Abstract
GGDEF and EAL domain proteins are involved in turnover of the novel secondary messenger cyclic di(3′→5′)-guanylic acid (c-di-GMP) in many bacteria. The rdar morphotype, a multicellular behavior of Salmonella enterica serovar Typhimurium characterized by the expression of the extracellular matrix components cellulose and curli fimbriae is controlled by c-di-GMP. In this work the roles of the EAL and GGDEF-EAL domain proteins on rdar morphotype development were investigated. Knockout of four of 15 EAL and GGDEF-EAL domain proteins upregulated rdar morphotype expression and expression of CsgD, the central regulator of the rdar morphotype, and partially downregulated c-di-GMP concentrations. More-detailed analysis showed that the EAL domain protein STM4264 and the GGDEF-EAL domain protein STM1703, which highly downregulated the rdar morphotype, have overlapping yet distinct functions. Another subset of EAL and GGDEF-EAL domain proteins influenced multicellular behavior in liquid culture and flagellum-mediated motility. Consequently, this work has shown that several EAL and GGDEF-EAL domain proteins, which act as phosphodiesterases, play a determinative role in the expression level of multicellular behavior of Salmonella enterica serovar Typhimurium.
Present evidence suggests that conventional GGDEF and EAL domain proteins, belonging to two of the most abundant superfamilies of proteins in members of the domain Bacteria, are dedicated to the synthesis and degradation of cyclic di(3′→5′)-guanylic acid (c-di-GMP), a novel secondary messenger (5, 27, 30, 39, 40, 44). Thereby, the diguanylate cyclase and phosphodiesterase activity of GGDEF and EAL domain proteins are regulated by environmental cues, such as oxygen (4, 19) and intra- and intercellular signaling pathways, such as phosphotransfer, starvation sensing through GTP, and spermidine (5, 21, 27, 39). Whole-genome sequencing uncovered the abundance of GGDEF and EAL domain proteins encoded by even a single bacterial genome (9). Snapshots of the function of individual GGDEF/EAL domain proteins showed that c-di-GMP signaling affects a variety of cellular processes from photosynthesis over biofilm formation and motility to virulence (6, 17, 33, 42, 45, 46). It has been well established that there is a positive correlation between the c-di-GMP concentration and bacterial phenotypes, such as sessility, biofilm formation, and the expression of adhesive extracellular matrix components, while there is a negative correlation between the c-di-GMP concentration and bacterial phenotypes, such as motility and virulence (3, 30, 42, 46). However, in contrast to overexpression studies, knockout of GGDEF domain proteins only occasionally showed a scorable phenotype, such as the activation of cellulose biosynthesis (10, 35) and expression of CsgD (20, 47). This suggests that the activity and/or expression of GGDEF/EAL domain proteins is to be strictly temporally regulated and/or highly spatially compartmentalized. In addition, redundancy of function can make the effects of knockout of individual GGDEF/EAL domain proteins to be subtle, since c-di-GMP signaling by individual GGDEF/EAL domain proteins affects the response to environmental cues, timing, and amplitude of the behavior but does not interfere with the basic mechanism of complex biological processes (14, 18, 20, 24, 45).
A multicellular morphotype in Salmonella enterica serovar Typhimurium, the rdar morphotype, is characterized by the expression of extracellular matrix components: the exopolysaccharide cellulose, adhesive curli fimbriae, and the large surface protein BapA (25, 31, 48). The rdar morphotype displays a red, dry, and rough colony morphology on Congo red (CR) agar plates (28) (Fig. 1). Expression of individual extracellular matrix components leads to the development of distinct colony morphology types. Expression of cellulose causes the development of a pink colony (pdar morphotype [Fig. 1]), while the expression of curli fimbriae triggers the development of a brown colony (bdar morphotype [Fig. 1]).
FIG. 1.
Colony morphology prototypes of Salmonella enterica serovar Typhimurium ATCC 14028 as described in the text. An example of a semiconstitutive rdar morphotype (MAE52 [UMR1 PcsgD1]), a regulated rdar morphotype (UMR1 [wild type]), a pdar morphotype (MAE97 [MAE52 ΔcsgBA102]) that is cellulose positive, a bdar morphotype (MAE171 [MAE52 ΔbcsA102]) with curli fimbriae, and a saw morphotype (MAE51 [UMR1 PcsgD2 ΔcsgD101]) that is negative for cellulose and curli fimbriae are shown. Cells were grown on CR agar plates at 28°C for 48 h.
Required for the expression of extracellular matrix components is the LuxR-type transcriptional regulator CsgD, the master regulator of the rdar morphotype (12). CsgD expression is regulated by a variety of environmental cues, global regulatory proteins, and c-di-GMP signaling (11, 13, 20, 47). In Salmonella serovar Typhimurium, the GGDEF-EAL domain proteins STM2123 and STM3388, 2 of 20 GGDEF/EAL domain proteins are responsible for ∼60% of the CsgD expression on a transcriptional and posttranscriptional level (20). On the other hand, CsgD regulates the transcription of the diguanylate cyclase AdrA (35, 42). AdrA in turn is responsible for the activation of cellulose biosynthesis on a posttranscriptional level and partially enhanced curli fimbria expression downstream of CsgD expression (20, 48).
Positive-feedback regulation of CsgD expression by AdrA-produced c-di-GMP does not occur in the wild-type strain Salmonella serovar Typhimurium UMR1, which expresses a highly regulated rdar morphotype only at ambient temperature (Fig. 1). However, in MAE52, a csgD promoter mutant with threefold-enhanced CsgD expression, which is temperature independent (36), positive-feedback regulation was observed (20).
Since CsgD is not required for c-di-GMP-regulated cellulose activation (48), cellulose biosynthesis can be uncoupled from CsgD expression. In a starvation medium, which does not support the growth of Salmonella serovar Typhimurium, the GGDEF domain protein STM1987 activated cellulose biosynthesis independent of CsgD (10). However, not only multicellular behavior and biofilm formation are regulated by c-di-GMP signaling in Salmonella serovar Typhimurium. STM1344, an EAL-like protein, is required for bacterial virulence in mice through withstanding oxygen-dependent killing and for macrophage cytotoxicity (17). Knockout of the EAL domain protein YhjH (STM3611) reduced swarming motility (8, 24, 42) and affected growth competition between different strains of S. enterica (37).
The outcome of c-di-GMP signaling is controlled by the synthesis and degradation of c-di-GMP (42). Previously, the role of diguanylate cyclases on multicellular behavior in Salmonella serovar Typhimurium has been investigated (20). However, the contribution of c-di-GMP-dependent phosphodiesterases to rdar morphotype expression is not clear. Therefore, in this work, the impact of the 15 chromosomally encoded EAL and GGDEF-EAL domain proteins, which presumably act as phosphodiesterases, was investigated. Knockout studies demonstrated functionally overlapping groups of EAL and GGDEF-EAL domain proteins that influenced rdar morphotype expression, biofilm formation in liquid culture, pellicle formation, and swimming and swarming motility. Importantly, the expression of phenotypes was controlled by the expression of a distinct EAL or GGDEF-EAL domain protein(s), which acts as a phosphodiesterase, while as previously shown, an individual diguanylate cyclase rarely leads to the significant alteration of a phenotype (20). This finding showed that in Salmonella serovar Typhimurium, the intracellular c-di-GMP signaling controlling multicellular behavior is determined mainly by the activity of phosphodiesterases. Most pronounced, expression of CsgD, the master regulator of rdar morphotype expression, is affected by four EAL and GGDEF-EAL domain proteins. In accordance with apparent phosphodiesterase activity in vivo, the absence of STM1703 and STM4264 significantly activated CsgD expression whereby STM1703 particularly was also required for temperature regulation of the rdar morphotype.
