MnCl2.
FIG. 4.
Effect on uridylylation of Mg2+ or Mn2+. The three PII paralogs (0.5 μM) were incubated with either 250 μM α-ketoglutarate and 25 mM Mg2+ (lanes 1, 3, and 5) or 60 μM α-ketoglutarate and 3 mM Mn2+ (lanes 2, 4, and 6). For both divalent cations used, 2 mM ATP, 0.13 μM GlnD, and 0.5 mM UTP, supplemented with [α-32P]UTP, were included in the reaction mixture. Samples were withdrawn from the reaction mixtures after 20 min of incubation and stopped by addition of SDS cocktail. (A) Autoradiogram showing incorporation of [α-32P]UMP into GlnB (lanes 1 and 2), GlnJ (lanes 3 and 4), or GlnK (lanes 5 and 6). (B) Coomassie-stained SDS-PAGE showing the levels of PII proteins loaded in panel A. (C) Histogram showing the difference in incorporation of [α-32P]UMP between the R. rubrum PII proteins with Mg2+ (dark) or Mn2+ (gray) in the uridylylation reaction. The amount of labeled, uridylylated GlnB, GlnJ, or GlnK was quantified using the Image Quant program. The data shown are from at least three independent experiments. UTase, uridylyltransferase.
A stimulating effect of Mn2+, compared to results with Mg2+, has also been observed for the E. coli system with GlnB, but this was regarded as nonphysiological (12). Our data may not reflect the physiological situation, but we believe that Mn2+ does have a role in uridylylation and most certainly in deuridylylation (see below). Further studies are required to distinguish whether the effect of Mg2+ and Mn2+ is related to interactions of the cations directly with GlnD or with the PII proteins or if they have an effect in the binding between PII proteins and GlnD.
The physiologically relevant explanation for the difference in uridylylation efficiencies of the PII proteins depending on which divalent cation used in the assay is not obvious. Also, the physiological role of Mn2+ is not established, although there are Mn2+-dependent enzymes in R. rubrum, e.g., glutamine synthetase and DRAG. However, results in our laboratory show that GlnJ-UMP in fact is deuridylylated in vivo in response to addition of, e.g., ammonium ions to the culture (data not shown). We and Zhang et al. (39) have also shown that GlnJ is uridylylated when the ammonium ions added are metabolized. This would indicate either that Mn2+ is present in sufficient concentrations or that in vivo unknown factors have an effect on the activities of GlnD with GlnJ as a substrate.
Uridylylation—the effect of glutamine.
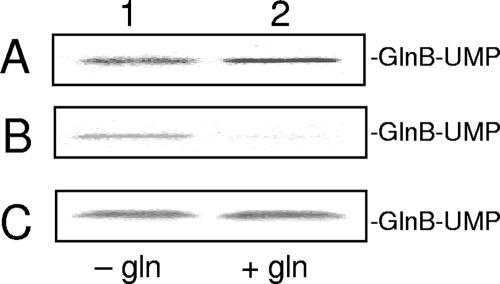
In contrast to results with the E. coli system, we could not see any inhibitory effect of glutamine on uridylylation catalyzed by purified R. rubrum GlnD (Fig. 5A) (12, 17). We considered that some component(s) necessary had been lost during the purification process of the recombinant protein, and therefore, we examined uridylylation in extracts from wild-type R. rubrum grown under either N+ or N− conditions. There was no effect of glutamine under these assay conditions, however, either with N+ extracts (Fig. 5C) or with extracts from N− cells (data not shown). To exclude the possibility that the observed effect was on the R. rubrum PII proteins, we used E. coli GlnK in the assay with both R. rubrum extracts and purified R. rubrum GlnD. No inhibition of uridylylation could be observed (data not shown). Interestingly, when using a partially purified E. coli GlnD fraction (see Materials and Methods), uridylylation of GlnB from R. rubrum was inhibited by addition of glutamine (Fig. 5B). The same results were obtained with R. rubrum GlnK and GlnJ and E. coli GlnK (data not shown). Taken together, these results suggest that the lack of response to glutamine is due to the characteristics of R. rubrum GlnD, and it raises the question of how the deuridylylation activity is regulated. From our results it would seem that the major effector of this activity is α-ketoglutarate.
