Abstract
The cydABCD operon of Bacillus subtilis encodes products required for the production of cytochrome bd oxidase. Previous work has shown that one regulatory protein, YdiH (Rex), is involved in the repression of this operon. The work reported here confirms the role of Rex in the negative regulation of the cydABCD operon. Two additional regulatory proteins for the cydABCD operon were identified, namely, ResD, a response regulator involved in the regulation of respiration genes, and CcpA, the carbon catabolite regulator protein. ResD, but not ResE, was required for full expression of the cydA promoter in vivo. ResD binding to the cydA promoter between positions −58 and −107, a region which includes ResD consensus binding sequences, was not enhanced by phosphorylation. A ccpA mutant had increased expression from the full-length cydA promoter during stationary growth compared to the wild-type strain. Maximal expression in a ccpA mutant was observed from a 3′-deleted cydA promoter fusion that lacked the Rex binding region, suggesting that the effect of the two repressors, Rex and CcpA, was cumulative. CcpA binds directly to the cydA promoter, protecting the region from positions −4 to −33, which contains sequences similar to the CcpA consensus binding sequence, the cre box. CcpA binding was enhanced upon addition of glucose-6-phosphate, a putative cofactor for CcpA. Mutation of a conserved residue in the cre box reduced CcpA binding 10-fold in vitro and increased cydA expression in vivo. Thus, CcpA and ResD, along with the previously identified cydA regulator Rex (YdiH), affect the expression of the cydABCD operon. Low-level induction of the cydA promoter was observed in vivo in the absence of its regulatory proteins, Rex, CcpA, and ResD. This complex regulation suggests that the cydA promoter is tightly regulated to allow its expression only at the appropriate time and under the appropriate conditions.
The cydABCD operon of Bacillus subtilis is responsible for the production of intact cytochrome bd oxidase (36). cydAB encodes the structural proteins of the bd oxidase, while cydCD is proposed to encode an ABC transporter which is required for the assembly of cytochrome bd. A single cydA transcriptional start site was identified for this operon from cells grown to stationary phase in nutrient sporulation medium with phosphate buffer (pH 7.0) and glucose (NSMPG) (36). Candidate sequences for the EσA −10 and −35 elements were identified. Expression of cytochrome bd was first observed under conditions of low oxygen availability and in cells grown in the presence of glucose (36). The role of YdiH (Rex) in the regulation of the cydABCD operon was determined through analysis of a suppressor of poor growth of a ΔresDE mutant. We showed that YdiH (Rex) binds downstream of the transcriptional start site in a long untranslated sequence and appears to negatively regulate the operon (28). It was recently proposed that YdiH (Rex) is a redox sensor whose activity is regulated by the levels of NAD+ and NADH in the cell (11, 17). YdiH has been renamed Rex (17), as it is an orthologue of Rex in Streptomyces coelicolor (2), which is a redox-sensitive transcription regulator that responds to the cellular NADH/NAD+ ratio, albeit via a different mechanism than that for Rex of B. subtilis (11). During anaerobic growth, Rex functions as a repressor of ldh, encoding NADH-linked fermentative lactate dehydrogenase (17), while during aerobic growth it represses ndh (formerly yjlD), encoding NADH dehydrogenase of the respiratory chain (11).
The ResD/ResE two-component signal transduction system plays a role in the regulation of both aerobic and anaerobic respiration. ResD, the response regulator, regulates the expression of fnr (23), hmp (23), nasDEF (23), hemN (14), hemZ (14), yclJK (13), and the sbo-alb operon (22) under anaerobic conditions and has a role in the regulation of ctaA (24, 38), ctaBCDEF (18), resABCDE (31), and qcrABC (formerly called petCBD) (31) under aerobic conditions. Because ResDE is essential for both ctaA and ctaB expression, which is required for heme A biosynthesis, ΔresDE strains lack cytochromes aa3 and caa3. ResD also activates the genes ctaCDEF, encoding the structural proteins for cytochrome caa3 (18). The role of ResD as a transcriptional activator of genes involved in terminal oxidase production led us to ask if ResD played a role in the activation of transcription of the cydABCD operon.
CcpA (Catabolite control protein A) is a member of the LacI/GalR family of repressor proteins (35) and has been shown to function as both an activator and a repressor in B. subtilis (1). A number of genes involved in aerobic respiration have been shown to be subject to catabolite regulation via CcpA, namely, ctaCDEF (18); qcrA, which produces a menaquinol:cytochrome c oxidoreductase (21); and cccA, which encodes cytochrome c550 (20). The requirement of glucose for growth under anaerobic conditions and for the expression of the cydABCD operon (36) raises the questions of whether this operon is subject to catabolite regulation and if CcpA plays a role in the regulation of the cydABCD operon. Larsson et al. (17) suggested that CcpA might be an indirect activator of this operon. A report by Zamboni et al. (37) suggested that cytochrome bd is important for growth in glucose-limited cultures under aerobic conditions. These conflicting data, albeit under different conditions, bring into question the regulatory role of CcpA.
In this paper, we report that ResD positively regulates transcription from the cydA promoter and that CcpA represses transcription from the cydA promoter. No promoter activity was found in the untranslated region where Rex binds. The cydA promoter has a low basal level of induction in vivo in the absence of its regulatory proteins, Rex, CcpA, and ResD.
MATERIALS AND METHODS
Strains and plasmids.