(This work was presented in part at the 158th Annual Conference of the Society for General Microbiology in Warwick, England; the 106th General Meeting of the American Society for Microbiology in Orlando, FL; and the ESF-EMBO Symposium Bacterial Networks 2006 in Sant Feliu de Guixols, Spain.)
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. For cloning purposes, Escherichia coli and Salmonella enterica serovar Typhimurium were grown on Luria-Bertani (LB) agar plates supplemented with the appropriate antibiotics. To carry out experiments, bacteria were precultured on LB agar plates at 37°C overnight and directly inoculated onto LB agar plates without salt. Alternatively, bacteria were resuspended in water, and the optical density at 600 nm (OD600) was adjusted. The antibiotics used were ampicillin (100 μg ml−1), chloramphenicol (20 μg ml−1), kanamycin (30 μg ml−1), and tetracycline (20 μg ml−1). For expression of genes, 0.1% arabinose or 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or descriptiona | Reference or source |
|---|---|---|
| Bacterial strains | ||
| Salmonella serovar Typhimurium ATCC 14028 | ||
| UMR1 | ATCC 14028-1s Nalr | 31 |
| MAE32 | UMR1 PcsgD2 | 36 |
| MAE50 | UMR1 ΔcsgD101 | 35 |
| MAE51 | MAE32 ΔcsgD101 | 35 |
| MAE52 | UMR1 PcsgD1 | 36 |
| MAE97 | MAE52 ΔcsgBA102 | 35 |
| MAE171 | MAE52 ΔbcsA102 | 48 |
| MAE120 | UMR1 STM3615::Kmr | 20 |
| MAE121 | UMR1 STM3388::Kmr | 20 |
| MAE258 | UMR1 STM1283::Cmr | 20 |
| MAE259 | UMR1 STM1987::Cmr | 20 |
| MAE260 | UMR1 ΔadrA103 | 20 |
| MAE262 | UMR1 STM2672::Cmr | 20 |
| MAE263 | UMR1 STM4551::Cmr | 20 |
| MAE272 | UMR1 STM2123::Cmr | 20 |
| MAE279 | UMR1 STM2410::Cmr | 20 |
| MAE280 | UMR1 STM2503::Cmr | 20 |
| MAE281 | UMR1 STM3375::Cmr | 20 |
| MAE282 | UMR1 STM1703::Cmr | 20 |
| MAE420 | UMR1 STM3611::Cmr | This study |
| MAE421 | UMR1 STM2215::Cmr | This study |
| MAE422 | UMR1 STM1827::Cmr | This study |
| MAE423 | UMR1 STM0468::Cmr | This study |
| MAE424 | UMR1 STM1344::Cmr | This study |
| MAE425 | UMR1 STM4264::Cmr | This study |
| MAE426 | UMR1 STM0343::Cmr | This study |
| MAE427 | UMR1 STM1697::Cmr | This study |
| MAE428 | UMR1 npt lacI PlacUV5 | This study |
| MAE430 | UMR1 ΔSTM4264::101 STM2672::Cmr | This study |
| MAE431 | UMR1 ΔSTM1703::101 STM2672::Cmr | This study |
| MAE432 | UMR1 ΔcsgD101 ΔSTM1703::Cmr | This study |
| MAE433 | UMR1 ΔSTM4264::101 STM4551::Cmr | This study |
| MAE434 | UMR1 ΔSTM1703::101 STM4551::Cmr | This study |
| MAE435 | UMR1 ΔSTM1703::101 STM4264::Cmr | This study |
| MAE437 | UMR1 ΔcsgD101 STM4264::Cmr | This study |
| MAE441 | UMR1 ΔSTM1703::101 STM1283::Cmr | This study |
| MAE442 | UMR1 ΔSTM4264::101 STM1283::Cmr | This study |
| MAE443 | UMR1 ΔSTM4264::101 STM2123::Cmr | This study |
| MAE444 | UMR1 ΔSTM1703::101 STM1223::Cmr | This study |
| MAE445 | UMR1 ΔSTM1703::101 STM1987::Cmr | This study |
| MAE446 | UMR1 ΔSTM4264::101 STM1987::Cmr | This study |
| MAE448 | UMR1 ΔadrA103 STM1703::Cmr | This study |
| MAE449 | UMR1 ΔadrA103 STM4264::Cmr | This study |
| MAE450 | UMR1 ΔSTM3611::101 STM3375::Cmr | This study |
| MAE451 | UMR1 ΔSTM3611::101 STM1344::Cmr | This study |
| MAE453 | UMR1 ΔSTM1703::101 | This study |
| MAE454 | UMR1 ΔSTM4264::101 | This study |
| MAE455 | UMR1 ΔSTM3611::101 | This study |
| MAE464 | UMR1 ΔSTM4264::101 STM3388::Cmr | This study |
| MAE465 | UMR1 ΔSTM1703::101 STM3388::Cmr | This study |
| MAE466 | UMR1 ΔcsgD101 STM4264::101 | This study |
| MAE265 | UMR1 ΔcsgD bcsA101::MudJ | 20 |
| MAE468 | UMR1 ΔcsgD bcsA101::MudJ STM4264::Cmr | This study |
| MAE469 | UMR1 ΔSTM4264::101 STM3375::Cmr | This study |
| MAE472 | UMR1 ΔSTM4264::101 STM3615::Kmr | This study |
| MAE473 | UMR1 ΔSTM4264::101 STM2410::Cmr | This study |
| MAE474 | UMR1 ΔSTM4264::101 STM2503::Cmr | This study |
| MAE475 | UMR1 ΔSTM1703::101 STM3375::Cmr | This study |
| MAE476 | UMR1 ΔSTM1703::101 STM3615::Kmr | This study |
| MAE477 | UMR1 ΔSTM1703::101 STM2410::Cmr | This study |
| MAE478 | UMR1 ΔSTM1703::101 STM2503::Cmr | This study |
| MAE490 | UMR1 ΔSTM1703 npt lacI PlacUV5 STM4264 | This study |
| Salmonella serovar Typhimurium LT2 LB5010 | metA22 metE551 ilv-452 leu-3121 trpO2 xyl-404 galE856 hsdLT6 hsdSA29 hsdSB121 rpsL120 | |
| Escherichia coli K-12 DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169(φ80lacZΔM15) | Laboratory collection |
| Plasmids | ||
| pBAD30 | Arabinose-regulated expression vector, Ampr | 15 |
| pEH1 | IPTG-inducible lac promoter; lacI repressor | 16 |
| pKD46 | λ red recombinase system, Ampr, temperature-sensitive replication | 7 |
| pKD3 | FRT-flanked cat cassette, Ampr Cmr | 7 |
| pRGS1 | pBAD30::yhjH, Ampr | 42 |
| pRGS2 | pBAD30::STM1827, Ampr | 42 |
| pRGS25 | pBAD30::STM1703, Ampr | This study |
| pWJB30 | pBAD30::adrA, Ampr | 48 |
Nalr, nalixidic acid resistant; Ampr, ampicillin resistant; FRT, FLP recombination target. Entire open reading frames, except the 40 nucleotides at the beginning and at the end of the gene, were replaced by either a kanamycin resistance marker (Kmr) or chloramphenicol resistance marker (Cmr).