FIG. 5.
The effect of glutamine on uridylylation. In panels A, B, and C, 3 mM MnCl2, 2 mM ATP, 60 μM α-ketoglutarate, and 0.5 mM UTP supplemented with [α-32P]UTP were included in the reaction mixture. (A) GlnB (0.5 μM) with 0.13 μM purified R. rubrum GlnD. (B) GlnB (15 μM) with 50 μg E. coli GlnD activity. (C) Fifteen micromolar GlnB with R. rubrum N+ extract (120 μg protein). Lane 1, no glutamine added; lane 2, 10 mM glutamine added. Samples were withdrawn after 0 or 20 min of incubation and stopped by addition of SDS cocktail.
Deuridylylation of R. rubrum PII proteins.
In E. coli, glutamine not only inhibits uridylylation but also stimulates the deuridylylation of GlnB and GlnK with purified components, albeit at a lower rate for GlnK (4, 12). In order to study the effect of glutamine on deuridylylation catalyzed by R. rubrum GlnD, GlnB and GlnJ were labeled with [α-32P]UMP, followed by purification on a Ni2+ chelating affinity column, and were then used as substrates. Initially we purified GlnD with NaCl in the buffer system used for GST-tagged proteins as recommended by the manufacturer (Amersham Biosciences), but we could not detect any deuridylylation activity of GlnD. However, when KCl was used instead of NaCl in the buffer system, the R. rubrum GlnD preparations were active in deuridylylation, in agreement with previous reports showing that GlnD from E. coli is more stable in buffers containing KCl (17).
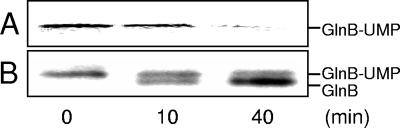
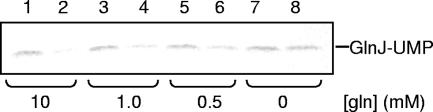
Figure 6 shows a representative deuridylylation experiment with GlnB-UMP as a substrate. In Fig. 6A, a decrease in the radioactivity corresponding to removal of the UMP group from GlnB is clearly seen. Figure 6B shows a Western blot of the same samples. It should be noted that 10 times more GlnD was required in the reaction mixture to detect deuridylylation than was required for uridylylation. The same results were obtained for [α-32P]UMP-GlnJ. As seen in Fig. 7, glutamine seems to give the greatest stimulating response for deuridylylation (Fig. 7, compare column pairs 1 and 4). When titrating the glutamine concentration required to detect deuridylylation of GlnJ-UMP, 1 mM was sufficient (Fig. 8), although 10 mM was used in the deuridylylation assays. The same results were obtained with [α-32P]UMP-GlnB. No deuridylylation after 40 min of incubation of either GlnB-UMP or GlnJ-UMP was observed when omitting all of ATP, α-ketoglutarate, and glutamine from the assay (data not shown). Furthermore, there was no deuridylylation in the absence of added Mn2+ with or without Mg2+ (data not shown). This is a notable difference from the E. coli system, where deuridylylation also can occur with Mg2+ alone, although with lower efficiency than with Mn2+ (12).
FIG. 6.
Deuridylylation activity of purified R. rubrum GlnD. The reaction mixture contained 0.4 μM GlnB-UMP, 1.3 μM GlnD, 3 mM MnCl2, 500 μM α-ketoglutarate, 2 mM ATP, and 10 mM glutamine. The activities were estimated as the decrease in labeled [α-32P]UMP-GlnB. Samples were withdrawn from the reaction mixtures after 0, 10, or 40 min and stopped by addition of SDS cocktail. The samples were subjected to SDS-PAGE and visualized by autoradiography (A) or Western blotting (B), immunoblotted with antibodies against R. rubrum GlnB.
FIG. 7.