Table 1 lists the strains and plasmids used in this study. Escherichia coli DH5α served as the host for all plasmid constructions. E. coli BL21(DE3)/pLysS (Novagen) served as the host for the overexpression of ResD and *ResE, a soluble form of ResE missing 226 N-terminal amino acids. E. coli M15(pREP4) (QIAGEN) served as the host for the overexpression of CcpA. B. subtilis JH642 served as the parental strain for all Bacillus constructions.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli strains | ||
| DH5α | Lab stock | |
| BL21(DE3)pLysS | Novagen | |
| M15(pREP4) | QIAGEN | |
| B. subtilis strains | ||
| JH642 | pheA1 trpC2 | J. A. Hoch |
| MH5202 | pheA1 trpC2 ΔresDE::Tetr | 31 |
| MH5427 | pheA1 trpC2 ΔresE::Spcr | E. Sharkova |
| MH5910 | pheA1 trpC2 ΔresD::SpcrctaB-lacZ | X. Zhang |
| QB5407 | pheA1 trpC2 ccpA::Tn917 Spcr | J. Stulke |
| MH5878 | pheA1 trpC2 amyE::pMS35 cydA-lacZ Cmr | 28 |
| MH5880 | pheA1 trpC2 ΔresDE::TetramyE::cydA-lacZ Cmr | 28 |
| MH5892 | pheA1 trpC2 rex::Spcr | 28 |
| MH5894 | pheA1 trpC2 ccpA::Tn917 SpcramyE::cydA-lacZ Cmr | This study |
| MH5895 | pheA1 trpC2 amyE::pMS46 cydA3′del-lacZ Cmr | This study |
| MH5896 | pheA1 trpC2 ΔresDE::TetramyE::pMS46 cydA3′del-lacZ Cmr | This study |
| MH5897 | pheA1 trpC2 ccpA::Tn917 SpcramyE::pMS46 cydA3′del-lacZ Cmr | This study |
| MH6064 | pheA1 trpC2 amyE::pMS35 cydA-lacZ Cmrrex::Spcr | This study |
| MH6065 | pheA1 trpC2 amyE::pMS46 cydA3′del-lacZ Cmrrex::Spcr | This study |
| MH6066 | pheA1 trpC2 amyE::pMS35 cydA-lacZ CmrresE::Spcr | This study |
| MH6067 | pheA1 trpC2 amyE::pMS46 cydA3′del-lacZ CmrresE::Spcr | This study |
| MH6077 | pheA1 trpC2 amyE::pAT21 cydAcre3′del-lacZ Cmr | This study |
| MH6079 | pheA1 trpC2 amyE::pMS46 cydA-lacZ CmrresD::Spcr | This study |
| MH6080 | pheA1 trpC2 amyE::pMS46 cydA3′del-lacZ CmrresD::Spcr | This study |
| MH6095 | pheA1 trpC2 amyE::pAT23 cydAcre-lacZ Cmr | This study |
| MH6301 | pheA1 trpC2 ΔresDE::TetrccpA::Tn917 SpcramyE::cydA-lacZ Cmr | This study |
| MH6302 | pheA1 trpC2 ΔresDE::TetrccpA::Tn917 SpcramyE::cydA3′del-lacZ Cmr | This study |
| Plasmids | ||
| pCR2.1 | Vector for cloning PCR products | Invitrogen |
| pDH32 | Vector for construction of promoter-lacZ fusions; Ampr Cmr | 29 |
| pET-16b | Vector for protein overexpression and His tagging; Ampr | Novagen |
| pGEX-2T | Vector for protein overexpression and glutathione S-transferase tagging | Pharmacia Biotech |
| pXH3 | Fragment of ResE in pGEX-2T for production of glutathione S-transferase-fused ResE | 38 |
| pES66 | Coding sequence of resD in pCR2.1; Ampr | E. Sharkova |
| pES67 | Coding sequence of resD in pET-16b for production of His-tagged ResD; Ampr | E. Sharkova |
| pQE30 with CcpA | Coding sequence of ccpA in pQE30 for production of His-tagged CcpA; Ampr | 19 |
| pMS34 | Full-length cydA promoter in pCR2.1; Ampr | 28 |
| pMS35 | cydA-lacZ fusion in pDH32; Ampr Cmr | 28 |
| pMS42 | Fragment of cydA promoter lacking Rex binding site in pCR2.1; Ampr | This study |
| pMS46 | cydA3′del-lacZ in pDH32; Ampr Cmr | This study |
| pAT20 | cydAcre3′del-lacZ in pCR2.1; Ampr | This study |
| pAT21 | cydAcre3′del-lacZ in pDH32; Ampr Cmr | This study |
| pAT22 | cydAcre-lacZ in pCR2.1; Ampr | This study |
| pAT23 | cydAcre-lacZ in pDH32; Ampr Cmr | This study |
Tetr, tetracycline resistant; Cmr, chloramphenicol resistant; Spcr, spectinomycin resistant; Ampr, ampicillin resistant.
The full-length cydA-lacZ fusion (pMS34) and strains bearing this fusion in a wild-type (WT) background (MH5878) and a ΔresDE background (MH5880) have been described previously (28). To construct MH5894 (ccpA::Tn917 cydA-lacZ), chromosomal DNA from QB5407 (ccpA::Tn917) was transformed into MH5878 (cydA-lacZ), with selection for spectinomycin resistance (Spcr).