Construction of mutants.
Knockout mutants were created by one-step gene inactivation as described by Datsenko and Wanner (7). In general, entire open reading frames except the 40 nucleotides at the beginning and at the end of the gene were replaced by either a kanamycin or chloramphenicol resistance marker. Approximately 300 ng of processed PCR product was electroporated into Salmonella serovar Typhimurium UMR1 containing pKD46. Recovered colonies were purified at least twice on LB medium containing appropriate antibiotics.
Phage transduction was carried out with phage P22 HT105/1 int-201 (41). Transductants were colony purified twice on LB agar plates containing EGTA (10 mM) and appropriate antibiotics. All constructed mutants were verified by PCR with control primers located in the flanking genes of the deleted open reading frame.
Plasmid construction.
Plasmids were constructed by cloning respective genes into pBAD30 with a C-terminal His tag unless otherwise stated. Primers used to construct the recombinant plasmids are available upon request. Plasmids were transformed into E. coli DH5α and passed through the restriction-deficient strain Salmonella serovar Typhimurium LB5010 before electroporation into Salmonella serovar Typhimurium ATCC 14028 derivatives.
STM4264 could not be cloned without mutation. In order to modulate the expression of this gene, STM4264 was put under the control of the lacUV5 promoter on the chromosome. The lacUV5 promoter, the lacI repressor gene, and the kanamycin resistance gene with its terminator sequence (npt-lacI-PlacUV5) were amplified from plasmid pEH1 (16) with primers consisting of a 5′ region of 40 nucleotides homologous to the target sequence and a 3′ region of 20 nucleotides complementary to the pEH1 plasmid. The Km-R/Elac cassette was inserted 30 bp upstream of the transcriptional start site of STM4264 (after bp 4502350 corresponding to the sequenced Salmonella serovar Typhimurium LT2 genome) by using Red recombinase on pKD46 (7). To verify the construct, the junction between the Km-R/Elac cassette and the target genes was sequenced. All primers are available upon request.
Phenotypic evaluation. (i) Congo red and calcofluor binding assays.
Five microliters of an overnight culture suspended in water (OD600 of 5) was spotted onto LB agar plates without NaCl supplemented with Congo red (40 μg ml−1) and Coomassie brilliant blue (20 μg ml−1) or calcofluor (fluorescence brightener 28; 50 μg ml−1). Plates were incubated at 28°C for 48 h or at 37°C for 24 h. The development of the colony morphology and dye binding was analyzed over time.
(ii) Multicellular behavior in liquid culture (biofilm formation).
The biofilm-forming ability was determined by inoculating bacteria from an overnight culture resuspended in water into 5 ml LB medium without NaCl or M9 minimal medium (starting OD600 of 0.01) in 12-ml glass tubes. The tubes, tilted at an angle of 50°, were incubated at 28°C for 48 h or at 37°C for 24 h with shaking at 150 rpm. The adherence of cells to the glass tube and the clumping ability of the mutants were visually compared. In order to quantify the amount of cells which were not able to form biofilms (cells in the planktonic state), the tubes were left standing in an upright position at room temperature for 30 min to let the cell clumps sediment. The overall amount of planktonic cells was quantified by measuring the OD600 of 1 ml culture collected at a depth of 1 cm below the surface. All mutants had identical growth rates at least up to mid-logarithmic phase.
(iii) Pellicle formation.
Cells were incubated in static culture in 24-well plates, whereby each well was filled with 2 ml of medium. The pellicle formed was inspected visually.
(iv) Swimming motility.
Swimming motility was observed on 0.3% LB agar plates for 6 h. The plates were inoculated with 5 μl of overnight culture resuspended at an OD600 of 5. Every hour, the diameter of the zone created by the swimming bacteria was measured.
(v) Swarming motility.
Swarming motility was analyzed on 0.5% LB agar plates supplemented with 0.5% glucose. The plates were inoculated with 5 μl overnight culture resuspended at an OD600 of 5. The radius from the spot of inoculation to the edge of the zone was measured every hour for up to 6 h.
Protein techniques.
For Western blot analysis, cells were grown on LB agar plates without salt. For CsgD analysis, 5 mg (wet weight) cells was harvested, resuspended in sample buffer, and heated to 95°C for 10 min. The protein content was analyzed by Coomassie blue staining (20% methanol, 10% acetic acid, 0.1% Coomassie brilliant blue G). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% resolving gel with 4% stacking gel) and were transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore). Detection of CsgD was carried out as described previously (35) using the polyclonal anti-CsgD peptide antibody (1:5,000) as the primary antibody and goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (1:2,000; Jackson ImmunoResearch Laboratories Inc.) as the secondary antibody. Chemiluminescence (Lumi-Light WB substrate; Roche) was recorded using the LAS-1000 system (FUJIFILM) and quantified using ImageQuant software (version 5.2).
Analysis of CsgA expression was carried out as described previously by two different methods (31, 32). Five milligrams (wet weight) cells was harvested, resuspended in 100 μl of 99% formic acid, and incubated for 10 min on ice. Liquid was removed by evaporation in a Speed Vac, and the pellet was resuspended in 200 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Three microliters was loaded on a gel (4% stacking gel and 15% separating gel). Detection of CsgA was carried out using a 1:4,000 dilution of an anti-CsgA antibody as described previously (31). Alternatively, CsgA was detected directly on a protein gel after enrichment (32). The pellet obtained by enrichment of curli fimbriae was treated with 99% formic acid for 10 min on ice to depolymerize the fimbriae. Depolymerized CsgA subunits were detected after the gel was stained with colloidal Coomassie brilliant blue overnight. Salmonella serovar Typhimurium MAE52 was used as a positive control.
Quantification of c-di-GMP.
Nucleotide extracts were prepared essentially as previously described (42). Bacteria were suspended in 0.19% ice-cold formalin, heated to 95°C for 10 min, and extracted by ethanol treatment. Nucleotide extracts equivalent to 10 mg (wet weight) were subjected to high-performance liquid chromatography (HPLC) separation using a reversed-phase column (Hypersil ODS 5μ; Hypersil-Keystone). Runs were carried out with a multistep gradient using 0.1 M triethyl ammonium acetate (pH 6.0) at 1 ml min−1 with increasing concentrations of acetonitrile. Relevant fractions were collected, lyophilized, and resuspended in 10 μl water. Fractions containing c-di-GMP were pinpointed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis and pooled. Synthetic c-di-AMP was added to the pooled fractions at a suitable concentration to be used as an internal standard. A standard curve was established using fractions spiked with known amounts of c-di-GMP, using a fixed amount of synthetic c-di-AMP as internal control. The isotope areas of c-di-GMP and c-di-AMP were calculated, and the ratio was determined. Each c-di-GMP measurement was carried out independently at least two times.
RESULTS
rdar morphotype expression and calcofluor binding by EAL and GGDEF-EAL protein mutants.
Previously, screening of GGDEF and GGDEF-EAL gene mutants identified GGDEF and GGDEF-EAL domain proteins that enhance expression of the rdar morphotype in Salmonella serovar Typhimurium strain UMR1 (20). In this study, the role of the 15 EAL and GGDEF-EAL domain proteins (28) on downregulation of the rdar morphotype was investigated. Salmonella serovar Typhimurium UMR1 expresses the rdar morphotype when cells are grown on LB agar plates without salt at ambient temperature (12, 36). Four of 15 mutants, STM1703 (yciR), STM1827, STM3611 (yhjH), and STM4264, led to a stronger expression of the rdar morphotype and higher calcofluor binding ability, indicating the activation of curli fimbriae and cellulose biosynthesis (Fig. 2). To exclude polar effects or secondary mutations, mutants were complemented by expression of respective cloned proteins. Since STM4264 could not be cloned without mutations, regulated expression of STM4264 from the chromosome was performed using the lacUV5 promoter in combination with the LacI repressor as described in Materials and Methods.
