Abstract
Bacillus subtilis forms dormant spores upon nutrient depletion. Under favorable environmental conditions, the spore breaks its dormancy and resumes growth in a process called spore germination and outgrowth. To elucidate the physiological processes that occur during the transition of the dormant spore to an actively growing vegetative cell, we studied this process in a time-dependent manner by a combination of microscopy, analysis of extracellular metabolites, and a genome-wide analysis of transcription. The results indicate the presence of abundant levels of late sporulation transcripts in dormant spores. In addition, the results suggest the existence of a complex and well-regulated spore outgrowth program, involving the temporal expression of at least 30% of the B. subtilis genome.
A number of bacterial species such as bacilli and clostridia have the ability to form dormant spores. The spore has a specialized and complex structure, enabling the organism to survive for a long time under harsh environmental conditions and in the absence of nutrients. When triggered by specific nutrients, the spore is capable of breaking dormancy (germination) and initiating vegetative growth (34, 52). The Bacillus subtilis spore is composed of a dehydrated central compartment (the spore core) engulfed by two protective outer layers: a thick spore-specific peptidoglycan layer known as the spore cortex and a multilayered protein structure known as the coat (12).
The process of endospore formation in Bacillus subtilis has been studied in great detail. Studies have revealed a highly ordered and strictly regulated program ensuring the correct coordination of various aspects of the sporulation process, such as asymmetric cell division, prespore engulfment, spore maturation, and mother cell lysis (22). The sporulation program involves the timed activation of several mother cell and forespore compartment- and sporulation stage-dependent RNA polymerase sigma factors that transcribe specific sets of sporulation genes. Eventually, the sporulation program results in the lysis of the mother cell and the release of a dormant spore (22).
The process of spore germination and outgrowth has been studies in less detail. Spore germination is initiated when the spore senses the appropriate trigger molecules, often simple sugars and/or amino acids. The germinant molecules are sensed by germination receptors. This, by an unknown mechanism, leads to an irreversible commitment of a spore to germination. The germinating spore initially releases Zn2+ and H+ (65). Simultaneously (and probably as a consequence), the pH of the spore core rises from 6.5 to 7.7. In a second stage, the germinating spore releases the spore core's large supply of dipicolinic acid (pyridine-2,6-dicarboxylic acid), and the spore core is rehydrated. Subsequently, cortex lytic enzymes are activated and the protective spore peptidoglycan cortex is degraded. This enables the germinating spore to hydrate the spore core further and to swell. These germination events coincide with a loss of heat resistance. This second stage of rehydration allows initiation of protein mobility and reactivation of biochemical processes during outgrowth (52). As of this stage, the spore has completed germination. The transition of the germinated spore to a growing cell is termed spore outgrowth.
In the first stage of outgrowth, ATP is generated through the conversion of 3-phosphoglycerate stored in the spore core (58). In a later stage, the outgrowing spore switches to the use of extracellular nutrients (54). Macromolecular synthesis, essential for the reconstitution of biochemical pathways, nutrient uptake, and replication, can be initiated upon the production of ATP. Protein synthesis in the outgrowing spore is dependent on de novo transcription and is initiated in the first minutes of germination (51, 55). Chromosomal replication is initiated after approximately 30 min (16). Studies on protein synthesis during outgrowth have revealed distinct patterns of expression, which perhaps suggest the existence of an ordered process for outgrowth of the germinated spore (21, 23, 28, 70). To date the regulatory process that underlies the ordered protein expression during outgrowth has remained obscure. The roles of a number of RNA polymerase sigma factors were analyzed by Horsburgh et al. through mutagenesis and Northern blotting (26). The work demonstrated the importance of the vegetative RNA polymerase sigma factor σA for the efficiency of outgrowth. However, none of the extracytoplasmic sigma factors tested was found to be crucial for spore germination and outgrowth. The extracytoplasmic function sigma factor σM appears to play a role in the osmotolerance of outgrowing spores. We have used a combination of microscopy, extracellular metabolite analysis, and genome-wide transcriptome analysis to explore the physiological and transcriptional changes that occur during germination and outgrowth of B. subtilis spores.
MATERIALS AND METHODS
Sporulation and germination conditions.
Spores of Bacillus subtilis 168 were generated by depletion of defined liquid medium containing 80 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 1.32 mM K2HPO4, 0.4 mM MgCl2, 0.276 mM K2SO4, 0.01 mM FeSO4, 0.14 mM CaCl2, 4 mM Tricine, 20 mM glucose, 10 mM NH4Cl, 3 nM (NH4)6Mo7O24, 0.4 μM H3BO3, 30 nM CoCl2, 10 nM CuSO4, 10 nM ZnSO4, 0.1 mM MnCl2, and 50 μg/ml tryptophan (19, 27, 37). The pH of the medium was adjusted to 7.4 with KOH. Cultures were incubated for 4 days at 37°C under continuous shaking (200 rpm). Spores were harvested and purified by extensive washing with MilliQ water at 4°C (40). The spore crops were inspected by phase-contrast microscopy and were free (>99%) of vegetative cells, germinating spores, and debris.
Spore germination was performed in an 80-ml bench top fermentor, which was aerated at a rate of 120 ml/minute and stirred continuously. The temperature was controlled at 37°C. The germination medium (tryptic soy broth [Difco] buffered with 80 mM MOPS at pH 7.4 and supplemented with 10 mM glucose, 1 mM fructose, 1 mM potassium chloride, and 10 mM l-asparagine) was prewarmed to 37°C. Spores were activated by thermal treatment at 70°C for 30 min. Subsequently, the fermentor was inoculated to a final optical density at 600 nm of approximately 10. The process of germination and outgrowth was monitored by optical density measurements of 20-fold-diluted samples at 600 nm. During germination and outgrowth, 2-ml samples for RNA isolation were drawn at regular intervals. The samples were rapidly spun down in a microcentrifuge, and the pellets were snap frozen in liquid nitrogen. The time needed for sampling was less than 40 seconds. Spent medium was kept for metabolite analysis. Samples drawn for microscopic analysis were fixed by incubation in a solution of 2.8% formaldehyde-0.04% glutaraldehyde for 15 min at room temperature, followed by incubation on ice (10).
Microarray construction.
B. subtilis microarrays were obtained by spotting a B. subtilis oligonucleotide library (Sigma-Genosys; no. BACLIB96) in duplicate onto UltraGAPS slides (Corning) with a Lucidea Array Spotter (GE Healthcare) according to standard protocols. Besides the control oligonucleotides included in the Sigma-Genosys oligonucleotide library, additional control oligonucleotides were spotted onto the microarray, such as the SpotReport Alien Oligo Array Validation System library (Stratagene) and ArrayControl Sense Oligo Spots (Ambion). The spotted oligonucleotides were immobilized onto the microarray by UV cross-linking.
RNA isolation, labeling, hybridization, and scanning.