Primers FMH792 (5′-TAGAATTCAGGAACGATGTGTTGATGTA-3′ [EcoRI site is underlined]) and FMH794 (5′-TAGGATCCGCCAGACTGACCAAATGAC-3′ [BamHI site is underlined]) were used to amplify a fragment of the cydA promoter region spanning from nucleotides (nt) −155 to +38 upstream of the start site of cydA (Fig. 1), using JH642 as the template. This fragment contains the transcriptional start site for the cydABCD operon and the −10 and −35 elements but lacks the previously described (28) binding sites for Rex. The resulting fragment was subcloned into pCR2.1 (Invitrogen) to construct pMS42. The cydA promoter fragment was released from pMS42 by digestion with EcoRI and BamHI and cloned into the complementary sites in pDH32 to construct pMS46. DNA sequencing confirmed the sequence of the truncated cydA3′del promoter. MH5895 (cydA3′del-lacZ) was constructed by transformation of JH642 with pMS46 linearized by digestion with PstI such that the promoter fusion was integrated into the amyE locus by double-crossover homologous recombination. Transformants were selected for chloramphenicol resistance, and the required insertion was confirmed by the amyE mutant phenotype. Chromosomal DNA from MH5202 (ΔresDE) was transformed into MH5895 (cydA3′del-lacZ), with selection for Tetr transformants, to construct MH5896 (ΔresDE cydA3′del-lacZ). To construct a strain bearing a mutation in ccpA and the cydA3′del-lacZ fusion, we transformed chromosomal DNA from QB5407 (ccpA::Tn917) into MH5895 (cydA3′del-lacZ) to construct MH5897 (ccpA::Tn917 cydA3′del-lacZ).
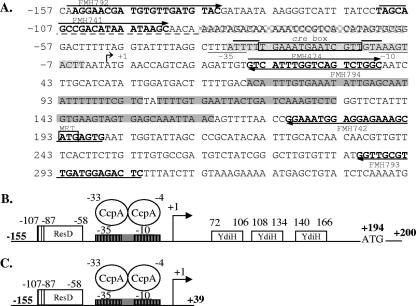
FIG. 1.
Locations of regulatory sites in the cydA promoter. (A) Sequence of the cydA promoter. All numbering is relative to the transcription start site of cydA at position +1. The transcriptional start site is identified by a bent arrow. The σA consensus −10 and −35 sites are underlined and labeled (36). Solid gray shading identifies the locations of the Rex binding sites. The location of the ResD binding site is identified with a grid (−58 to −87), and the dashed line under the adjacent sequence (−88 to −107) identifies the sequence more weakly protected by ResD. The stippled shading identifies the location of the CcpA binding site. The consensus for CcpA binding is shown within a rectangle, with the 3′ and 5′ AT-rich flanking sequences indicated by overlining. Primer sequences are shown in bold, with arrows indicating the 5′-to-3′ orientation. (B) Cartoon showing the locations of regulatory regions in the cydA promoter. Numbering is relative to the transcription start site of cydA at position +1. The transcriptional start site is denoted by a bent arrow. The consensuses for σA −10 and −35 elements are denoted by boxes with vertical lines. The locations of Rex and ResD binding sites are marked by boxes bearing the names of the proteins binding at those sites. The location of the CcpA binding site is denoted by a gray box overlapping the binding site at the σA −10 and −35 elements. (C) Cartoon showing cydA promoter lacking Rex binding sites.
MH6064 (cydA-lacZ Cmr rex::Spcr) and MH6065 (cydA3′del-lacZ Cmr rex::Spcr) were constructed by transforming DNA from the rex strain, MH5892, into MH5878 (cydA-lacZ Cmr) and MH5895 (cydA3′del-lacZ Cmr), respectively, with selection for Spcr transformants. MH6066 (cydA-lacZ Cmr resE::Spcr) and MH6067 (cydA3′del-lacZ Cmr resE::Spcr) were obtained by transforming chromosomal DNA from the resE mutant strain MH5427 into MH5878 (cydA-lacZ Cmr) and MH5895 (cydA3′del-lacZ Cmr), respectively, with selection for Spcr transformants. MH6079 (cydA-lacZ Cmr resD::Spcr) and MH6080 (cydA3′del-lacZ Cmr resD::Spcr) were constructed by transforming chromosomal DNA from the resD mutant strain MH5910 into MH5878 (cydA-lacZ Cmr) and MH5895 (cydA3′del-lacZ Cmr), respectively, with selection for Spcr transformants. MH6301 (ΔresDE ccpA::Tn917 cydA-lacZ) was constructed by transformation of chromomosomal DNA from MH5202 (ΔresDE) into MH5894 (ccpA::Tn917 cydA-lacZ), with selection for Tetr transformants. MH6302 (ΔresDE ccpA::Tn917 cydA3′del-lacZ) was made by transformation of chromosomal DNA from MH5202 (ΔresDE) into MH5897 (ccpA::Tn917 cydA3′del-lacZ), with selection for Tetr transformants.
Strains bearing a single-base-pair mutation in the putative cre box in the full-length or truncated cydA promoter were constructed by first subjecting plasmids pMS34 and pMS42, respectively, to site-directed mutagenesis, using a QuikChange II site-directed mutagenesis kit (Stratagene). The primers used were FMH884 (5′-CTTTATTTTTGAAATGAAcCGTTGTAAAGTACTTAAT-3′) and FMH885 (5′-ATTAAGTACTTTACAACGgTTCATTTCAAAAATAAAG-3′) (mutations are indicated with lowercase letters). The mutagenized plasmids were called pAT22 and pAT20, respectively. The full-length and 3′-truncated promoters from pAT22 and pAT20 were then digested with EcoRI and BamHI and ligated into EcoRI and BamHI sites of pDH32 to construct pAT23 and pAT21, respectively. pAT23 and pAT21 were then transformed into JH642, and strains MH6095 and MH6077 were obtained, which contained cydAFL-lacZ and cydA3′del-lacZ fusions with cre box mutations, respectively.
Primers FMH238 (5′-TTCATATGGACCAAACGAACGAAAC-3′ [NdeI site is underlined]) and FMH230 (5′-TTGGATCCTCATTCAGCGCCGACCTC-3′ [BamHI site is underlined]) were used to amplify the coding sequence of resD. The resulting PCR product was cloned into pCR2.1 to create pES66. DNA sequencing confirmed that the sequence of ResD was the WT sequence. The coding sequence of ResD was released from pES66 by digestion with NdeI and BamHI, and the fragment was cloned into pET-16b to construct pES67. pES67 was transformed into BL21(DE3)/pLysS to allow the overexpression and purification of His-tagged ResD.