FIG. 2.
Knockout mutants of EAL and GGDEF-EAL genes showing upregulated rdar morphotype expression of Salmonella serovar Typhimurium UMR1. (A) Domain structure of EAL and GGDEF-EAL domain proteins as indicated in the SMART database (http://smart.embl-heidelberg.de/) with signaling peptide (red rectangle) and transmembrane domain (blue rectangle). (B and C) rdar morphotype (B) and calcofluor binding (C) of Salmonella serovar Typhimurium UMR1 and isogenic EAL and GGDEF-EAL domain protein mutants after growth on LB agar plates without salt at 28°C. The STM1703 (1703) and STM4264 (4264) mutants displayed highly upregulated rdar morphotypes, which clearly differed in structure. The STM3611 (3611) and STM1827 (1827) mutants were temporally upregulated, displaying the largest difference compared to UMR1 at time points before and after 24 h of growth, respectively.
The knockout mutants in the EAL domain proteins STM1827 and STM3611 showed a small, but consistent upregulation of rdar morphotype expression and calcofluor binding. The STM1827 knockout mutant is upregulated from 20 h until the end of the observation period at 48 h. On the other hand, the STM3611 mutant showed an upregulated rdar morphotype at early stages up to 24 h, but its morphotype is close to the wild-type morphotype after 48 h.
Knockout mutants in the GGDEF-EAL domain protein STM1703 and the EAL domain protein STM4264 displayed considerable upregulation of the rdar morphotype expression and calcofluor binding throughout the growth phase. Curiously, there is a difference in the structure of the colony morphology of the STM1703 and STM4264 mutants. The knockout mutant in STM1703 highly resembled MAE52 (Fig. 1 and 2) (36) in color and structural development. The MAE52 strain has a promoter mutation, which results in threefold-higher, temperature-independent expression of csgD and the rdar morphotype (36). The STM4264 mutant showed a more reddish rdar morphotype at the earlier stage compared to the deep purple color of MAE52. In addition, the STM4264 mutant showed a colony morphology which is more compact and displayed a more pronounced structural development. In conclusion, although the STM1703 and STM4264 mutants showed an upregulation of the rdar morphotype, the differences in rdar morphology indicated distinct functions for the two encoded proteins.
Molecular analysis of CsgA and CsgD expression in STM1703, STM1827, STM3611, and STM4264 protein mutants.
To assess rdar morphotype expression on a molecular level in STM1703, STM1827, STM3611, and STM4264 mutants, expression of intact curli fimbriae was analyzed after 20 h of growth by monitoring the amount of curli subunit CsgA depolymerized by formic acid (32). CsgA expression coincided with rdar morphotype expression, supporting the visual assessment of rdar morphotype expression by molecular data (Fig. 3A and B). In particular, the STM1703 and STM4264 mutants showed a significant upregulation of CsgA expression, which was more pronounced in the STM1703 mutant than in the STM4264 mutant.
FIG. 3.
Molecular analysis of central biofilm components of Salmonella serovar Typhimurium UMR1 and STM1703 (1703), STM1827 (1827), STM3611 (3611), and STM4264 (4264) mutants. (A) rdar morphotype development on CR agar plates. (B and C) Western blot analysis of CsgA (B) and CsgD (C) expression. (D) Relative c-di-GMP concentration in mutants compared to the wild type. c-di-GMP concentrations were determined using quantification by MALDI-TOF analysis after HPLC separation of cell extracts and pooling of appropriate fractions. The c-di-GMP concentration in Salmonella serovar Typhimurium UMR1 was arbitrarily set at 1. Values represent the averages of two independent experiments. Error bars indicate standard deviations. All analysis was performed after cell growth on LB agar plates without salt at 28°C for 20 h.
rdar morphotype expression in Salmonella serovar Typhimurium is controlled by the global regulator CsgD (12, 28, 36). To correlate rdar morphotype expression with CsgD expression, CsgD expression in the mutants was analyzed by Western blotting after 10, 16, 20, and 36 h of growth (Fig. 3C and data not shown) and compared to that in the wild-type UMR1. Consistent with the phenotypic and CsgA expression data, the knockout mutants in genes encoding the EAL domain-containing proteins STM1827 and STM3611 showed upregulation of the CsgD protein levels after 20 h of growth. The STM1703 and STM4264 mutants displayed highly increased expression of CsgD (Fig. 3C). These data indicate that in STM1703, STM1827, STM3611, and STM4264 mutants, the rdar morphotype and CsgD expression is upregulated, whereby STM1703 and STM4264 mutants showed the most pronounced upregulation of the rdar morphotype and expression of its master regulator CsgD.
c-di-GMP concentrations in STM1703, STM1827, STM3611, and STM4264 protein mutants.
It has been well established that orthodox GGDEF domains act as diguanylate cyclases, while EAL domains possess phosphodiesterase activity (27, 39, 40, 44). Consequently, diguanylate cyclase activity of GGDEF domain proteins led to enhanced intracellular concentrations of c-di-GMP, while the phosphodiesterase activity of EAL domain proteins lowered the intracellular c-di-GMP concentration (20, 42). To establish the impact of STM1703, STM3611, STM1827, and STM4264 on the regulation of physiological levels of c-di-GMP during rdar morphotype expression, the knockout mutants were analyzed for total intracellular c-di-GMP concentration (Fig. 3D).
The c-di-GMP concentrations in the STM1703, STM1827, and STM4264 mutants were increased consistent with a proposed phosphodiesterase activity of the proteins. The STM4264 mutant showed an approximately 5.5-fold-higher c-di-GMP concentration than the wild type, suggesting that STM4264 possesses the high c-di-GMP phosphodiesterase activity in the cell under the conditions investigated. The STM1827 and STM1703 mutants showed a moderate increase in c-di-GMP concentration (84% over wild-type level), while the STM3611 mutant did not show a significant increase in c-di-GMP concentration compared to the wild type. Since it was previously shown that STM3611 possesses phosphodiesterase activity (42), this finding indicated that STM3611 most likely leads to a local downregulation of c-di-GMP concentration.
Since CsgD expression is enhanced by c-di-GMP concentrations, the amount of CsgD expression was correlated with c-di-GMP concentration in the cell (Fig. 3C and D). Although the STM4264 mutant showed the highest c-di-GMP concentration, CsgD expression was lower than in the STM1703 mutant. Although the STM1827 and STM1703 mutants showed the same increase in c-di-GMP concentration, the increase in rdar morphotype and CsgD expression was substantially higher in the STM1703 mutant than in the STM1827 mutant. These observations indicated that the c-di-GMP pool present in the STM1703 mutant is dedicated to a higher extent to CsgD expression than the c-di-GMP pools in the STM4264 and STM1827 mutants.
Distinct roles for STM1703 and STM4264 in rdar morphotype and CsgD expression.