RNA was isolated from spores and outgrowing spores by using the FastRNA Pro Blue kit (BIO101/Q-BIOgene) according to the manufacturer's recommendations. Samples were processed three times for 40 seconds each in the FastPrep machine at setting 6.0. Between the processing stages, the samples were cooled on ice-water for at least 1 min. After ethanol precipitation, samples were treated with RNase-free DNase I (Boehringer Mannheim) and subsequently purified by phenol-chloroform extractions. Residual phenol was removed by a final chloroform extraction, and RNA was precipitated with ethanol (2.5 volumes) and potassium acetate (0.3 M, pH 5.2). Finally, the RNA was pelleted by centrifugation, washed with cold 75% ethanol, and dissolved in an appropriate volume of RNase-free water (Ambion). The quality and quantity were determined by nanodrop UV spectroscopy (Ocean Optics) and analysis on a RNA 6000 Nano LabChip (Agilent Technologies) using a 2100 bioanalyzer (Agilent Technologies). Cy-labeled cDNA was made by direct incorporation of Cy-labeled dUTP. For labeling, RNA (15 μg) was incubated with 1 μg of random hexamers [pd(N)6; GE Healthcare] and spike control RNA at 70°C for 10 min. Next, the mixture was placed on ice for 2 min. A labeling mix containing 2× reverse transcription buffer (Life Technologies), 5 mM MgCl2, 20 mM dithiothreitol, deoxynucleoside triphosphates (1 mM dATP, 1 mM dGTP, 1 mM dCTP, and 0.4 mM dTTP), and either Cy3-dUTP or Cy5-dUTP (Perkin-Elmer Life Sciences) was added to the RNA-primer mixture. After incubation of the mixture at 25°C for 5 min, Superscript II reverse transcriptase (300 U) (Life Technologies) was added. The mixture was then incubated at 25°C for 10 min, followed by incubation for 140 min at 42°C. The reaction was stopped by the addition of 1.5 μl of 20 mM EDTA. To hydrolyze the RNA, 15 μl 0.1 M NaOH was added and the samples were incubated at 70°C for 10 min. Subsequently, 15 μl 0.1 M HCl was added for neutralization. Unincorporated nucleotides were removed using QiaQuick purification spin columns (QIAGEN). Labeled cDNA was dried and resuspended in hybridization buffer (25 mM HEPES [pH 8.0], 1 mM EDTA, 0.8 μg of yeast tRNA/μl, 3× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.2% (wt/vol) sodium dodecyl sulfate [SDS]). The experiment was carried out in duplicate (biological duplicate) and each sample was hybridized in duplicate on the microarray (technical duplicate).
Prior to hybridization, the microarray slides were prehybridized by incubation in 2× SSPE (0.3 M sodium chloride, 0.02 M sodium hydrogen phosphate, 2 mM EDTA, pH 7.4) and 0.2% SDS at 52°C for 1.5 h. Subsequently, the slides were washed in MilliQ water and dried by centrifugation. Hybridization was performed in an automated slide processor (GE Healthcare) for 16 h at 37°C.
The slides were washed in 1× SSC-0.2% SDS (10 min), 0.1× SSC-0.2% SDS (10 min), and 0.1× SSC and flushed with isopropanol prior to drying under a nitrogen stream. Microarrays were scanned using an Agilent G2505 scanner. The Cy3 and Cy5 fluorescence mean intensity and surrounding median background from each spot were obtained with ArrayVision (v6.1) (Imaging Research, Inc.).
Data analysis.
Data preprocessing was performed using Microsoft Excel software and the gene expression pattern analysis suite GEPAS (69). Data normalization for samples obtained during the first 20 min was performed using the added spike controls. At later time points global normalization was shown to provide the best consistency for duplicate experiments and therefore was used. Low-intensity fluorescence data were floored at 2 times the average background fluorescence level, and the data were log2 transformed. Inconsistent replicate values (duplicate spots on the microarray and duplicate hybridization) were removed if the distance to the median was greater than 1 on a log2 scale. Of the remaining replicas, the median value was calculated. Genes were omitted if the number of missing values was more than 20%. These filtering steps resulted in the removal of data for 48 of the 4,060 genes. Missing values of the remaining genes were inferred using the KNNInpute algorithm, which determines the average value of genes with expression profiles similar to that of the gene of interest (K nearest neighbors) (67). Ratios of gene expression were calculated over the average of the mean value of the time series between t = 10 and 100 min. This data set was used for functional analysis as outlined below. For the analysis of patterns of coregulation, flat patterns were filtered by excluding genes that did not show a two fold increase in expression over their average, leaving 1,130 genes. To facilitate the comparison of patterns of gene expression, the patterns were brought to the same range by subtracting the mean of the pattern and dividing it by the standard deviation. Data were analyzed using a number of tools implemented in the Microarray Expression Viewer software (MEV-TIGR; http://www.tm4.org/mev.html) (47), such as hierarchical clustering (13) and K-means cluster analysis (60). The optimal number of K-means clusters was estimated by principal-component analysis (45). Since the differences between successive principal-component analysis components (eigenvalues) were found to rapidly go to near zero after the 12th component, the genes were subdivided into 12 groups with different expression patterns. Functional interpretation of the microarray data was performed by the analysis of overrepresented gene ontology terms using (JProGO) (2, 49). The unpaired Wilcoxon test was selected as method for analysis, the significance level was set at 0.05, and the Benjamini-Hochberg control for false discovery rate was used to correct for the multiple-testing effect (24).
Analysis of dipicolinic acid and extracellular metabolites.
The release of dipicolinic acid in the medium during spore germination was monitored by using the terbium fluorescence assay described previously (29).
For extracellular metabolite analysis, supernatants were deproteinized by acid precipitation with 35% HClO4 (0.1 ml to 1 ml supernatant) and neutralized with cold 7 M KOH. After centrifugation (4 min at 10,000 rpm), the supernatants were filtered through a 0.22-μm membrane. The filtered supernatants were injected into an Aminex HPX 87H organic acid analysis column (Bio-Rad) at 65°C. The eluent was 5 mM H2SO4 at a flow rate of 0.5 ml·h−1 Residual carbon source, pyruvate, and lactate concentrations were determined by high-pressure liquid chromatography using an LKB 2142 refractive index detector.
Microscopy.
The as above-mentioned glutaraldehyde-formaldehyde-fixed cells were pelleted and resuspended in 1 ml phosphate-buffered saline with 1 nM 4′,6′-diamidino-2-phenylindole (DAPI). After 10 min of incubation in the dark, cells were immobilized on agarose slides as described by Van Helvoort et al. (68) and photographed with a cooled charge-coupled device camera (Princeton Instruments, SARL, Utrecht, The Netherlands) mounted on an Olympus BX-60 fluorescence microscope. In all experiments, the cells were photographed first in the phase-contrast mode and then with a DAPI fluorescence filter (U-MWU; excitation at 330 to 385 nm).
Microarray accession number.
Microarray data are deposited in the GEO database (http://www.ncbi.nlm.nih.gov/projects/geo/) under accession number GSE 6865.
RESULTS AND DISCUSSION
Synchronous spore germination and outgrowth.
In order to understand the mechanism by which the dormant spore reactivates the cellular processes and resumes vegetative growth, germination and outgrowth of Bacillus subtilis spores were studied by microscopy, metabolite analysis, and genome-wide gene expression analysis. Bacillus subtilis spores were obtained from cells that had been cultured in a defined synthetic medium. This defined sporulation medium was selected on the basis of our observations that spores generated were shown to be homogenous in their outgrowth characteristics and thermal resistance properties (29). After harvesting and extensive washing at 4°C with MilliQ water, spore crops were inspected by phase-contrast microscopy and shown to be free (>99%) of vegetative cells. Spores were heat activated (30 min, 70°C) and immediately transferred to prewarmend germination medium to an optical density (600 nm) of approximately 10. This dense inoculation enabled rapid sampling of sufficient cells in a small volume. Rapid sampling and snap freezing of samples were found to constitute the most effective way to stabilize the RNA in the germinating spores (data not shown).