Genetic manipulations.
Transformation of B. subtilis was done by the two-step transformation method of Cutting and Vander Horn (3). Transformants were selected on tryptose blood agar base medium supplemented with 0.5% glucose and the appropriate antibiotic. Antibiotics were added to the medium for selection of B. subtilis transformants at the following concentrations: chloramphenicol, 5 μg/ml; spectinomycin, 100 μg/ml; and tetracycline, 10 μg/ml. Transformation of E. coli was done according to the method of Hanahan (12). Transformants were selected on LB plates containing ampicillin (100 μg/ml).
Growth conditions and enzyme assays.
For measurement of cydA-lacZ expression, cells were grown in NSMPG (6). Four-hundred-milliliter cultures in 1,000-ml baffle-bottomed flasks were maintained at 37°C on a rotary shaker (Infors Multitron) at 200 rpm. β-Galactosidase activity was detected using the method of Ferrari et al. (5). The activity unit was defined as 0.33 nmol of ortho-nitrophenol produced min−1, and the specific activity was calculated as units per mg of protein.
Preparation of His-ResD.
E. coli BL21(DE3)/pLysS harboring pES67 was incubated overnight at 37°C in LB medium containing ampicillin (100 μg/ml) and then inoculated into 1 liter of the same medium at a ratio of 1 to 100. The cells were grown at 32°C until the optical density of the culture at 600 nm reached 0.4. IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) was then added to the culture, and growth was continued for an additional 5 h. The cells were harvested by centrifugation at 4°C and washed with buffer A (1 M NaCl, 5 mM MgCl2, 10 mM dithiothreitol [DTT], 50 mM Tris-HCl [pH 7.8]). The cell pellets were suspended on ice in 50 ml of buffer A containing 1 mM phenylmethylsulfonyl fluoride and were immediately subjected to sonication. The disruption of cells was confirmed by microscopy. After centrifugation at 40,000 × g for 1 h at 4°C, the supernatant fraction was filtered through a 0.45-μm-pore-size membrane. A 1/50 volume of 0.5 M imidazole in buffer A was added, and the clear cell lysate was mixed with 4 ml Talon resin (Clontech) preequilibrated with buffer A. Gentle shaking at 4°C for 30 min was carried out before the mixture was loaded onto an Econo column (2.5 cm by 10 cm [inner diameter by height]; Bio-Rad). The column was washed with buffer A until the eluate contained no detectable protein by the Bio-Rad protein assay. The column was further washed with 10 ml of buffer A containing 10 mM imidazole. The protein bound to the column was eluted using 500 mM imidazole in buffer A. The protein fractions were dialyzed stepwise at 4°C against buffer A containing 20% glycerol with decreasing concentrations of NaCl, from 1 M to 0.8, 0.6, 0.5, 0.2, and finally, 0.1 M. The purity of His-ResD proteins was over 95%, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Production of GST-*ResE.
The plasmid pXH3 was used for the overexpression and purification of *ResE as described previously (38).
Production of His-CcpA.
The plasmid pQE30, containing the coding sequence of ccpA (19), was used for the overexpression and purification of His-CcpA. His-CcpA was purified as described previously (15).
Preparation of cydA promoter probe.
The labeling and purification of the cydA promoter probe for DNase I footprinting were done as described previously (28). For the purposes of the gel shift assay, the DNA probe was prepared by subjecting the plasmid pMS42 to EcoRI and BamHI restriction digestion, and the promoter fragment was purified from the agarose gel by using a QIAquick gel extraction kit (QIAGEN). The overhangs of the 194-bp fragment were filled with a mix of deoxynucleoside triphosphates containing radiolabeled [α-32P] dATP, using the Klenow fragment supplied by Invitrogen. The probe was purified by phenol-chloroform extraction.
Electromobility shift assay (EMSA) with CcpA.
In each reaction mix, 20,000 cpm of labeled probe was incubated with various concentrations of CcpA in buffer containing 10 mM Tris-HCl (pH 7.5), 1 mM EDTA (pH 7.5), 50 mM KCl, 0.05% (vol/vol) NP-40, 10% glycerol, 1 mM DTT (modified from the work of Kim et al. [16]), and 30 mM glucose-6-phosphate (G-6-P) (10) at room temperature for 30 min. Tartrate buffer was added according to the method of Gosseringer et al. (10). The samples were then separated by electrophoresis, using a 6% polyacrylamide gel made in 1× Tris-borate-EDTA (27). The gel was run at 80 V for 1 h at 4°C and vacuum dried, and the radioactivity was detected using a PhosphorImager or X-ray film.
DNase I footprinting of the cydA promoter.
In each reaction mix, the required protein (CcpA or ResD) at the indicated concentrations and the probe at a 50 nM final concentration were incubated at room temperature for 30 min in binding buffer (20 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 10% glycerol). DNase I (3 μl of 0.04-U/μl DNase I in 5 mM MgCl2, 5 mM CaCl2) was added to each reaction mixture, and digestion was conducted for 60 s for protein-containing samples and 30 s for protein-free samples. The reaction was stopped, and the DNA fragments were purified by phenol extraction followed by ethanol precipitation. The DNA fragments were separated by electrophoresis, using a 4% polyacrylamide gel in 1× Tris-borate-EDTA containing 7 M urea. The gel was run at 65 mA for 2 h and dried, and the radioactivity was detected by PhosphorImager analysis and/or X-ray film (Fuji) radiography.
Preparation of ResD∼P and ResD for footprinting.