Since STM1703 and STM4264 had the most pronounced effect on rdar morphotype expression, the roles of the two proteins were investigated in more detail. The findings described above indicated that STM1703 and STM4264 have overlapping yet distinguishable roles in rdar morphotype and CsgD expression. To investigate whether STM1703 and STM4264 act additively on CsgD expression, an STM1703 STM4264 double mutant was constructed. rdar morphotype expression in the double mutant resembled the phenotype of the STM1703 mutant. The effect on CsgD expression was analyzed quantitatively (Fig. 4). The double mutant showed a 10.5-fold increase in CsgD expression compared to the wild type. This CsgD expression level is only slightly higher than in the STM1703 mutant (9.5-fold increase in CsgD expression compared to the wild type) but significantly higher than in the STM4264 mutant (4.5-fold increase in CsgD expression). These findings showed that the effect of the STM1703 and STM4264 knockout on CsgD expression is not additive.
FIG. 4.
Effect of the STM1703 STM4264 double mutant on CsgD expression. (A) Western blot analysis of CsgD expression in the STM1703 (1703), STM4264 (4264), and STM1703 STM4264 (1703 4264) mutants. Analysis was performed after cell growth on LB agar plates without salt at 28°C for 20 h. (B) Quantification of CsgD expression. CsgD expression in Salmonella serovar Typhimurium UMR1 was arbitrarily set at 1. Values are the averages of two experiments. Error bars indicate standard deviations.
The capacity of STM1703 and STM4264 to cross-complement each other was also tested. Expression of STM1703 in the STM4264 mutant converted the highly upregulated rdar morphotype to a saw morphotype (smooth and white colony) (Fig. 1) and almost abolished CsgD expression (Fig. 5). On the other hand, overexpression of STM4264 in the STM1703 mutant did not diminish rdar morphotype and CsgD expression, although STM4264 overexpression led to a saw morphotype and abolished CsgD expression in the wild type. These findings showed that STM1703 acts downstream of STM4264.
FIG. 5.
Results of cross-complementation experiments of STM1703 and STM4264 mutants. (A) Expression of STM4264 (4264) from the chromosome in Salmonella serovar Typhimurium UMR1 using the lacUV5 promoter in combination with the LacI repressor abolished CsgD expression. (B) Overexpression of STM1703 from pBAD30 left only traces of CsgD in the STM4264 (4264) mutant. On the other hand, STM4264 expression could not downregulate CsgD in the STM1703 (1703) mutant. CsgD was detected by Western blot analysis as described in Materials and Methods. Analysis was performed after cell growth on LB agar plates without salt at 28°C for 20 h.
rdar morphotype expression in the STM1703 and STM4264 mutants is regulated by csgD-dependent and -independent pathways.
In order to demonstrate that upregulation of the rdar morphotype in the STM1703 and STM4264 mutants is mediated by CsgD, csgD was knocked out in the STM1703 and STM4264 mutants. The STM1703 csgD double mutant showed a saw morphotype, indicating that in the STM1703 mutant, upregulation of the rdar morphotype is mediated entirely via csgD (Fig. 6). The STM4264 csgD double mutant, however, possessed a pas morphotype (pink and smooth colony) (Fig. 1) and still bound calcofluor. This finding indicated that, although the majority of c-di-GMP signaling in the STM4264 mutant goes via csgD, a csgD-independent activation pathway for cellulose biosynthesis exists (Fig. 7). To demonstrate that the pas morphotype indeed is derived from the expression of cellulose, the STM4264 csgD bcsA triple mutant was created. The saw morphotype of the triple mutant and lack of Calcofluor binding confirmed that cellulose was expressed by a csgD-independent pathway in the STM4264 mutant (Fig. 6).
FIG. 6.
Upregulation of rdar morphotype development of Salmonella serovar Typhimurium UMR1 mutants STM1703 (1703) and STM4264 (4264) is mediated by csgD-dependent and csgD-independent pathways. (A) Congo red phenotype as observed after 20 h of growth on LB agar plates without salt at 28°C. (B) Calcofluor binding phenotype as observed after 20 h of growth on LB agar plates without salt at 28°C. The calcofluor binding phenotype is consistent with the rdar morphotype expression shown in panel A.
FIG. 7.
Temperature-dependent regulation of the rdar morphotype expression in Salmonella serovar Typhimurium UMR1 is overcome by mutations in STM1703 or STM4264. (A) rdar morphotype on CR plate. (B and C) Calcofluor binding (B) and expression of CsgA (C) demonstrated that both cellulose and curli fimbriae are upregulated. (D) The master regulator of rdar morphotype expression, CsgD, is also upregulated in the STM1703 and STM4264 mutants. (E) c-di-GMP concentration in STM1703 (1703) and STM4264 (4264) mutants compared to UMR1. c-di-GMP concentrations were determined using quantification by MALDI-TOF analysis after HPLC separation of cell extracts and pooling of appropriate fractions. Values represent the averages of two independent experiments. Error bars indicate standard deviations. All analysis was performed using cells grown on LB agar plates without salt at 37°C for 16 h.
Regulatory network of GGDEF/EAL domain proteins in rdar morphotype expression.
The EAL-domain proteins STM1703 and STM4264 upregulated the rdar morphotype significantly by displaying apparent phosphodiesterase activity in vivo. In order to identify the corresponding GGDEF domain protein that produced c-di-GMP, double knockouts were constructed. The double knockouts adrA STM1703 and adrA STM4264 displayed an upregulated bdar morphotype (data not shown), indicating that AdrA produces the c-di-GMP required for the activation of cellulose biosynthesis and that adrA is epistatic to STM1703 and STM4264. Double knockout mutants with all other GGDEF domain proteins did not lead to a change in phenotype (data not shown), indicating that none of the GGDEF domain proteins alone is responsible for the c-di-GMP, which is subsequently degraded by STM1703 and STM4264.
Cellulose production is slightly activated in the STM4264 csgD double mutant (Fig. 6). AdrA was demonstrated to be required for cellulose expression during rdar morphotype development whereby adrA is activated by CsgD (35). To investigate whether cellulose production is stimulated by residual AdrA activity in the STM4264 csgD double mutant, the STM4264 csgD adrA triple mutant was constructed. However, cellulose was still produced in the STM4264 csgD adrA triple mutant as well as it was in the STM4264 csgD STM1987 triple mutant. STM1987 is the only other GGDEF domain protein in Salmonella serovar Typhimurium, which was demonstrated to activate cellulose production when expressed at physiological concentrations from the chromosome (10).
STM1703 and STM4264 mutations lead to temperature-independent expression of the rdar morphotype.
At 37°C, UMR1 does not express the rdar morphotype, the phenotype is saw on CR plates, and no calcofluor binding and CsgD expression are observed (31) (Fig. 7). Investigation of the knockout mutants for rdar morphotype expression at 37°C showed a dye binding phenotype for the STM1703 and the STM4264 mutant, which were rdar and ras (red and smooth), respectively (Fig. 7A). Upregulation of cellulose in the STM1703 and STM4264 mutants was independently demonstrated by enhanced calcofluor binding (Fig. 7B), while in the respective bcsA mutants, calcofluor binding was abolished (data not shown). The CsgD and CsgA levels were analyzed after 16 h of growth at 37°C on LB agar plates without salt. Consistent with the ras phenotype, the STM4264 mutant showed higher CsgA expression levels than UMR1 (Fig. 7C). The STM4264 mutant also clearly demonstrated CsgD expression in comparison to Salmonella serovar Typhimurium UMR1 (Fig. 7D).
In the STM1703 mutant, CsgA and CsgD expression was significantly higher than in the STM4264 mutant, which correlates with the more pronounced expression of the rdar morphotype of STM1703 than that of STM4264 at this temperature. Overcoming the temperature regulation of Salmonella serovar Typhimurium UMR1 by mutations in STM1703 and STM4264 demonstrated that both gene products act as suppressors of the rdar morphotype expression upstream of CsgD at 37°C. However, STM1703 contributes significantly more to the temperature regulation of the rdar morphotype.