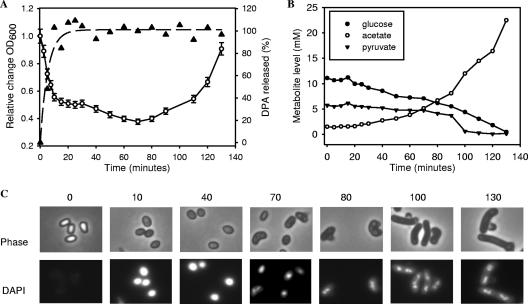
Upon inoculation of the fermentor, the spores rapidly and synchronously initiated germination. Indicative of immediate germination are the rapid release of the spore's supply of dipicolinic acid and a decrease in optical density (Fig. 1A). After approximately 10 minutes following the inoculation of the fermentor, spore germination appeared to have been completed. The dipicolinic acid levels in the supernatant reached a maximum level, and microscopic analysis of samples obtained at this time point showed a nearly complete transition from phase-bright spores to phase-dark germinated cells (Fig. 1C). The phase-dark spore also became susceptible to DAPI DNA staining (Fig. 1C). At 10 minutes into germination, the optical density of the germinated culture had decreased to approximately 55% of the initial value. The decrease in the optical density during germination is believed to coincide with the rehydration of the spore during germination. During later stages of outgrowth (t = 10 to 70 min), a further decrease in optical density to approximately 40% of the initial value was observed (Fig. 1A). Since the low level of remaining phase-bright spores at this stage did not change (Fig. 1C) and no additional dipicolinic acid was released in the medium (Fig. 1A), this decrease is likely to be due to further swelling of the germinated spore.
FIG. 1.
Spore germination and outgrowth. (A) After the addition of Bacillus subtilis spores to prewarmed germination medium (see Materials and Methods), germination and outgrowth were monitored by measuring changes in the optical density at 600 nm (OD600) (○). In addition, the release of dipicolinic acid (DPA) (▴) was monitored as a measure for the efficiency of spore germination. (B) Extracellular glucose, pyruvate, and acetate levels were monitored during germination and outgrowth. The switch from an endogenous metabolism to the use of extracellular metabolites was indicated by the decrease of the glucose concentration and increase of the acetate concentration in the medium. Pyruvate levels were found to decrease rapidly late in outgrowth. (C) Morphological changes during spore germination and outgrowth were investigated by microscopic analysis. Cells harvested at various time points during germination and outgrowth were fixed and monitored by phase-contrast microscopy (top row) and fluorescence microscopy following DNA DAPI (4′,6′-diamidino-2-phenylindole) straining (bottom row).
After approximately 70 min, the optical density was found to increase (Fig. 1A). Microscopic analysis revealed that at this time point, cells burst out of the remaining protective outer spore structures (spore cortex and/or coat) and initiated chromosome segregation, indicating the near completion of the first round of cell division (Fig. 1C). The increase in optical density thus appears to coincide with cell growth. Remnants of the spore coat and/or cortex were often observed to remain attached to the polar ends of the outgrowing cell, as has been observed previously by electron microscopy (48). After approximately 100 min, the young vegetative Bacillus cells had adopted their characteristic rod shape and appeared to have undergone an additional round of DNA replication (Fig. 1C). At this stage, the young vegetative cells became motile, as was observed by microscopy of nonfixed cells (not shown). The stabilization of the optical density of the culture and the characteristics of the gene expression pattern indicated that after approximately 150 min the cells entered stationary phase (data not shown).
Metabolite analysis indicated that the uptake of extracellular glucose was initiated after approximately 15 min (Fig. 1B). The uptake of glucose marks the transition from the use of intracellular metabolites stored within the spore to the use of extracellular metabolites. Simultaneously with the uptake of glucose, acetate was produced, as is often observed for Bacillus subtilis fermentations under conditions of excess carbon (5, 9). Since the medium was buffered sufficiently, acetate production did not affect the pH of the medium. This was confirmed by pH measurements. During outgrowth, the glucose uptake and acetate production rates appeared to increase. In the period between 15 and 70 min, glucose consumption and acetate production reached rates of near 4.2 and 3.9 mmol/h, respectively. In the period between 70 and 130 min, the glucose consumption rate increased to approximately 6.3 mmol/h, while the acetate production rate increased to 12 mmol/h. The increase in the rate of glucose uptake coincided with chromosomal segregation and the formation of rod-shaped cells (Fig. 1C). The increase in the glucose uptake/acetate efflux rate during outgrowth suggests an increase in the flux towards the generation of ATP.
Transcriptional analysis of spore germination and outgrowth.
During germination and outgrowth of spores, samples for RNA isolation were rapidly drawn and snap frozen. The method used for RNA isolation from spores and germinating and outgrowing cells is similar to that published by Moeller et al. (33). In agreement with their observations, we found that by using this method, nucleic acids could be isolated efficiently from spores and germinating spores. Microscopic analysis of processed samples could reveal only cellular debris and no intact spores. In addition, quantitative PCR on chromosomal DNA isolated from spore suspensions of known densities using the same method for bead beating indicated an extraction efficiency of between 98 and 99%. After RNA isolation, the integrity of the RNA was verified by bioanalyzer (Agilent RNA 6000 Nano) analysis (see Fig. S1 in the supplemental material). During germination and outgrowth, the amount of RNA extracted was found to increase by approximately a factor of 4 (data not shown), which is in agreement with recent publications (33). While two prominent bands, corresponding to the 16S and 23S rRNA subunits, were observed on the bioanalyzer pseudogel in all samples, two additional bands were observed in only those samples obtained during the first 70 min of germination and outgrowth (see Fig. S1 in the supplemental material). One band migrated with an apparent size of approximately 2,250 nucleotides, and a second one migrated at an apparent size of 530 nucleotides. These bands, which may represent abundant transcripts or fragments of the rRNA, could not be discerned at later stages of outgrowth.
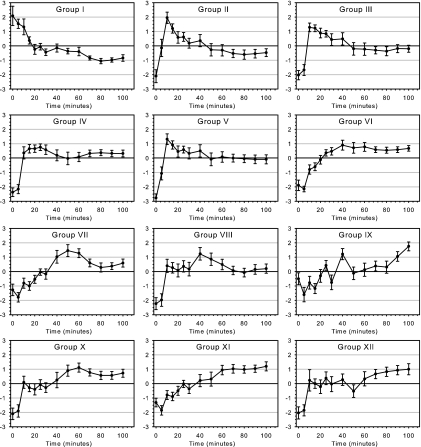
RNA isolated from germinating and outgrowing spores was used to prepare fluorescently labeled cDNA which was hybridized onto spotted 65-mer oligonucleotide slides. RNA isolated from vegetative cells was used as common reference in all experiments. After washing and scanning, fluorescence intensity data were extracted and analyzed. Since germination and outgrowth of spores are likely to coincide with changes in the rRNA/mRNA ratio and total RNA was used for labeling, exogenous RNA spike controls were used for normalization of the early-outgrowth transcription data. Gene expression was found to be highly dynamic during outgrowth of the germinated spore and was found to involve a large number of genes. Approximately 27% of all B. subtilis genes were found to be overexpressed at one ore more time points during outgrowth in comparison to the average level observed. To reveal patterns of temporal gene expression and to identify potential coregulated genes, the individual expression profiles were clustered into 12 groups of genes with similar expression profiles by K-means clustering (Fig. 2). The optimal number of groups that was used to subdivide the genes was revealed by principal-component analysis (45, 60). The groups were subsequently ordered by the timing of expression. The first (group I) consisted of approximately 23 genes, the transcripts of which were present in the dormant spores and disappeared rapidly during later stages of outgrowth. Groups II to IV consisted of approximately 350 genes, the expression of which occurred transiently between the first 5 and 40 min of outgrowth. Important household genes, encoding proteins such as elongation factors and ribosomal proteins, were found to be transcribed after approximately 10 min and showed ample variation in their level of expression during outgrowth (group IV in Fig. 2). Approximately 580 genes, subdivided into five groups, were found to be overexpressed in the time frame between 40 and 70 min following germination. Approximately 165 genes, subdivided into two groups, were found to be overexpressed at late stages of outgrowth and the initiation of vegetative growth (70 to 100 min following germination).