ResD was mixed at the required concentrations with equimolar *ResE in the presence of ATP (molar ratio of ResD to ATP, 1/1.5) or without ATP in P buffer (50 mM KCl, 5 mM MgCl2, 50 mM HEPES [pH 8.0]) and incubated for 20 to 30 min at room temperature.
RESULTS
Deletion of Rex binding sites leads to increased expression of the cydA-lacZ fusion in a WT strain, similar to that observed in rex mutant strains.
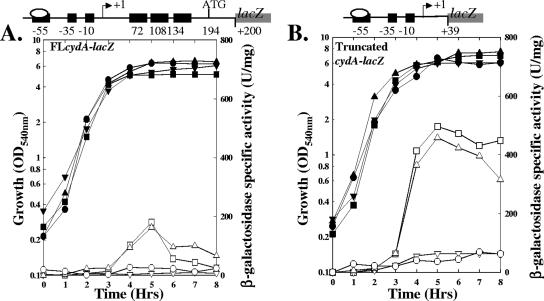
Rex regulates the expression of the cydA promoter by binding to the three sites located in a long untranslated region downstream of the transcriptional start site for the cydA promoter (28). This binding appears to lead to repression of the cydABCD operon, as a strain bearing a loss-of-function mutation in rex leads to expression of the cydABCD operon in a medium (LB-glucose) where it is not expressed in a rex+ strain. To further explore the regulation of the cydABCD operon by Rex, we asked how the deletion of Rex binding sites in the cydA untranslated region would affect expression of the cydABCD operon (Fig. 2). MH5895 (WT carrying cydA3′del-lacZ), which contains a 3′ deletion in the cydA leader region removing all three previously identified Rex binding sites, and MH5878 (WT carrying cydAFL-lacZ) were grown in NSMPG and assayed for expression from the cydA promoter. NSMPG was previously shown to allow the expression of the cydABCD operon (36). β-Galactosidase expression from the full-length and truncated promoters occurred during post-exponential phase; deletion of the Rex binding sites led to increased expression compared to that from the full-length promoter (Fig. 2). The most dramatic difference was observed during late-stationary-phase growth, a time when expression from the full-length cydA promoter fusion in a WT strain (MH5878) decreased but the expression of the truncated promoter (MH5895) remained elevated, suggesting that Rex represses cydA during late-stationary-phase growth. The expression of the cydA3′del-lacZ (MH6065) or cydAFL-lacZ (MH6064) fusion in the rex mutant strain was similar to that of the cydA3′del-lacZ fusion in the WT strain (MH5895). These data predict that a second promoter in the Rex binding region that is expressed in the absence of Rex in the rex mutant strain does not exist and are consistent with the previously identified promoter being the promoter regulated by Rex. To further check for another promoter in the untranslated region, we constructed a lacZ fusion including most of the untranslated region, from positions +20 to +304, from which there was no expression (data not shown). These data are consistent with the model that Rex acts as a repressor of the cydABCD operon.
FIG. 2.
Similar increases in expression of cydA are observed in a rex strain and by deletion of the binding sites in the cydA promoter fusion in a WT strain. Growth and β-galactosidase expression in cells cultured for 8 h in NSMPG are shown. Solid symbols, growth; open symbols, β-galactosidase activity. Symbols: ▪, MH5878 (JH642 cydA-lacZ); •, MH5895 (JH642 cydA3′del-lacZ); ▴, MH6064 (rex cydA-lacZ); ▾, MH6065 (rex cydA3′del-lacZ). Cartoons above the graph show regions of the promoter fused to lacZ. OD540, optical density at 540 nm.
ResD positively regulates expression of the cydABCD operon.
The ResD-ResE two-component signal transduction system plays a crucial role in the activation of genes involved in aerobic respiration (18, 24, 31, 38). Previously (28), we showed that the cydABCD operon was not expressed in a resDE mutant strain grown in LB supplemented with glucose, a medium in which the WT strain also failed to express the cydA fusion. Hence, we examined the expression of the full-length and truncated forms of the cydA-lacZ fusion in ΔresDE, ΔresD, and ΔresE strains cultured in NSMPG, a medium which allows expression from the WT cydA-lacZ fusion. Expression of the full-length cydA promoter (Fig. 3A) was decreased significantly in a ΔresD (MH6079) or ΔresDE (MH5880) strain compared to expression in the WT strain (MH5878). Expression of the truncated cydA promoter was also decreased (Fig. 3B) in a ΔresD (MH6080) or ΔresDE (MH5896) strain compared to expression in the parental strain (MH5895) (Fig. 2), but it retained low-level induction. This level of induction from the truncated but not the full-length cydA fusion suggests that the cydA promoter has a low basal level of expression independent of ResD that is observed in the absence of Rex repression. In contrast to the resD strain, the resE strain expressed either the full-length or truncated cydA promoter at a level similar to that of the WT strains. These data support an activator role for ResD, but not ResE, at the cydABCD operon promoter.
FIG. 3.
cydA expression requires ResD but not ResE. (A) In vivo expression of full-length cydABCD promoter. (B) In vivo expression of cydABCD promoter lacking Rex binding sites. Growth and β-galactosidase expression in cells cultured for 8 h in NSMPG are shown. Symbols: ▪, WT (MH5878 [A] or MH5895 [B]); ▴, resE mutant (MH6066 [A] or MH6067 [B]); ▾, resD mutant (MH6079 [A] or MH6080 [B]); •, resDE mutant (MH5880 [A] or MH5896 [B]). Solid symbols, growth; open symbols, β-galactosidase activity. The cartoon above each graph shows the region of the promoter fused to lacZ. OD540, optical density at 540 nm.
ResD binds the cydA promoter.