To demonstrate by genetic means that rdar morphotype expression is upregulated via CsgD in the absence of STM1703 and STM4264, the STM4264 csgD and STM1703 csgD double mutants and the STM4264 csgD bcsA triple mutant were investigated for rdar morphotype expression at 37°C. As demonstrated for 28°C, rdar morphotype expression in the STM1703 mutant is entirely dependent on CsgD, while cellulose expression in the STM4264 mutant occurs to a minor part via a CsgD-independent pathway (data not shown).
Biofilm formation in liquid media by EAL and GGDEF-EAL domain protein mutants.
rdar morphotype expression and calcofluor binding indicate alterations in multicellular behavior while the bacteria are grown on a solid medium, the agar plates. As a second approach to investigate changes in multicellular behavior, the EAL and GGDEF-EAL domain protein knockout mutants were screened for their ability to display biofilm behavior (clumping, adherence to the glass surface, and amount of planktonic cells) in liquid culture at 28°C using LB medium without salt and M9 minimal medium (Fig. 8 and Table 2; also data not shown).
FIG. 8.
Biofilm formation of Salmonella serovar Typhimurium UMR1 and isogenic mutants in EAL and GGDEF-EAL domain proteins. Cells were incubated in LB medium without salt at 28°C for 48 h with shaking at 150 rpm. The five mutants that display an altered phenotype compared to wild-type Salmonella serovar Typhimurium UMR1, namely, STM1703 (1703), STM4264 (4264), STM3611 (3611), STM1827 (1827), and STM3375 (3375), are shown. (A) Adherence to the glass surface and the clumping ability observed. (B) Quantification of planktonic cells by optical density measurements from the top layer of the cultures after sedimentation of the cell clumps for 30 min. Values represent the averages of two independent experiments. Error bars indicate standard deviations.
TABLE 2.
Summary of phenotypes affected by EAL and GGDEF-EAL domain proteins
| Mutant | Change in regulation of the phenotypea
|
|||||
|---|---|---|---|---|---|---|
| Rdar morphotype | Biofilm in liquid medium
|
Pellicle in LB medium without salt | Motility
|
|||
| LB medium without salt | M9 minimal medium | Swimming | Swarming | |||
| STM1703 | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ||
| STM4264 | ↑↑ | ↑ | ↑ | ↑↑ | ||
| STM3611 | ↑ | ↓↓ | ↓↓ | |||
| STM1827 | ↑ | ↑ | ↑ | |||
| STM3375 | ↓↓ | ↓ | ↓↓ | |||
The arrows indicate upregulation (arrow pointing up) or downregulation (arrow pointing down) of the phenotype of the mutant compared to the phenotype of Salmonella serovar Typhimurium UMR1. A moderate effect is shown by one arrow, and a large effect is shown by two arrows. No arrow indicates no difference compared to the wild-type UMR1.
In agreement with an upregulated rdar morphotype expression on agar plates, the knockout mutants STM1703 and STM4264 showed upregulated clumping ability in LB without salt medium. Consistent with plate growth, the clumping ability was substantially upregulated in the STM1703 mutant, and few cells were in the planktonic state. The STM4264 mutant, in contrast to plate growth, was only slightly upregulated in its clumping capacity, while quantification of the amount of planktonic cells showed that there were consistently slightly fewer planktonic cells than in the wild type. On the other hand, the clumping and adherence to the glass surface are noticeably downregulated in the knockout mutant οf the GGDEF-EAL domain-like protein STM3375. From visual inspection, adherence to the glass seems slightly more brittle and the clumps formed are somewhat smaller in size than in Salmonella serovar Typhimurium UMR1. In agreement with the visual examination, the OD600 measurements of the supernatant for the STM3375 mutant demonstrated more cells in the planktonic state than in the wild-type Salmonella serovar Typhimurium UMR1. In summary, downregulation of multicellular behavior on plates and in liquid culture required partially the same gene products.
In M9 medium at 28°C, the STM1703 mutant was greatly upregulated in both clumping and adhesiveness to the glass surface, while the STM4264 mutant was considerably upregulated in the adhesiveness to the glass surface at the air liquid interface. The STM1827 mutant displayed slightly increased clumping and adherence capacity (data not shown).
Pellicle formation in standing culture by EAL and GGDEF-EAL domain protein mutants.
The pellicle formation (bacterial mat formation at the air-liquid interface) in a standing culture was also analyzed (34). At 28°C in LB medium without salt, the wild type and all the mutants formed pellicles although to different extents (Table 2). STM4264 and STM1703 formed leather-like pellicles comparable to the pellicle formed by Salmonella serovar Typhimurium MAE52, which expresses an upregulated rdar morphotype (36) (Fig. 1), as observed by eye. The STM1827 mutant formed a pellicle with a thicker structure at the air-liquid interface than that of the wild-type strain UMR1. In M9 medium, no pellicle could be detected for the wild type and any of the mutants (data not shown).
Motility phenotypes of EAL and GGDEF-EAL domain mutants.
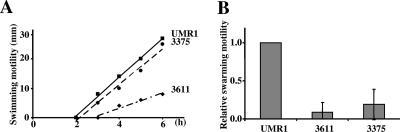
Regulation of motility contributes to the development of multicellular behavior and biofilm formation (23). To study the effects of the inactivation of genes encoding EAL and GGDEF-EAL domain proteins on the regulation of motility, the mutants were investigated for onset of swimming and the motility diameter in semisolid agar at 37°C (similar results were obtained at 28°C). Two of 15 mutants had a consistent downregulation in their swimming behavior compared to the wild-type Salmonella serovar Typhimurium UMR1. The STM3611 mutant showed a later onset of swimming and a reduction in motility. The mutant of STM3375 started to swim later than the wild type did, but the mutant swam at approximately the same speed as Salmonella serovar Typhimurium UMR1 did (Fig. 9A; also data not shown). The STM3375 and STM3611 mutants also displayed obvious defects in their swarming ability (Fig. 9B; also data not shown).
FIG. 9.
Flagellum-mediated motility by Salmonella serovar Typhimurium UMR1 and isogenic mutants. The isogenic mutants were STM3375 (3375) and STM3611 (3611). (A) Swimming motility of Salmonella serovar Typhimurium UMR1 and isogenic mutants. (B) Swarming motility of Salmonella serovar Typhimurium UMR1 and isogenic mutants analyzed after 5 h of incubation. Shown are representative experiments done in triplicate. Error bars indicate standard deviations. At least three independent experiments were done.
The different phenotypes of the mutants suggested that flagellum-mediated motility is regulated by at least two separate signaling pathways, which affect the onset of swimming and the swimming speed. To study the relative contributions of STM3611 and STM3375 to the regulation of the swimming ability, the STM3611 STM3375 double mutant was constructed. The STM3611 STM3375 double mutant displayed onset of swimming and speed similar to that of the STM3611 mutant, suggesting that the STM3611 and STM3375 proteins act in the same signaling pathway (data not shown).