FIG. 2.
Gene expression profiles during spore germination and outgrowth. By K-means clustering, genes were grouped in 12 clusters according to their gene expression pattern. Plotted is the mean log2 ratio of the individual genes in the 12 K-means clusters (I to XII) at the various time points (minutes into outgrowth) over the average value. Indicated by the bars are the standard deviations of the individual genes in the 12 K-means clusters.
Functional analysis.
For a functional interpretation of the transcriptional activities during spore outgrowth, overrepresented groups of functionally related genes (gene ontology groups) were identified using JProGO (49). The individual gene ontology groups were subsequently ordered by hierarchical clustering (13). The most prominent functional categories in relation to their expression pattern will be discussed below.
Spore transcripts.
Although spores are believed to be virtually devoid of stable transcripts (1, 8, 11, 20), transcripts of approximately 23 genes were found to be present abundantly in the dormant spore (Fig. 2, group I; see Table S1 in the supplemental material). The late sporulation transcripts found in dormant spores included ykzE, ymfJ, yhcV, yqfX, ythC, ythD, and coxA and genes encoding the minor small acid-soluble proteins (SASPs) (sspN, sspO, and tlp) and the major SASP (sspE). To explore the relationship between late sporulation forespore gene expression and the spore transcript abundance, transcriptome data for dormant spores was compared with prespore late sporulation transcription data from a previous study by Steil et al. (61) (Fig. 3). It was clear that all transcripts identified in the dormant spores belonged to a distinct subclass of σG-controlled genes expressed at a late stage of prespore formation (61). In a few cases, discrepancies were found between the relative transcript level observed during sporulation and those found in spores. Examples are sspA and sspB, encoding major acid-soluble spore proteins, which displayed relative high transcript levels during sporulation but did not appear be abundant in the dormant spore. In contrast, the microarray signal intensities of transcripts of the minor SASP genes, sspN, sspH, and tlp indicated relatively abundant levels, whereas the microarray intensity levels observed during sporulation were relatively low. These observed discrepancies between spore transcript abundance from this study and prespore transcript levels reported by Steil et al. (61) may be due to differences in the sporulation conditions. However, the apparent abundance of SspA and SspB and low abundance of the minor SASPs has been reported under a number of sporulation conditions (6, 7, 30, 50). Taken together, the data suggest that the 23 spore-specific transcripts that we identified are a specific subset of forespore transcripts that are longer lived.
FIG. 3.
Comparison of microarray signal intensity levels of genes of the sigma G regulon during late sporulation, derived from reference 61, and those found in dormant spores. The scatter plot shows the microarray signal intensity values of late-sporulation transcripts of the prespore after 6.5 h of sporulation and the microarray signal intensity derived from the spore transcripts. Indicated are the early (•) and late (○) subclasses of σG, expressed at relatively early and late stages, respectively, of prespore gene expression.
The levels of the spore transcripts were found to decrease rapidly during germination and outgrowth and became undetectable after approximately 30 min into outgrowth. The presence of these spore transcripts and their disappearance during outgrowth was confirmed for all nine spore transcripts that were tested by quantitative reverse transcription-PCR (L. M. Hornstra et al., unpublished data).
In Bacillus megaterium it has been shown that material released through the breakdown of existing RNA is the major source for de novo RNA synthesis (53). These stored mRNA molecules as well as rRNA may provide the initial source of nucleotides. However, these spore transcripts may also have a more dedicated role. Stored mRNA is also found in a number of dormant biological systems, such as sporangiospores of the fungus Mucor racemosus, seeds of Arabidobsis thaliana, spores of the fern Onoclea sensibilis, and cysts (31, 36, 42, 66). In these cases, the stored mRNA is rapidly translated upon activation and germination Alternatively, the stored late-sporulation transcripts have a regulatory role, similar to the case for the small regulatory RNA molecules identified in prokaryotic and eukaryotic cells (17, 63, 71, 72). Further research is necessary to reveal the role of these RNA molecules in sporulation and germination.
Transcriptional processes during early stages of outgrowth (5 to 30 min).
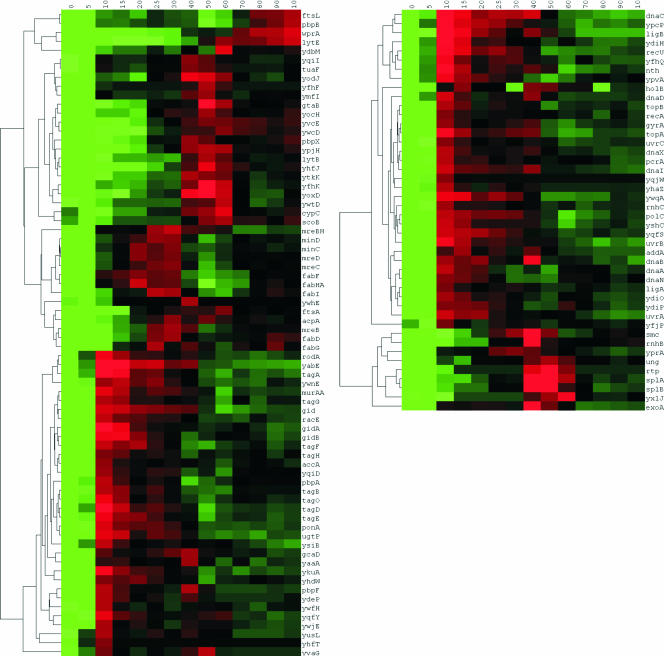
Approximately 350 genes, separated into three transcriptional groups (groups II to IV), were found to be overexpressed during the first 25 min of outgrowth (Fig. 2). During this stage of outgrowth, the germinated spore had a phase-dark round appearance (Fig. 1C). Interestingly, pbpA, encoding penicillin-binding protein 2A, was found to be overexpressed during this early outgrowth stage (group II) (Fig. 3; see Fig. 5). Disruption of this gene has been shown to delay outgrowth and to affect the efficiency of the formation of an elongated cell during outgrowth (35). A number of overrepresented gene ontology groups during early stages of outgrowth were identified (Fig. 4). These included transport functions, regulation of transcription, DNA repair, replication, and heterocycle (porphyrin) biosynthesis.
FIG. 5.
Hierarchic clustering of transcriptional profiles of genes associated with cell division, cell wall functions, and membrane biosynthesis (left) and DNA replication/repair (right). Rows represent time points from 0 to 100 min. Red and green indicate genes that are induced and repressed, respectively.
FIG. 4.
Analysis of overrepresented gene ontology (GO) groups during germination and outgrowth. Significantly overrepresented gene ontology groups are indicated in black.
Gene transcription and regulation.