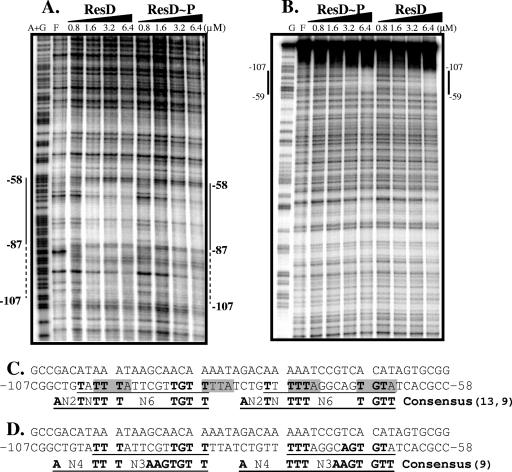
To determine if the role of ResD in the activation of the cydA promoter was direct or indirect, we asked if ResD or ResD∼P could bind the cydA promoter, using a DNase I protection assay. We found that similar concentrations of ResD and ResD∼P protected a single site on the cydA promoter, giving protection of the region spanning from nt −107 to −58 relative to the start of transcription of cydA, on both the coding and noncoding strands (Fig. 4A and B). Protection, starting at 1.6 μM ResD, was greater between bp −58 and −87 than between bp −88 and −107. In the case of the cydA promoter, phosphorylation of ResD did not enhance its ability to bind DNA. This result corroborates the in vivo expression studies, strongly suggesting a role for ResD, but not ResE, in the activation of transcription of the cydABCD operon.
FIG. 4.
(A) DNase I footprinting of cydA promoter coding strand, using ResD and ResD∼P. End-labeled FMH792 was used to create the probe. The concentration of ResD or ResD∼P is found at the top of each lane. Lane F, free of ResD; lane A+G, Maxam-Gilbert A+G sequencing reaction as a marker. (B) Footprinting of the noncoding strand. Lane G, Maxam-Gilbert G sequencing reaction as a marker. End-labeled FMH793 was used to create the probe. (C) ResD binding region in cydA promoter. Hydroxyl radical protection bases (8) are shaded in gray. The ResD binding consensus, according to bioinformatics (13), is shown in the bottommost line in bold, underlined capitals. The region containing the consensus in the cydA promoter is underlined, and the conserved bases are shown in bold on the noncoding strand. (D) ResD binding region compared to the consensus from the work of Geng et al. (9). The ResD binding consensus is shown in the bottommost line in bold, underlined capitals. The region containing the consensus in the cydA promoter is underlined, and the conserved bases are shown in bold on the noncoding strand.
Several suggestions for ResD binding consensuses have been made. Hydroxy radical footprinting of ResD on the nasD and hmp promoters (8) revealed that ResD binds in tandem to the same face of the helix, most commonly protecting A(AT)TT sequences. Figure 4C shows the occurrence of these sequences on the noncoding strand with approximately 10-bp spacing within the ResD-protected region of the cydA promoter. A bioinformatics approach (13) yielded a consensus that was subsequently reinterpreted as TTGTN6TTTNTN2A (9), with different consensus sequences at two half-sites, i.e., TTGT at the 5′ half-site and TTTNTN2A at the 3′ half-site. Figure 4C shows two proposed consensus sequences aligned within the ResD-protected cydA sequence on the noncoding strand, with one in the more fully protected region at bp −66 to −79 and the other in the more weakly protected region at bp −87 to −101. Base pairs identical to the consensus sequence are shown in bold. Unexpectedly, the more strongly protected region showed 7 of 9 bp conserved from bp −66 to −79, while the more weakly protected region had 8 of 9 bp conserved from bp −87 to −101. Recently, another consensus for ResD binding was proposed (TTGTGAAN3TTTN4A) (9). The original ResD binding sequence proposed in 2000, TTTGTGAAT (23, 38), corresponds more closely to the 5′ half-site of this consensus proposal, TTGTGAA, than to the bioinformatics-derived sequence, TTGT. Interestingly, the 5′ half-site of this consensus (9) is more conserved in the strongly protected region of the cydA promoter, bp −65 to −79 (5 of 7 bp), than in the weakly protected region, bp −87 to −99 (3 of 7 bp) (Fig. 4D). These data are consistent with the TTGTGA sequence being responsible for the enhanced ResD binding at the −65-to-−79 consensus sequence on the cydA promoter.
A poor carbon source causes increased expression of the cydA promoter.
It has been reported that glucose is required for expression of the cydABCD operon (36), suggesting that the cydA promoter may be subject to catabolite regulation. Because other studies have reported that cydA is positively (17, 21) or negatively (37) regulated or not regulated (1) by catabolite regulation, we asked if or how a poor carbon source would affect cydA expression under the conditions studied here. The strain containing the cydAFL-lacZ fusion (MH5878) was grown in NSMP with gluconate as an alternate carbon source, and its expression was compared with that of the same strain grown in NSMPG. Gluconate can serve as a poor carbon source since the genes for gluconate metabolism encoded by gntRKPZ are under strong catabolite repession (7), indicating the preference for glucose over gluconate. As shown in Table 2, during all hours of growth tested, the expression of the cydAFL promoter fusion was two- to threefold higher when cells were grown in gluconate, suggesting that cydA is subject to catabolite repression.
TABLE 2.
Effect of a poor carbon source, gluconate, on cydA promoter expression
| Time (h)a | β-Galactosidase sp act
|
Fold Change | |
|---|---|---|---|
| 0.5% Glucose | 0.5% Gluconate | ||
| 2 | 222 | 397 | 1.8 |
| 3 | 143 | 411 | 2.8 |
| 4 | 138 | 452 | 3.3 |
Hours recorded post-transition state (0 h).
CcpA negatively regulates the expression of the cydABCD operon.