DISCUSSION
c-di-GMP metabolism is reasonably complex in Salmonella enterica serovar Typhimurium with five GGDEF proteins, eight EAL proteins, and seven proteins with both GGDEF and EAL domains. In this work, a reverse genetic approach was taken to elucidate the role of the 15 EAL and GGDEF-EAL domain proteins (28) that presumably act as phosphodiesterases on rdar morphotype development. Four of 15 EAL and GGDEF-EAL gene knockouts, STM1703, STM1827, STM3611, and STM4264, displayed enhanced rdar morphotype expression. Since rdar morphotype expression is stimulated by c-di-GMP, this is the expected phenotype for genes encoding proteins with c-di-GMP-specific phosphodiesterase activity. Recently, in vitro phosphodiesterase activity was demonstrated for YciR, the STM1703 homologue in E. coli (47).
However, knockout of STM1344 encoding an EAL domain-like protein led to downregulation of rdar morphotype expression (data not shown). Since this is an unconventional behavior, STM1344 was not analyzed further. Another protein with GGDEF- and EAL-like domains, STM3375, is most likely not involved in c-di-GMP turnover (43).
The c-di-GMP signaling response of a certain phenotype reflects production and degradation of the molecule, which is the sum of diguanylate cyclase and phosphodiesterase activities under certain conditions. The same response can be achieved by high or low activities of diguanylate cyclases and phosphodiesterases. Under the conditions investigated, higher (STM4264) and lower (STM1703, STM1827, and STM3611) apparent phosphodiesterase activities were present that affected CsgD expression. This enables the cell to specifically tailor the responsiveness of the c-di-GMP-signaling system and to allow for its highly dynamic adjustment.
Nonlinearity between phosphodiesterase activity and CsgD expression was demonstrated. Knockout of STM4264 led to a significant (5.5-fold) increase in total intracellular c-di-GMP concentration, which triggered a 4.5-fold-enhanced CsgD expression. Thus, CsgD expression responds linearly to the c-di-GMP pool present in the STM4264 mutant. On the other hand, knockout of STM1703 led to a 9.5-fold CsgD expression, although the rise in intracellular c-di-GMP was moderate (84% of wild-type concentration). This finding indicated that the c-di-GMP pool present in the STM1703 mutant is to a larger extent dedicated to CsgD expression than the c-di-GMP pool present in the STM4264 mutant. Indeed, microarray analysis in E. coli has recently shown that knockout of yciR affected almost exclusively the expression of genes of the csg operon (47). The nonlinear relationship between the phosphodiesterase activity and output response (CsgD expression) shows that the response to c-di-GMP is complex and cannot be explained only by the absolute activities, as it has also been demonstrated for diguanylate cyclases in the same system (20). Spatial compartmentalization of proteins (33) or specific protein-protein interactions (1) are possibly affecting the response. A c-di-GMP binding protein has recently been characterized in enterobacteria (38). This protein or alternative c-di-GMP binding proteins might specifically interact with certain phosphodiesterases and/or target proteins further downstream.
The results of cross-complementation experiments suggested that STM1703 and STM4264 do not have redundant functions but affect distinct, yet overlapping c-di-GMP pools. The inability of STM4264 to downregulate CsgD in the absence of STM1703 suggests that, despite the high phosphodiesterase activity of STM4264, a large part of the c-di-GMP pool required for CsgD expression is not accessible for degradation by STM4264. On the other hand, overexpression of STM1703 in the STM4264 mutant could almost abolish CsgD expression.
Phosphodiesterases are involved in temperature expression of the rdar morphotype in Salmonella serovar Typhimurium. The activity of the EAL domain protein STM4264 and, in particular, the GGDEF-EAL domain protein STM1703 was responsible not only for dramatic downregulation of rdar morphotype expression at 28°C but also for lack of expression at 37°C. In general, different scenarios for the downregulation of the rdar morphotype at 37°C can be envisaged, such as lack of diguanylate cyclase activity. However, the findings described in this work indirectly showed that GGDEF domain proteins are active and possess diguanylate cyclase activity at 37°C, which is downregulated by the expression of the phosphodiesterase activity of EAL domain proteins. Temperature regulation of biofilm formation by an EAL domain protein was also observed recently in Yersinia pestis (22).
However, species-specific variation of the role of EAL domain proteins was observed. It has been shown recently that in E. coli, YciR downregulated CsgD at 28°C but did not have any effect at 37°C (47). Therefore, the role of STM1703/YciR is different in these closely related organisms.
The role of CsgD as a master regulator of rdar morphotype expression (12) was confirmed. Most of the c-di-GMP signaling required for activation of cellulose and curli fimbria expression in rdar morphotype development acts via CsgD. In addition, the CsgD concentration is positively correlated with the expression of the rdar morphotype (Fig. 3 and 6). Therefore, this work together with previous findings (20, 35, 42) demonstrates the intimate link between c-di-GMP metabolism, expression of CsgD, and the rdar morphotype. At least 7 of 20 GGDEF/EAL domain proteins are involved in rdar morphotype expression (Fig. 10). Six of them are involved in CsgD expression: the diguanylate cyclases STM2123 and STM3388 (20) and the phosphodiesterases STM1703, STM4264, STM3611, and STM1827 (this work). This demonstrates a highly sophisticated signaling network converging at CsgD, which integrates a variety of external and internal signals and thus modulates the response. Development of the rugose colony morphology in Vibrio cholerae, which is associated with enhanced exopolysaccharide production and biofilm formation, is also coordinated by several GGDEF/EAL domain proteins (26). CsgD, in turn, is required for the transcriptional activation of adrA encoding a GGDEF domain protein, which is required for the expression of cellulose (29, 35) and, partially, curli fimbriae (20).
FIG. 10.
Working model of the regulatory network of c-di-GMP signaling leading to rdar morphotype expression in Salmonella serovar Typhimurium. At least seven GGDEF and/or EAL-domain proteins are involved in the regulation of the rdar morphotype in Salmonella serovar Typhimurium. STM2123, STM3388 and other unidentified diguanylate cyclases produce c-di-GMP (blue dots). STM1703, STM4264, STM1827, and STM3611 lead to c-di-GMP degradation. STM4264 with the major apparent phosphodiesterase activity degrades c-di-GMP required for CsgD expression, cellulose biosynthesis, and (presumably) other phenotypes. Via STM1703, degraded c-di-GMP is dedicated almost exclusively to the expression of CsgD. STM4264 is located upstream of STM1703, since STM1703 can complement an STM4264 defect by downregulation of CsgD but not vice versa. Via STM1827, degraded c-di-GMP contributes only partially to CsgD expression. STM3611 hardly leads to degradation of c-di-GMP but affects CsgD expression as much as STM1827 does. Therefore, through STM3611, degraded c-di-GMP is to a greater part dedicated to CsgD expression than the c-di-GMP degraded by STM1827. AdrA regulated on the transcriptional level by CsgD is necessary for cellulose biosynthesis and partially involved in the production of curli fimbriae.
There is a clear task distribution among EAL and GGDEF-EAL domain proteins in Salmonella serovar Typhimurium with distinct overlapping groups of EAL and GGDEF-EAL domain proteins involved in rdar morphotype expression, multicellular behavior in liquid medium, pellicle formation, and swimming and swarming motility. For example, the GGDEF-EAL domain protein STM1703, which displays apparent phosphodiesterase activity, strongly affects all forms of biofilm formation (rdar morphotype, pellicle formation, and clumping in liquid media) but does not influence swimming and swarming motility. On the other hand, the EAL domain protein STM3611 stimulates mostly swimming and swarming motility and has a minimal effect on rdar morphotype expression but does not affect pellicle formation. A similar task distribution has recently been described for a subset of EAL and GGDEF-EAL domain proteins encoded by the Vibrio cholerae genome (26).