A large number of genes involved in transcription and the regulation of transcription were found to be overexpressed during the early stages of outgrowth (5 to 30 min). This group included genes for the RNA polymerase sigma factors SigY and SigI and the transcription antiterminator factor NusG. A large number of transcriptional regulators were found to be overexpressed in this stage, several of which are believed to be involved in the regulation of transport. Examples are the repressors for iron (fur) and zinc (zur) uptake and manganese transport (mntR) and also azlB, encoding the repressor of the azlBCD-brnQ-yrdK operon, which has been shown to be involved in branched-chain amino acid transport (3).
Transport.
One of the earliest significantly overrepresented gene ontology groups encoded proteins involved in transport of various molecules. This group included putative multidrug transporters, ABC transporters, and Na+/H+ antiporters. The encoded transporters are devoted to the transport of ions, amino acids, sugars, and other organic compounds (multidrug transporters). While most transport genes were activated after approximately 10 min of outgrowth (groups III to IV), a few genes were expressed already after 5 minutes of outgrowth (group II). Examples of these early activated genes are the putative copper transporter-encoding operon (yvgZYXW), the putative zinc ABC transporter genes ycdHI-yceA, the arsenate resistance genes arsBC, the yuaA potassium transporter gene, and the mntH manganese transporter gene. In all cases, expression showed a maximum at around 10 minutes after the onset of germination and diminished at later times.
The immediate initiation of transport functions is likely to be necessary to rapidly supply the germinated spore with the essential elements (cofactors and metabolites) for efficient outgrowth. The central role of transporters during germination and outgrowth has been illustrated by the observation that during early stages of germination, a large efflux of cations (H+, K+, Na+, and Zn+) occurs. Potassium ions have been shown to be subsequently reabsorbed in an energy-dependent and likely transcription-dependent process (65). This process may depend on the ykrM (ktrD) and yuaA (ktrA) K+ transporter genes, which were found to be expressed in this early stage of outgrowth. In vegetative cells, KtrA and KtrD have been suggested to play a role in the defense against osmotic stress (25). The simultaneous expression of the glycine betaine transport protein OpuAB may suggest that osmotic defense is important in the earliest stages of outgrowth.
Active export during early stages of outgrowth may also provide the germinated spore with a transient resistance against antimicrobial complexes. A large number of transporter genes encoded putative multidrug transporters, such as lmrB, yttB, yubD, yfhI, ykuC, and ydgH, which were overexpressed during the first 20 min of outgrowth.
DNA repair, replication, and RNA modification.
From the analysis of overrepresented gene ontology terms it was suggested that DNA replication and DNA repair functions are overrepresented during the early stages of outgrowth (5 to 25 min) (Fig. 4 and 5B). Upon close inspection of the expression of the DNA repair genes, it was found that expression occurred during two distinct stages during outgrowth (Fig. 5, bottom). During early stages of outgrowth the nucleotide excision repair enzyme genes uvrAB, the base excision repair enzyme genes yqfS and nth, the 5′-3′ exonuclease homolog gene ypcP, and the helixase-exonuclease genes addAB were found to be overexpressed. During a later outgrowth stage (40 and 50 min following the onset of germination (group IX)) the base excision repair enzyme genes exoA, the spore photoproduct lyase genes splAB, the uracil-DNA glycosylase gene ung, and the putative DNA-3-methyladenine glycosidase gene yxlJ were found to be overexpressed. This second stage of DNA repair is discussed below.
DNA of dormant spores is extremely well protected against damage resulting from heat, oxidizing agents, and UV. Passive protection mechanisms include the low permeability of spores to toxic chemicals and the decreased spore core water content. Importantly, the abundant α/β-type small acid-soluble spore proteins saturate the negatively supercoiled spore DNA, reducing the DNA's chemical and enzymatic reactivity and changing its UV photochemistry (38).
A number of DNA repair genes are expressed actively during sporulation and are likely to exert their function upon spore germination. Nucleotide excision repair, the spore photoproduct lyase, and more recently also base excision repair have been shown to be involved in the protection of spores against damaging UV radiation and wet heat resistance (38). The involvement of recombinational repair and SOS-mediated repair systems in the protection of spores has remained unclear.
Expression of (spore-specific) DNA repair genes during outgrowth may be part of an intrinsic outgrowth gene expression program and may enforce the activity of DNA repair enzymes produced during sporulation. Expression of DNA repair enzymes during sporulation may not under all conditions provide the spore with sufficient protection. The de novo expression of DNA repair enzymes during outgrowth may be essential for the recovery of sublethally damaged spores.
The ATP dependent helicase genes ypvA and pcrA and the SNF2-like helicase gene ywqA were found to be overexpressed during early stages (10 to 30 min) of outgrowth (Fig. 5, bottom). DNA of dormant spores is believed to be in a supercoiled state, providing protection against damage (15, 39). During outgrowth, the chromosomal DNA is required to relax rapidly to allow an efficient reactivation of transcription. Relaxation of the supercoiled DNA is accomplished partly by the degradation of the SASPs that coat the supercoiled DNA (43). Increased helicase activity during early stages of outgrowth may be necessary to complete the unwinding of the negatively coiled spore DNA and may also be important for DNA repair.
A prominent group of genes that were overexpressed between 25 and 30 min into outgrowth encoded proteins involved in modification of RNA. This group of genes included the 5S rRNA maturase gene rnmV; the putative RNA methylase genes ybxB, cspR, and ypsC; the putative tRNA methyltransferase gene trmU, and the ykvJKM operon, which is involved in queuosine synthesis (46). The function of queuosinilation of specific tRNA molecules is not fully known, but experimental evidence suggests a regulatory effect of the queuosine content of specific tRNAs by influencing their affinity for wobble codons (for a review, see reference 59). Interestingly, in conjunction with the expression of the RNA-modifying enzymes, a small shift was observed in the motility of the 23S and 16S rRNA subunits in samples obtained after 30 min into outgrowth (see Fig. S1 in the supplemental material).
Twenty-five to 50 min into outgrowth (groups VI to VIII).
Approximately 440 genes, separated into three transcriptional groups (groups VI, VII, and VIII), were found to be overexpressed at between 25 and 60 min of outgrowth (Fig. 2). During this stage of outgrowth, the germinated spore underwent the first round of DNA replication, as suggested by the occurrence of DNA segregation at t = 70 (Fig. 1C) and an average time for DNA replication of 40 min (56). The outgrowing spore had a round/oval appearance and increased in size (Fig. 1C). From a transcriptional point of view, this time frame appears to be the next phase in outgrowth. Cells appear to prepare for lateral wall and cell membrane expansion and cell division. During this stage of outgrowth, the expression of a number of general stress response genes was observed as well (group VII in Fig. 2).
Cell growth and cell division.
Following the early stage of outgrowth (5 to 25 min), the functional characteristics of the genes overexpressed at this point suggest that the outgrowing spore prepares for cylindrical growth, chromosomal segregation, and cell division. Cell cycle- and cell division-associated genes, such as the mreBHCD and minCD genes, as well as fatty acid biosynthesis genes are overexpressed during this stage (Fig. 4 and 5A). Also the spo0J-like gene yaaA and the smc gene, encoding the chromosome condensation and segregation protein, were overexpressed in this time frame (18, 57, 64). However, it was not until 70 min into outgrowth that fully segregated chromosomes became apparent. This coincided with the bursting of the round germinated spore from the spore coat remnants and the formation of an elongated cell type (Fig. 1C).
Second stage of DNA repair.