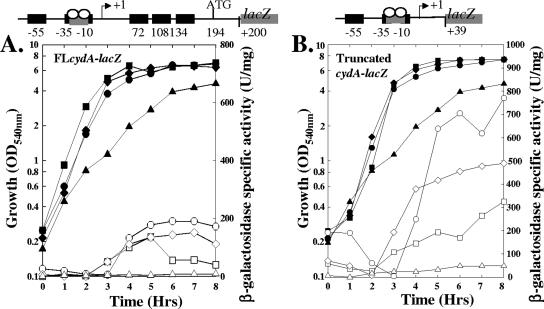
The above results led us to ask what role CcpA might have in regulating the expression of the full-length and truncated forms of the cydA promoter in NSMPG (Fig. 5). Induction from the WT promoter fusion occurred at approximately the same time in the WT and ccpA mutant strains, but expression in the ccpA mutant strain continued later into stationary phase than that in the WT strain with the full-length (MH5878) promoter, where β-galactosidase specific activity decreased after 5 h of growth (Fig. 5A). Expression in MH5894 (ccpA::Tn917 cydA-lacZ) was as much as fourfold higher than that from the WT promoter (Fig. 5A, hours 6 to 10). Expression of the truncated promoter (Fig. 5B) was >2.5-fold higher in the ccpA mutant strain (MH5897) than in the ccpA+ strain (MH5895). The maximal expression observed from the cydA promoter occurred in MH5897 (ccpA::Tn917 cydA3′del-lacZ), indicating that the actions of both repressors, CcpA and Rex, are cumulative. These data suggest that CcpA serves as a repressor of the cydABCD operon, with the maximum difference in expression between the WT and ccpA mutant strains occurring during the late stationary phase of growth (Fig. 5).
FIG. 5.
Effect of CcpA or cre box mutation on expression of the cydABCD operon. (A) Expression of full-length cydABCD promoter. (B) Expression of cydABCD promoter lacking Rex binding sites. Growth and cydA-lacZ expression in cells cultured for 8 h in NSMPG are shown. Solid symbols, growth; open symbols, β-galactosidase activity. Symbols: ▪, WT (MH5878 [A] or MH5895 [B]); •, ccpA mutant (MH5894 [A] or MH5897 [B]); ⧫, cydAcre1-lacZ mutant (MH6095 [A] or MH6077 [B]); ▴, resDE ccpA mutant (MH6301 [A] or MH6302 [B]). The cartoon above each graph shows the region of the promoter fused to lacZ. OD540, optical density at 540 nm.
ResD is required for elevated expression in a ccpA mutant strain. Using a resDE ccpA double mutant strain, we found that expression of the full-length cydA promoter fusion required ResD (Fig. 5A, MH6301), while the truncated form of the cydA promoter (Fig. 5B, MH6302) induced a low level of expression in a resDE ccpA double mutant, which experienced a 15- to 20-fold decrease in expression in the absence of ResD. These data support the observations that ResD plays a critical role in activation of the cydA promoter and that Rex can block expression of the promoter fusion containing the Rex binding site but that low-level expression persists for the 3′-deleted promoter fusion in the resDE ccpA double mutant. The reduced growth rate of the resDE ccpA double mutant is likely because a ccpA mutation causes defective phosphoenolpyruvate:sugar phosphotransferase system sugar uptake, which may expose the resD growth requirement for glucose (31).
CcpA binds the cydA promoter region.
To determine if the regulation of the cydA promoter by CcpA was direct or indirect, we asked if CcpA binds the cydA promoter region, using DNase I footprinting (Fig. 6). The results showed that CcpA (2.3 μM) protected the cydA promoter sequence from bp −33 to −4 upstream of the start of the cydA transcription start site (Fig. 6A), including the sequences for the −10 and −35 promoter elements (36) (Fig. 1). A hypersensitive site just upstream of the protected region was observed at about bp −36. We identified a weak cre site (the consensus binding sequence for CcpA) at positions −12 to −32 in the CcpA binding region (Fig. 6C).
FIG. 6.
CcpA binds to the cydA promoter at a sequence bearing significant similarity to the consensus cre box. (A) DNase I footprinting on the coding strand. The end-labeled primer FMH792 was used to create the probe. Labeled DNA fragments are the PCR products obtained using the FMH792 and FMH793 primers, with JH642 chromosomal DNA as the template. The CcpA concentration is shown at the top of each lane. A hypersensitive site is marked with an arrowhead. Lane F, free of CcpA; lane A+G, Maxam-Gilbert A+G sequencing reaction as a marker. (B) Footprinting on the noncoding strand. End-labeled FMH793 was used to create the probe. (C) Alignment of CcpA binding site with a consensus for the cre box and the cydA promoter showing the single point mutation created in the cre box, cre1. Bases identical to the consensus are shown in bold, and the point mutation in the cre box is shown in gray. The cre box consensus sequence is shown using IUPAC (International Union of Pure and Applied Chemistry) ambiguity codes for DNA sequences, as follows: W = A or T, R = A or G, Y = C or T, and N = A, T, C, or G. (D) CcpA binding to cydA promoter without any cofactor. (E) CcpA binding to cydA promoter in the presence of G-6-P, a putative cofactor for CcpA. (F) CcpA binding to cydA promoter with cre1 mutation in the presence of G-6-P.
The presence of a weak cre sequence and a requirement for elevated levels of CcpA to complete the footprint on the cydA promoter suggested the possibility of the requirement for a cofactor for CcpA to obtain more efficient binding. This hypothesis was tested using G-6-P, a putative cofactor shown to enhance CcpA binding (10). EMSAs performed using CcpA alone with the cydA promoter exhibited a significant shift at 0.9 μM, a concentration somewhat lower than that seen via footprinting (Fig. 6A, B, and D). The addition of 30 mM G-6-P to the binding buffer enhanced the binding by CcpA at least 10-fold (Fig. 6E). The addition of other known small-molecule cofactors of CcpA (fructose-1,6-bisphosphate and NADP) had no effect on its binding to cydA (data not shown).