In conclusion, this work has significantly contributed to elucidating the role of EAL domain proteins and to constructing a regulatory network of GGDEF/EAL domain proteins in the development of the rdar morphotype in Salmonella serovar Typhimurium. Further experimentation aims to elucidate the effect of c-di-GMP on rdar morphotype expression on a molecular level.
Acknowledgments
We thank Michael Morr for providing the c-di-AMP.
This work was supported by the Karolinska Institutet (Elitforskartjänst to U.R.) and Vetenskapsrådet (621-2004-3979).
Footnotes
Published ahead of print on 23 February 2007.
REFERENCES
- 1.Andrade, M. O., M. C. Alegria, C. R. Guzzo, C. Docena, M. C. Rosa, C. H. Ramos, and C. S. Farah. 2006. The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv. citri. Mol. Microbiol. 62:537-551. [DOI] [PubMed] [Google Scholar]
- 2.Bullas, L. R., and J. I. Ryu. 1983. Salmonella typhimurium LT2 strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J. Bacteriol. 156:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 311:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, A. L., J. R. Tuckerman, G. Gonzalez, R. Mayer, H. Weinhouse, G. Volman, D. Amikam, M. Benziman, and M. A. Gilles-Gonzalez. 2001. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 40:3420-3426. [DOI] [PubMed] [Google Scholar]
- 5.Christen, M., B. Christen, M. Folcher, A. Schauerte, and U. Jenal. 2005. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280:30829-30837. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. A., and S. I. Miller. 2004. Cyclic di-GMP as a bacterial second messenger. Microbiology 150:2497-2502. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frye, J., J. E. Karlinsey, H. R. Felise, B. Marzolf, N. Dowidar, M. McClelland, and K. T. Hughes. 2006. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galperin, M. Y. 2004. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 6:552-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia, B., C. Latasa, C. Solano, F. Garcia-del Portillo, C. Gamazo, and I. Lasa. 2004. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 54:264-277. [DOI] [PubMed] [Google Scholar]
- 11.Gerstel, U., C. Park, and U. Römling. 2003. Complex regulation of csgD promoter activity by global regulatory proteins. Mol. Microbiol. 49:639-654. [DOI] [PubMed] [Google Scholar]
- 12.Gerstel, U., and U. Römling. 2003. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 154:659-667. [DOI] [PubMed] [Google Scholar]
- 13.Gerstel, U., and U. Römling. 2001. Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ. Microbiol. 3:638-648. [DOI] [PubMed] [Google Scholar]
- 14.Gronewold, T. M., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for Myxococcus xanthus development. Mol. Microbiol. 40:744-756. [DOI] [PubMed] [Google Scholar]
- 15.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashemzadeh-Bonehi, L., F. Mehraein-Ghomi, C. Mitsopoulos, J. P. Jacob, E. S. Hennessey, and J. K. Broome-Smith. 1998. Importance of using lac rather than ara promoter vectors for modulating the levels of toxic gene products in Escherichia coli. Mol. Microbiol. 30:676-678. [DOI] [PubMed] [Google Scholar]
- 17.Hisert, K. B., M. MacCoss, M. U. Shiloh, K. H. Darwin, S. Singh, R. A. Jones, S. Ehrt, Z. Zhang, B. L. Gaffney, S. Gandotra, D. W. Holden, D. Murray, and C. Nathan. 2005. A glutamate-alanine-leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: role of cyclic diGMP. Mol. Microbiol. 56:1234-1245. [DOI] [PubMed] [Google Scholar]
- 18.Huang, B., C. B. Whitchurch, and J. S. Mattick. 2003. FimX, a multidomain protein connecting environmental signals to twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 185:7068-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenal, U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185-191. [DOI] [PubMed] [Google Scholar]
- 20.Kader, A., R. Simm, U. Gerstel, M. Morr, and U. Römling. 2006. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 60:602-616. [DOI] [PubMed] [Google Scholar]
- 21.Karatan, E., T. R. Duncan, and P. I. Watnick. 2005. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 187:7434-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirillina, O., J. D. Fetherston, A. G. Bobrov, J. Abney, and R. D. Perry. 2004. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol. Microbiol. 54:75-88. [DOI] [PubMed] [Google Scholar]
- 23.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50:61-68. [DOI] [PubMed] [Google Scholar]
- 24.Ko, M., and C. Park. 2000. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J. Mol. Biol. 303:371-382. [DOI] [PubMed] [Google Scholar]
- 25.Latasa, C., A. Roux, A. Toledo-Arana, J. M. Ghigo, C. Gamazo, J. R. Penades, and I. Lasa. 2005. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol. Microbiol. 58:1322-1339. [DOI] [PubMed] [Google Scholar]
- 26.Lim, B., S. Beyhan, J. Meir, and F. H. Yildiz. 2006. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 60:331-348. [DOI] [PubMed] [Google Scholar]
- 27.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Römling, U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 62:1234-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Römling, U. 2002. Molecular biology of cellulose production in bacteria. Res. Microbiol. 153:205-212. [DOI] [PubMed] [Google Scholar]
- 30.Römling, U., and D. Amikam. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9:218-228. [DOI] [PubMed] [Google Scholar]
- 31.Römling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Römling, U., W. Bokranz, W. Rabsch, X. Zogaj, M. Nimtz, and H. Tschape. 2003. Occurrence and regulation of the multicellular morphotype in Salmonella serovars important in human disease. Int. J. Med. Microbiol. 293:273-285. [DOI] [PubMed] [Google Scholar]
- 33.Römling, U., M. Gomelsky, and M. Y. Galperin. 2005. c-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 34.Römling, U., and M. Rohde. 1999. Flagella modulate the multicellular behavior of Salmonella typhimurium on the community level. FEMS Microbiol. Lett. 180:91-102. [DOI] [PubMed] [Google Scholar]
- 35.Römling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinköster. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 36.Römling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 37.Rychlik, I., G. Martin, U. Methner, M. Lovell, L. Cardova, A. Sebkova, M. Sevcik, J. Damborsky, and P. A. Barrow. 2002. Identification of Salmonella enterica serovar Typhimurium genes associated with growth suppression in stationary-phase nutrient broth cultures and in the chicken intestine. Arch. Microbiol. 178:411-420. [DOI] [PubMed] [Google Scholar]
- 38.Ryjenkov, D. A., R. Simm, U. Romling, and M. Gomelsky. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310-30314. [DOI] [PubMed] [Google Scholar]
- 39.Ryjenkov, D. A., M. Tarutina, O. V. Moskvin, and M. Gomelsky. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt, A. J., D. A. Ryjenkov, and M. Gomelsky. 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol. 187:4774-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 42.Simm, R., M. Morr, A. Kader, M. Nimtz, and U. Römling. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123-1134. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, K., P. Babitzke, S. R. Kushner, and T. Romeo. 2006. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 20:2605-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamayo, R., A. D. Tischler, and A. Camilli. 2005. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J. Biol. Chem. 280:33324-33330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, C., C. R. Andersson, S. R. Canales, and S. S. Golden. 2004. PsfR, a factor that stimulates psbAI expression in the cyanobacterium Synechococcus elongatus PCC 7942. Microbiology 150:1031-1040. [DOI] [PubMed] [Google Scholar]
- 46.Tischler, A. D., and A. Camilli. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber, H., C. Pesavento, A. Possling, G. Tischendorf, and R. Hengge. 2006. Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol. Microbiol. 62:1014-1034. [DOI] [PubMed] [Google Scholar]
- 48.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Römling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]