As indicated above, the transcriptional activation of DNA repair genes occurred in two time frames: one between 10 and 30 min and one between 40 and 50 min of outgrowth (Fig. 5, bottom). Genes encoding DNA repair enzymes that were expressed in the time frame between 40 and 50 min include the base excision repair enzyme gene exoA, the spore photoproduct lyase genes splAB, the uracil-DNA glycosylase gene ung, and the putative DNA-3-methyladenine glycosidase gene yxlJ. Strikingly, rtp, encoding the replication termination protein, and the smc chromosome condensation and segregation gene were found to be coexpresssed with splAB and exoA. Perhaps this suggests that DNA repair during this stage of outgrowth is coupled to the initial round of replication.
The expression of splAB is surprising since during sporulation transcription is driven by the prespore-specific sigma G-dependent RNA polymerase (41). SplB is involved in the repair of spore photoproduct, a spore-specific type of DNA damage, and is known to be expressed during sporulation in a sigma G-dependent manner. The TRAP-like SplA protein is a trans-acting negative regulator of spore photoproduct lyase synthesis during Bacillus subtilis sporulation (14). The splAB operon was found to be coexpressed with exoA and rtp during a narrow time frame between 40 and 50 min of outgrowth, prior to the occurrence of chromosomal separation. Whether sigma G plays a role in the expression of splAB during outgrowth remains to be determined.
Do extracellular proteases play a role in the unzipping of the spore coat?
The ykwD and ykoJ genes were found to be overexpressed transiently between 30 and 60 min of outgrowth. Both genes encode proteins with an N-terminal signal peptide, suggesting extracellular activity. The ykwD gene product carries an SCP domain, which has been proposed to be a Ca2+-chelating serine protease. ykoJ carries two PepSY protease domains. The PepSY domain has been shown to have a role in regulating the activity of a number of proteases. The domain is also found in the YpeB protein, a regulator of SleB spore cortex lytic enzyme (74). Whether ykoJ has a similar role in the regulation of the coexpressed ykwD remains to be determined.
Activation of the general stress response.
A prominent group overexpressed after 40 to 50 min of outgrowth was the general stress sigma B regulon. It is not clear what stress factor triggered activation of sigma B. During the experiment, no changes in temperature, aeration, or pH occurred. Furthermore, at the moment the sigma B stress occurred, approximately 10 mM glucose was still present in the complex growth medium, excluding carbon or energy depletion as a likely cause for the stress response. Transient expression of the general stress response coincided with a transient decrease in the expression of essential genes encoding proteins such as elongation factors and ribosomal proteins (Fig. 4).
Fifty to 80 min into outgrowth.
In the time frame between 50 and 80 min into outgrowth, the outgrown spores burst out of an outer spore layer, possible remnants of the spore coat (Fig. 1C). Caps of spore coat fragments often remained attached to the polar ends of the dividing cell, as has been observed previously by electron microscopy, (48). The overall cell morphology changed from a round cell to an elongated cell. The burst of the germinated spore from the spore coat remnants coincided with the near completion of the first round of chromosomal replication, as indicated by the segregation of the two daughter chromosomes (Fig. 1C). After approximately 50 min of outgrowth, the purine biosynthetic cluster (pur) was activated. Specific for this event is the overexpression of the yrhDE operons and yrhG. The putative yxeKLMNOPQR operon encodes a putative monooxygenase and components of an ABC transporter which may be involved in the translocation of a polar amino acid. The specific roles of these gene products during outgrowth remain to be investigated.
Eighty to 100 min into outgrowth (groups XI and XII).
In the time frame between 80 and 100 min, the sigma D regulon appears to be activated and changes in the medium are a major attribute of changes in gene expression. Activation of the sigma D regulon was revealed by the transcriptional activation of motility and septation genes during this phase. This was confirmed by results obtained by direct microscopy of cells following this stage which were motile (data not shown). In addition, the overrepresented gene ontology groups included groups associated with sulfur amino acid, aspartate, and serine metabolism; glycolysis; and the tricarboxylic acid cycle (Fig. 4). The metabolic change was also indicated by the sudden drop in extracellular pyruvate levels after 90 min of outgrowth (Fig. 1B). Analysis showed that purine biosynthetic genes were activated during late outgrowth stages (group XI) (Fig. 2 and 4). In contrast, pyrimidine biosynthetic genes were activated in three waves, with maximum levels of expression at 10, 50, and 90 min. The enhanced expression of metabolic routes during late stages of outgrowth may reflect changes in the medium and a depletion of the initial sources for amino acids or a metabolic change. During late stages of outgrowth, the cells appeared to prepare for septation, as indicated by the overexpression of the septation genes ftsL and pbpB (Fig. 5, top). Expression of ftsL and pbpB coincided with the penicillin-binding protein 3 gene pbpC, the peptidoglycan hydrolase gene lytE, and the cell wall-bound protease gene wprA. Activity of WprA is believed to be necessary for the correct localization of LytF and septation (73). With the activation of cell septation, the transition of the dormant spore to an actively growing vegetative cell appears to be completed.
Concluding remarks.
This study has provided a detailed view on physiological processes that occur during the process of spore germination and outgrowth through the application of a combination of microscopy and analysis of metabolites and genome-wide expression. An important question following this work is what the relation is between the observed gene expression events during outgrowth and cellular activities. In prokaryotes, transcriptional regulation is the main mechanism for an adaptive response, often relating directly to cellular activity. However, to what extent this is also true for the transcriptional events observed during spore outgrowth will need to be established during future functional studies. Furthermore, it is important to take into account the composition of the dormant spore. Proteome studies of dormant spores have revealed not only spore-specific proteins, such as structural components of the spore coat, but also a large number of proteins essential for growth, such as proteins involved in protein synthesis, metabolism, transport, secretion, etc. (30, 32). In Bacillus anthracis some of these proteins are expressed specifically late during sporulation, suggesting a loading mechanism that equips the spore with enzymes that may be essential in the early events during germination and outgrowth (32). Nevertheless, active, transcription-dependent protein synthesis is an absolute requirement for the outgrowth of B. subtilis spores, as has been demonstrated by the effects of transcription- and translation-inhibiting antibiotics on germination and outgrowth (44, 62). Key transcriptional events during the outgrowth of the germinated spore were identified, which often coincided with important steps in the process of outgrowth, suggesting a direct correlation between transcription and cellular activity. The mechanism for relating the progression of outgrowth to the transcriptional events remains to be elucidated. It seems apparent that a well-developed regulatory mechanism must exist for the appropriate integration of the important physiological events during germination and outgrowth. Checkpoins may rely on DNA integrity, metabolic status, and the breakdown of the spore's protective layers. We hope that the work presented here provides a framework for the understanding of the regulation and integration of various physiological, morphological, and transcriptional stages during the transition of the dormant spore to a vegetative cell.
ADDENDUM
After submission of this paper, Bettegowda et al. (4) reported the finding that dormant Clostridium novyi spores contained mRNA, as has been shown in this paper for B, subtilis spores. While 60% of the Clostridium novyi spore mRNAs had no known function, many others were predicted to encode proteins with redox activity. Proteins encoded by the abundant spore transcripts identified in B. subtilis spores showed little homology with those found in C. novyi. Despite these differences, the finding of abundant levels of mRNA in B. subtilis as well as C. novyi spores may indicate that spore mRNA is a common component of bacterial spores. Perhaps these spore-specific transcripts may be used in molecular diagnostics to rapidly discriminate between dormant spores and vegetative cells.
Supplementary Material
Acknowledgments
This work was financially supported through the Ecology, Economy and Technology program (EET), which is a joint program of the Dutch Ministry of Economic Affairs; the Ministry of Education, Culture and Science; and the Ministry of Housing, Spatial Planning and the Environment.