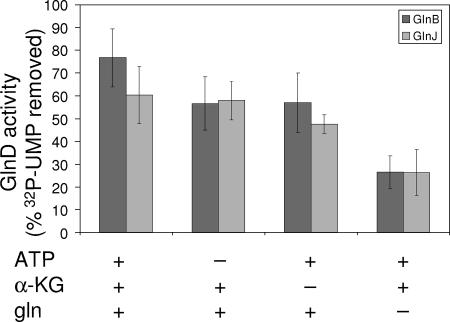
Stimulation of the deuridylylation activity of purified R. rubrum GlnD. The data are from at least three independent experiments showing percentages of [α-32P]UMP removed after 40 min of incubation for both GlnB-UMP (dark) and GlnJ-UMP (gray). For every pair of columns, 0.4 μM GlnB-UMP/GlnJ-UMP, 1.3 μM GlnD, and 3 mM MnCl2 were added to the deuridylylation mixture. The first pair of columns shows deuridylylation with 500 μM α-ketoglutarate, 2 mM ATP, and 10 mM glutamine. The second pair shows deuridylylation without 2 mM ATP, and the third without 500 μM α-ketoglutarate. The last pair of columns shows deuridylylation without 10 mM glutamine. The data are from at least three independent experiments showing percent [α-32P]UMP removed after 40 min of incubation for both GlnB-UMP and GlnJ-UMP.
FIG. 8.
Effect of glutamine on deuridylylation activity of purified R. rubrum GlnD. The uridylyl-removing activity was estimated as a decrease in labeled [α-32P]UMP-GlnJ after 40 min of incubation. The reaction mixture contained 0.4 μM GlnJ-UMP, 1.3 μM GlnD, 3 mM MnCl2, 500 μM α-ketoglutarate, 2 mM ATP, and 0 to 10 mM glutamine. Samples were withdrawn from reaction mixtures after time zero (lanes 1, 3, 5, and 7) or 40 min (lanes 2, 4, 6, and 8). Reactions were stopped by addition of SDS cocktail.
Figure 7 shows that some deuridylylation activity of GlnD could be detected when only ATP and α-ketoglutarate were added to the assay without glutamine (Fig. 7, column pairs 2 and 3), but the activity was three times higher when glutamine was included (Fig. 7, column pair 1). Our data suggest that when the in vivo glutamine levels increase, deuridylylation of GlnB-UMP and GlnJ-UMP is stimulated almost irrespective of the α-ketoglutarate and ATP concentrations. In the E. coli system, α-ketoglutarate, ATP, and glutamine are all required for the deuridylylation activity when Mg2+ is used in the assay. On the other hand, when Mn2+ is the cation used, only glutamine is required, showing characteristics similar to those of the R. rubrum system (12).
We also wanted to study the effect of unmodified PII protein on the deuridylylation reaction. We therefore produced His-tagged GlnB-UMP, which migrates detectably slower than untagged GlnB-UMP in the SDS-PAGE system used. As shown in Fig. 9 (lanes 1 and 2), GlnB does inhibit deuridylylation of His-GlnB-UMP, and it is also uridylylated. When GlnB was omitted from the reaction mixture, His-GlnB-UMP was demodified (Fig. 9, lanes 3 and 4). These results indicate a higher affinity for unmodified GlnB under the conditions used. It should be noted that UTP and glutamine were included in all reaction mixtures, showing that deuridylylation can occur in the presence of UTP (Fig. 9, lanes 3 and 4) and that uridylylation can proceed even in the presence of glutamine (Fig. 9, Lanes 1 and 2). The results in Fig. 9 (lane 1) show substantial uridylylation already after 5 min that is faster than under the conditions used in Fig. 1. This is due to the addition of 10 times more GlnD in the deuridylylation assay.
With E. coli, similar experiments also showed inhibition of deuridylylation when GlnB was added to the deuridylylation reaction (12). On the other hand, UTP strongly inhibited the deuridylylation reaction, but this is not the case for the R. rubrum system. We believe that the activities of GlnD occur within the same domain and at overlapping active sites, as has also been suggested for E. coli GlnD (25). Furthermore, our results indicate a preference of GlnD for the unmodified GlnB over GlnB-UMP, since deuridylylation occurred only in the absence of GlnB.