We thank Jurgo Verkooijen and Tessa Dillerop-van der Hoeven of the Microarray Department of the University of Amsterdam for their excellent technical assistance. We thank Anne Moir, Peter Setlow, and Remco Kort for fruitful discussions on the manuscript.
Footnotes
Published ahead of print on 23 February 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Armstrong, R. L., and N. Sueoka. 1968. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc. Natl. Acad. Sci. USA 59:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner, M., C. A. Ball, J. A. Blake, D. Botstein, H. Butler, J. M. Cherry, A. P. Davis, K. Dolinski, S. S. Dwight, J. T. Eppig, M. A. Harris, D. P. Hill, L. Issel-Tarver, A. Kasarskis, S. Lewis, J. C. Matese, J. E. Richardson, M. Ringwald, G. M. Rubin, G. Sherlock, et al. 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belitsky, B. R., M. C. Gustafsson, A. L. Sonenshein, and W. C. Von. 1997. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J. Bacteriol. 179:5448-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettegowda, C., X. Huang, J. Lin, I. Cheong, M. Kohli, S. A. Szabo, X. Zhang, L. A. Diaz, Jr., V. E. Velculescu, G. Parmigiani, K. W. Kinzler, B. Vogelstein, and S. Zhou. 2006. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat. Biotechnol. 24:1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencke, H. M., G. Homuth, H. Ludwig, U. Mader, M. Hecker, and J. Stulke. 2003. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5:133-149. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Hernandez, A., J. L. Sanchez-Salas, M. Paidhungat, and P. Setlow. 1999. Regulation of four genes encoding small, acid-soluble spore proteins in Bacillus subtilis. Gene 232:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera-Hernandez, A., and P. Setlow. 2000. Analysis of the regulation and function of five genes encoding small, acid-soluble spore proteins of Bacillus subtilis. Gene 248:169-181. [DOI] [PubMed] [Google Scholar]
- 8.Chambon, P., M. P. Deutscher, and A. Kornberg. 1968. Biochemical studies of bacterial sporulation and germination. X. Ribosomes and nucleic acids of vegetative cells and spores of Bacillus megaterium. J. Biol. Chem. 243:5110-5116. [PubMed] [Google Scholar]
- 9.Dauner, M., T. Storni, and U. Sauer. 2001. Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J. Bacteriol. 183:7308-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Den Blaauwen, T., N. Buddelmeijer, M. E. Aarsman, C. M. Hameete, and N. Nanninga. 1999. Timing of FtsZ assembly in Escherichia coli. J. Bacteriol. 181:5167-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi, R. H., and R. T. Igarashi. 1964. Ribonucleic acids of Bacillus subtilis spores and sporulating cells. J. Bacteriol. 87:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fajardo-Cavazos, P., and W. L. Nicholson. 2000. The TRAP-like SplA protein is a trans-acting negative regulator of spore photoproduct lyase synthesis during Bacillus subtilis sporulation. J. Bacteriol. 182:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenkiel-Krispin, D., R. Sack, J. Englander, E. Shimoni, M. Eisenstein, E. Bullitt, R. Horowitz-Scherer, C. S. Hayes, P. Setlow, A. Minsky, and S. G. Wolf. 2004. Structure of the DNA-SspC complex: implications for DNA packaging, protection, and repair in bacterial spores. J. Bacteriol. 186:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrick-Silversmith, L., and A. Torriani. 1973. Macromolecular syntheses during germination and outgrowth of Bacillus subtilis spores. J. Bacteriol. 114:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman, S., G. Storz, C. Rosenow, N. Majdalani, F. Repoila, and K. M. Wassarman. 2001. Small RNA regulators of translation: mechanisms of action and approaches for identifying new small RNAs. Cold Spring Harb. Symp. Quant. Biol. 66:353-362. [DOI] [PubMed] [Google Scholar]
- 18.Graumann, P. L. 2001. SMC proteins in bacteria: condensation motors for chromosome segregation? Biochimie 83:53-59. [DOI] [PubMed] [Google Scholar]
- 19.Hageman, J. H., G. W. Shankweiler, P. R. Wall, K. Franich, G. W. McCowan, S. M. Cauble, J. Grajeda, and C. Quinones. 1984. Single, chemically defined sporulation medium for Bacillus subtilis: growth, sporulation, and extracellular protease production. J. Bacteriol. 160:438-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen, J. N., G. Spiegelman, and H. O. Halvorson. 1970. Bacterial spore outgrowth: its regulation. Science 168:1291-1298. [DOI] [PubMed] [Google Scholar]
- 21.Hecker, M. 1983. Molecular biology of the germination of Bacillus spores. Z. Allg. Mikrobiol. 23:517-535. [DOI] [PubMed] [Google Scholar]
- 22.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano, Y., M. Matsuda, and T. Kameyama. 1991. Two-dimensional polyacrylamide gel electrophoresis of proteins synthesized during early germination of Bacillus subtilis 168 in the presence of actinomycin D. J. Basic Microbiol. 31:429-436. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg, Y., and Y. Benjamini. 1990. More powerful procedures for multiple significance testing. Stat. Med. 9:811-818. [DOI] [PubMed] [Google Scholar]
- 25.Holtmann, G., E. P. Bakker, N. Uozumi, and E. Bremer. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185:1289-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horsburgh, M. J., P. D. Thackray, and A. Moir. 2001. Transcriptional responses during outgrowth of Bacillus subtilis endospores. Microbiology 147:2933-2941. [DOI] [PubMed] [Google Scholar]
- 27.Hu, P., T. Leighton, G. Ishkhanova, and S. Kustu. 1999. Sensing of nitrogen limitation by Bacillus subtilis: comparison to enteric bacteria. J. Bacteriol. 181:5042-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, C. M., K. W. Foster, T. S. DeSilva, K. R. Van Kampen, C. A. Elmets, and D. C. Tang. 2004. Identification of Bacillus anthracis proteins associated with germination and early outgrowth by proteomic profiling of anthrax spores. Proteomics 4:2653-2661. [DOI] [PubMed] [Google Scholar]
- 29.Kort, R., A. C. O'Brien, S. van Stokkum, I. S. J. Oomes, W. Crielaard, K. J. Hellingwerf, and S. Brul. 2005. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl. Environ. Microbiol. 71:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuwana, R., Y. Kasahara, M. Fujibayashi, H. Takamatsu, N. Ogasawara, and K. Watabe. 2002. Proteomics characterization of novel spore proteins of Bacillus subtilis. Microbiology 148:3971-3982. [DOI] [PubMed] [Google Scholar]
- 31.Linz, J. E., and M. Orlowski. 1982. Stored mRNA in sporangiospores of the fungus Mucor racemosus. J. Bacteriol. 150:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. A. Rasko, J. Ravel, T. D. Read, S. N. Peterson, J. Yates III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186:164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller, R., G. Horneck, P. Rettberg, H. J. Mollenkopf, E. Stackebrandt, and W. L. Nicholson. 2006. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr. Microbiol. 53:227-231. [DOI] [PubMed] [Google Scholar]
- 34.Moir, A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526-530. [DOI] [PubMed] [Google Scholar]
- 35.Murray, T., D. L. Popham, and P. Setlow. 1997. Identification and characterization of pbpA encoding Bacillus subtilis penicillin-binding protein 2A. J. Bacteriol. 179:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakabayashi, K., M. Okamoto, T. Koshiba, Y. Kamiya, and E. Nambara. 2005. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J. 41:697-709. [DOI] [PubMed] [Google Scholar]
- 37.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, W. L., and P. Setlow. 1990. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J. Bacteriol. 172:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons Ltd, Chichester, United Kingdom.