In summary, we have shown that GlnD from R. rubrum exhibits characteristics that differ from those of GlnD from E. coli, the most interesting being that glutamine does not inhibit the uridylylation activity. This raises the question of how the uridylylation/deuridylylation activities are regulated in R. rubrum in response to changes in the nitrogen status in the cell. From our results, it would seem that the concentration of α-ketoglutarate plays a central role, since this metabolite is required for and stimulates uridylylation. Interestingly, our studies on GlnE from R. rubrum show that glutamine does not have a direct effect on this enzyme but that α-ketoglutarate is an important effector, although exerting its function on the PII protein (Jonsson et al., unpublished). Addition of ammonium ions to nitrogen-fixing R. rubrum has been shown to cause a transient increase in the cellular glutamine concentration (19), but there are no studies on the concomitant effect on the α-ketoglutarate concentration. Another interesting feature of R. rubrum GlnD is the difference in the rates at which the R. rubrum PII proteins are uridylylated with different cations, which may have implications for their different regulatory roles in R. rubrum. In conclusion, we believe that the R. rubrum system with the three PII proteins and the metabolic regulation of nitrogen fixation offers an interesting variation of regulatory features with respect to nitrogen metabolism in bacteria. To determine if the differences are related to R. rubrum being a diazotroph, a phototroph, or both will require extended in vitro studies with other bacteria. It is striking, however, that in R. rubrum both nitrogenase activity and ammonium assimilation are regulated at the metabolic level by mechanisms that are dependent on the presence of PII proteins, thus assigning a central role for GlnD.
Acknowledgments
We thank Wally C. Van Heeswijk for generously providing the E. coli strain RB 9040 and Gary P. Roberts for generously providing R. rubrum GlnJ antibodies. Also, we thank Tatiana Pisareva for matrix-assisted laser desorption ionization-time-of-flight analysis and Tomas Edgren and Pedro Teixeira for helpful discussions.
This work was supported by grants from the Swedish Research Council to S.N.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Araujo, M. S., V. A. Baura, E. M. Souza, E. M. Benelli, L. U. Rigo, M. B. Steffens, F. O. Pedrosa, and L. S. Chubatsu. 2004. In vitro uridylylation of the Azospirillum brasilense N-signal transducing GlnZ protein. Protein Expr. Purif. 33:19-24. [DOI] [PubMed] [Google Scholar]
- 2.Arcondeguy, T., R. Jack, and M. Merrick. 2001. P(II) signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 65:80-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson, M. R., E. S. Kamberov, R. L. Weiss, and A. J. Ninfa. 1994. Reversible uridylylation of the Escherichia coli PII signal transduction protein regulates its ability to stimulate the dephosphorylation of the transcription factor nitrogen regulator I (NRI or NtrC). J. Biol. Chem. 269:28288-28293. [PubMed] [Google Scholar]
- 4.Atkinson, M. R., and A. J. Ninfa. 1999. Characterization of the GlnK protein of Escherichia coli. Mol. Microbiol. 32:301-313. [DOI] [PubMed] [Google Scholar]
- 5.Bueno, R., G. Pahel, and B. Magasanik. 1985. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J. Bacteriol. 164:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colnaghi, R., P. Rudnick, L. He, A. Green, D. Yan, E. Larson, and C. Kennedy. 2001. Lethality of glnD null mutations in Azotobacter vinelandii is suppressible by prevention of glutamine synthetase adenylylation. Microbiology 147:1267-1276. [DOI] [PubMed] [Google Scholar]
- 7.Connelly, H. M., D. A. Pelletier, T. Y. Lu, P. K. Lankford, and R. L. Hettich. 2006. Characterization of pII family (GlnK1, GlnK2, and GlnB) protein uridylylation in response to nitrogen availability for Rhodopseudomonas palustris. Anal. Biochem. 357:93-104. [DOI] [PubMed] [Google Scholar]
- 8.Contreras, A., M. Drummond, A. Bali, G. Blanco, E. Garcia, G. Bush, C. Kennedy, and M. Merrick. 1991. The product of the nitrogen fixation regulatory gene nfrX of Azotobacter vinelandii is functionally and structurally homologous to the uridylyltransferase encoded by glnD in enteric bacteria. J. Bacteriol. 173:7741-7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner, E., D. Wolf, C. Gancedo, S. Elsasser, and H. Holzer. 1970. ATP: glutamine synthetase adenylyltransferase from Escherichia coli B. Purification and properties. Eur. J. Biochem. 14:535-544. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, R., and M. Merrick. 1995. The role of uridylyltransferase in the control of Klebsiella pneumoniae nif gene regulation. Mol. Gen. Genet. 247:189-198. [DOI] [PubMed] [Google Scholar]
- 11.Jaggi, R., W. C. van Heeswijk, H. V. Westerhoff, D. L. Ollis, and S. G. Vasudevan. 1997. The two opposing activities of adenylyl transferase reside in distinct homologous domains, with intramolecular signal transduction. EMBO J. 16:5562-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, P., J. A. Peliska, and A. J. Ninfa. 1998. Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry 37:12782-12794. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, P., J. A. Peliska, and A. J. Ninfa. 1998. Reconstitution of the signal-transduction bicyclic cascade responsible for the regulation of ntr gene transcription in Escherichia coli. Biochemistry 37:12795-12801. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, P., J. A. Peliska, and A. J. Ninfa. 1998. The regulation of Escherichia coli glutamine synthetase revisited: role of 2-ketoglutarate in the regulation of glutamine synthetase adenylylation state. Biochemistry 37:12802-12810. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, P., P. Zucker, M. R. Atkinson, E. S. Kamberov, W. Tirasophon, P. Chandran, B. R. Schefke, and A. J. Ninfa. 1997. Structure/function analysis of the PII signal transduction protein of Escherichia coli: genetic separation of interactions with protein receptors. J. Bacteriol. 179:4342-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson, M., and S. Nordlund. 1999. Purification of P(II) and P(II)-UMP and in vitro studies of regulation of glutamine synthetase in Rhodospirillum rubrum. J. Bacteriol. 181:6524-6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamberov, E. S., M. R. Atkinson, J. Feng, P. Chandran, and A. J. Ninfa. 1994. Sensory components controlling bacterial nitrogen assimilation. Cell. Mol. Biol. Res. 40:175-191. [PubMed] [Google Scholar]
- 18.Kamberov, E. S., M. R. Atkinson, and A. J. Ninfa. 1995. The Escherichia coli PII signal transduction protein is activated upon binding 2-ketoglutarate and ATP. J. Biol. Chem. 270:17797-17807. [DOI] [PubMed] [Google Scholar]
- 19.Kanemoto, R. H., and P. W. Ludden. 1987. Amino acid concentrations in Rhodospirillum rubrum during expression and switch-off of nitrogenase activity. J. Bacteriol. 169:3035-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingdon, H. S., B. M. Shapiro, and E. R. Stadtman. 1967. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc. Natl. Acad. Sci. USA 58:1703-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrick, M. J., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, R. E., and E. R. Stadtman. 1972. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J. Biol. Chem. 247:7407-7419. [PubMed] [Google Scholar]
- 23.Ninfa, A. J., and M. R. Atkinson. 2000. PII signal transduction proteins. Trends Microbiol. 8:172-179. [DOI] [PubMed] [Google Scholar]
- 24.Ninfa, A. J., and P. Jiang. 2005. PII signal transduction proteins: sensors of α-ketoglutarate that regulate nitrogen metabolism. Curr. Opin. Microbiol. 8:168-173. [DOI] [PubMed] [Google Scholar]
- 25.Ninfa, A. J., P. Jiang, M. R. Atkinson, and J. A. Peliska. 2000. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr. Top. Cell Regul. 36:31-75. [DOI] [PubMed] [Google Scholar]