- 41.Pedraza-Reyes, M., F. Gutierrez-Corona, and W. L. Nicholson. 1997. Spore photoproduct lyase operon (splAB) regulation during Bacillus subtilis sporulation: modulation of splB-lacZ fusion expression by P1 promoter mutations and by an in-frame deletion of splA. Curr. Microbiol. 34:133-137. [DOI] [PubMed] [Google Scholar]
- 42.Raghavan, V. 1991. Gene activity during germination of spores of the fern, Onoclea sensibilis: RNA and protein synthesis and the role of stored mRNA. J. Exp. Bot. 42:251-260. [DOI] [PubMed] [Google Scholar]
- 43.Ragkousi, K., A. E. Cowan, M. A. Ross, and P. Setlow. 2000. Analysis of nucleoid morphology during germination and outgrowth of spores of Bacillus species. J. Bacteriol. 182:5556-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rana, R. S., and H. O. Halvorson. 1972. Nature of deoxyribonucleic acid synthesis and its relationship to protein synthesis during outgrowth of Bacillus cereus T. J. Bacteriol. 109:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raychaudhuri, S., J. M. Stuart, and R. B. Altman. 2000. Principal components analysis to summarize microarray experiments: application to sporulation time series. Pac. Symp. Biocomput. 455-466. [DOI] [PMC free article] [PubMed]
- 46.Reader, J. S., D. Metzgar, P. Schimmel, and V. Crecy-Lagard. 2004. Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. J. Biol. Chem. 279:6280-6285. [DOI] [PubMed] [Google Scholar]
- 47.Saeed, A. I., V. Sharov, J. White, J. Li, W. Liang, N. Bhagabati, J. Braisted, M. Klapa, T. Currier, M. Thiagarajan, A. Sturn, M. Snuffin, A. Rezantsev, D. Popov, A. Ryltsov, E. Kostukovich, I. Borisovsky, Z. Liu, A. Vinsavich, V. Trush, and J. Quackenbush. 2003. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34:374-378. [DOI] [PubMed] [Google Scholar]
- 48.Santo, L. Y., and R. H. Doi. 1974. Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J. Bacteriol. 120:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheer, M., F. Klawonn, R. Munch, A. Grote, K. Hiller, C. Choi, I. Koch, M. Schobert, E. Hartig, U. Klages, and D. Jahn. 2006. JProGO: a novel tool for the functional interpretation of prokaryotic microarray data using Gene Ontology information. Nucleic Acids Res. 34:W510-W515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Setlow, B., K. A. McGinnis, K. Ragkousi, and P. Setlow. 2000. Effects of major spore-specific DNA binding proteins on Bacillus subtilis sporulation and spore properties. J. Bacteriol. 182:6906-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setlow, P. 1975. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J. Biol. Chem. 250:631-637. [PubMed] [Google Scholar]
- 52.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 53.Setlow, P., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. 23. Nucleotide metabolism during spore germination. J. Biol. Chem. 245:3645-3652. [PubMed] [Google Scholar]
- 54.Setlow, P., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J. Biol. Chem. 245:3637-3644. [PubMed] [Google Scholar]
- 55.Setlow, P., and G. Primus. 1975. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J. Biol. Chem. 250:623-630. [PubMed] [Google Scholar]
- 56.Sharpe, M. E., P. M. Hauser, R. G. Sharpe, and J. Errington. 1998. Bacillus subtilis cell cycle as studied by fluorescence microscopy: constancy of cell length at initiation of DNA replication and evidence for active nucleoid partitioning. J. Bacteriol. 180:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sievers, J., B. Raether, M. Perego, and J. Errington. 2002. Characterization of the parB-like yyaA gene of Bacillus subtilis. J. Bacteriol. 184:1102-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh, R. P., and P. Setlow. 1979. Regulation of phosphoglycerate phosphomutase in developing forespores and dormant and germinated spores of Bacillus megaterium by the level of free manganous ions. J. Bacteriol. 139:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slany, R. K., and H. Kersten. 1994. Genes, enzymes and coenzymes of queuosine biosynthesis in procaryotes. Biochimie 76:1178-1182. [DOI] [PubMed] [Google Scholar]
- 60.Soukas, A., P. Cohen, N. D. Socci, and J. M. Friedman. 2000. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 14:963-980. [PMC free article] [PubMed] [Google Scholar]
- 61.Steil, L., M. Serrano, A. O. Henriques, and U. Volker. 2005. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology 151:399-420. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg, W., H. O. Halvorson, A. keynan, and E. weinberg. 1965. Timing of protein synthesis during germination and outgrowth of spores of Bacillus cereus strain T. Nature 208:710-711. [Google Scholar]
- 63.Storz, G., S. Altuvia, and K. M. Wassarman. 2005. An abundance of RNA regulators. Annu. Rev. Biochem. 74:199-217. [DOI] [PubMed] [Google Scholar]
- 64.Strunnikov, A. V., and R. Jessberger. 1999. Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur. J. Biochem. 263:6-13. [DOI] [PubMed] [Google Scholar]
- 65.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J. Bacteriol. 148:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tourancheau, A. B., L. Morin, T. Yang, and R. Perasso. 1999. Messenger RNA in dormant cells of Sterkiella histriomuscorum (Oxytrichiade): indentification of putative regulatory gene transcripts. Protist 150:137-147. [DOI] [PubMed] [Google Scholar]
- 67.Troyanskaya, O., M. Cantor, G. Sherlock, P. Brown, T. Hastie, R. Tibshirani, D. Botstein, and R. B. Altman. 2001. Missing value estimation methods for DNA microarrays. Bioinformatics 17:520-525. [DOI] [PubMed] [Google Scholar]
- 68.Van Helvoort, J. M., P. G. Huls, N. O. Vischer, and C. L. Woldringh. 1998. Fused nucleoids resegregate faster than cell elongation in Escherichia coli pbpB(Ts) filaments after release from chloramphenicol inhibition. Microbiology 144:1309-1317. [DOI] [PubMed] [Google Scholar]
- 69.Vaquerizas, J. M., L. Conde, P. Yankilevich, A. Cabezon, P. Minguez, R. Diaz-Uriarte, F. Al Shahrour, J. Herrero, and J. Dopazo. 2005. GEPAS, an experiment-oriented pipeline for the analysis of microarray gene expression data. Nucleic Acids Res. 33:W616-W620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wachlin, G., and M. Hecker. 1982. Activation of protein biosynthesis in outgrowing spores of Bacillus subtilis. Z. Allg. Mikrobiol. 22:495-502. [DOI] [PubMed] [Google Scholar]
- 71.Wassarman, K. M. 2002. Small RNAs in bacteria: diverse regulators of gene expression in response to environmental changes. Cell 109:141-144. [DOI] [PubMed] [Google Scholar]
- 72.Wassarman, K. M., A. Zhang, and G. Storz. 1999. Small RNAs in Escherichia coli. Trends Microbiol. 7:37-45. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto, H., S. Kurosawa, and J. Sekiguchi. 2003. Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and stability of these enzymes to cell wall-bound or extracellular proteases. J. Bacteriol. 185:6666-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeats, C., N. D. Rawlings, and A. Bateman. 2004. The PepSY domain: a regulator of peptidase activity in the microbial environment? Trends Biochem. Sci. 29:169-172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.