- 26.Nordlund, S., and P. W. Ludden. 2004. Post-translational regulation of nitrogenase in photosynthetic bacteria, p. 175-196. In W. Klipp, B. Masephol, J. R. Gallon, and W. E. Newton (ed.), Genetics and regulation of nitrogen fixation in free-living bacteria, vol. 2. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 27.Ormerod, J. G., K. S. Ormerod, and H. Gest. 1961. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch. Biochem. Biophys. 94:449-463. [DOI] [PubMed] [Google Scholar]
- 28.Perlova, O., R. Nawroth, E. M. Zellermann, and D. Meletzus. 2002. Isolation and characterization of the glnD gene of Gluconacetobacter diazotrophicus, encoding a putative uridylyltransferase/uridylyl-removing enzyme. Gene 297:159-168. [DOI] [PubMed] [Google Scholar]
- 29.Pioszak, A. A., and A. J. Ninfa. 2003. Genetic and biochemical analysis of phosphatase activity of Escherichia coli NRII (NtrB) and its regulation by the PII signal transduction protein. J. Bacteriol. 185:1299-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitzer, L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57:155-176. [DOI] [PubMed] [Google Scholar]
- 31.Rhee, S. G., P. B. Chock, and E. R. Stadtman. 1989. Regulation of Escherichia coli glutamine synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 62:37-92. [DOI] [PubMed] [Google Scholar]
- 32.Rudnick, P. A., T. Arcondeguy, C. K. Kennedy, and D. Kahn. 2001. glnD and mviN are genes of an essential operon in Sinorhizobium meliloti. J. Bacteriol. 183:2682-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Tondervik, A., H. R. Torgersen, H. K. Botnmark, and A. R. Strom. 2006. Transposon mutations in the 5′ end of glnD, the gene for a nitrogen regulatory sensor, that suppress the osmosensitive phenotype caused by otsBA lesions in Escherichia coli. J. Bacteriol. 188:4218-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dommelen, A., V. Keijers, E. Somers, and J. Vanderleyden. 2002. Cloning and characterisation of the Azospirillum brasilense glnD gene and analysis of a glnD mutant. Mol. Genet. Genomics 266:813-820. [DOI] [PubMed] [Google Scholar]
- 36.Wang, H., C. C. Franke, S. Nordlund, and A. Noren. 2005. Reversible membrane association of dinitrogenase reductase activating glycohydrolase in the regulation of nitrogenase activity in Rhodospirillum rubrum; dependence on GlnJ and AmtB1. FEMS Microbiol. Lett. 253:273-279. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y., E. L. Pohlmann, P. W. Ludden, and G. P. Roberts. 2001. Functional characterization of three GlnB homologs in the photosynthetic bacterium Rhodospirillum rubrum: roles in sensing ammonium and energy status. J. Bacteriol. 183:6159-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, Y., E. L. Pohlmann, P. W. Ludden, and G. P. Roberts. 2000. Mutagenesis and functional characterization of the glnB, glnA, and nifA genes from the photosynthetic bacterium Rhodospirillum rubrum. J. Bacteriol. 182:983-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Y., E. L. Pohlmann, and G. P. Roberts. 2005. GlnD is essential for NifA activation, NtrB/NtrC-regulated gene expression, and posttranslational regulation of nitrogenase activity in the photosynthetic, nitrogen-fixing bacterium Rhodospirillum rubrum. J. Bacteriol. 187:1254-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, Y., D. M. Wolfe, E. L. Pohlmann, M. C. Conrad, and G. P. Roberts. 2006. Effect of AmtB homologues on the posttranslational regulation of nitrogenase activity in response to ammonium and energy signals in Rhodospirillum rubrum. Microbiology 152:2075-2089. [DOI] [PubMed] [Google Scholar]
- 41.Zhu, Y., M. C. Conrad, Y. Zhang, and G. P. Roberts. 2006. Identification of Rhodospirillum rubrum GlnB variants that are altered in their ability to interact with different targets in response to nitrogen status signals. J. Bacteriol. 188:1866-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]