Footprinting analysis provided us with a sequence approximately 68% identical to the consensus. A single-base-pair mutation was created in the conserved residue of this cre box (Fig. 6C) to test its validity. CcpA failed to bind to the cydAcre1 probe as efficiently as it did to the WT promoter, requiring an approximately 10-fold higher concentration of CcpA (Fig. 6F).
WT strains with either the full-length or truncated cydA promoter fusion containing the cre1 mutation were grown in NSMPG. In either MH6095 (Fig. 5A) or MH6077 (Fig. 5B), the in vivo expression of the cydAcre1 promoter fusion was higher than the expression observed for a nonmutated promoter fusion in strain MH5878 (Fig. 5A) or MH5895 (Fig. 5B). Together, these observations strongly suggest a repressor function for CcpA directly on the cydA promoter.
DISCUSSION
Rex does not repress an unknown promoter in the untranslated region of the cydA operon.
The architecture of the cydA 5′ region includes the promoter and a long untranslated sequence that follows the transcriptional start at position +1. The absence of activity from a transcriptional fusion containing the untranslated region only (data not shown) and the similar patterns of expression of a cydA-lacZ fusion and a cydA3′del-lacZ or cydAFL-lacZ fusion in a rex mutant strain argue against an additional promoter and suggest that the cydA untranslated region is devoted to a regulatory cis element for the promoter via Rex rather than being a transcriptional element by itself. Rex has also been shown to bind to the untranslated regions downstream of the ndh promoter (11) and the ldh promoter (17); in the latter case, a second Rex binding site was located over the −10 promoter element.
ResD stimulates the basal level of expression of the cydA promoter.
Previously, we have shown (28) that in a resD rex double mutant strain, the production of cytochrome bd is sufficient to complement a number of phenotypes associated with a resDE deletion. The fact that ResD stimulates activation of the cydABCD operon leads one to ask how enough cytochrome bd is produced in a resD rex background to accomplish phenotypic complementation. During growth in NSMPG, the expression of cytochrome bd in strain MH5896 (ΔresDE cydA3′del-lacZ) (Fig. 3B), in which the effects of both ResD and Rex on the cydA promoter are removed, was approximately 50% (75 U/mg) that from the full-length promoter in the WT background (Fig. 2). This level of expression is sufficient to complement the phenotypes associated with the loss of terminal oxidase expression in a ΔresDE strain by allowing cytochrome bd to compensate for the loss of cytochromes caa3 and aa3.
ResD directly regulates cydA expression.
The presence of ResD in a strain with a cydA promoter fusion lacking the Rex binding sites stimulated the expression of that cydA fusion approximately 10-fold over that observed in the resD mutant strain, while ResD was required for expression of a cydAFL promoter fusion containing the Rex binding sites in a WT strain. Evidence suggests that ResD regulation is direct, as ResD binds to the cydA promoter upstream of the −35 element in a sequence that contains two sequences similar to proposed ResD consensus binding sequences. It was predicted that an activator protein might function directly downstream of position −80 on the cydA promoter because an approximately 15-fold decrease in expression resulted upon deletion of nt −79 to −74 relative to the transcription start site (17). Our data suggest that this regulator is likely ResD because the 5′ deletion of nt −74 to −79 would remove the 3′ half-site of the conserved ResD binding sequence between positions −65 and −79 in the cydA promoter sequence most strongly protected by ResD.
In contrast to our results, a previous study (17) found that ResD had no significant role in cydA regulation. There may be several reasons for the differences in the results. The previous growth conditions were anaerobic, while our studies were done under aerobic conditions. Perhaps more importantly, the Bacillus strains in the previous study were maintained on tryptose blood agar base, a condition identified during early phenotypic analysis of resDE mutant strains that led to lysis of those strains or the acquisition of a secondary mutation that allowed growth of the resDE mutants on tryptose blood agar base (31). The secondary mutations were later shown to be loss-of-function mutations in rex (28). Thus, the impact of ResD on cydA activation would be minimized in a strain containing a rex mutation. Our results indicated that the full-length promoter required ResD for expression and that deletion of the Rex binding sites from the promoter fusion in a resD strain allowed 40 to 60% of the expression observed for the full-length promoter fusion in a WT strain.
Binding of ResD to the cydA promoter was not enhanced by ResD phosphorylation. This is not unprecedented. Previous work has shown that phosphorylation of ResD can enhance its ability to bind at some regulated sites but not at adjacent ResD-regulated sites (38). The ctaA-ctaB intergenic region contains three ResD binding sites. Binding to one of the three sites was enhanced by phosphorylation of ResD, while phosphorylation made no significant difference in the KD of binding at either of the other two sites. The in vivo data assessing the role of ResE in cydA promoter expression indicated that ResE was not important for cydA promoter function. Thus, if ResD requires phosphorylation in vivo for cydA activation, it is not via the cognate histidine kinase, ResE. Unphosphorylated ResD was previously shown to function in vivo (8) when D57A ResD in a resDE double mutant was able to stimulate expression of nasD, hmp, and fnr, although the stimulating effect was lower than that of native ResD. Expression from nasD and hmp was in response to oxygen limitation, suggesting that unphosphorylated ResD is capable of responding to the redox state of the cell. It remains to be determined for the cydA promoter whether ResD is phosphorylated by another histidine kinase or a small high-energy phosphorylated compound or functions in the unphosphorylated state.
CcpA represses the cydA promoter directly.
In vivo expression assays in a ccpA mutant background (Fig. 5) showed that CcpA repressed the cydA promoter. CcpA repression was judged to be direct because a mutation in the cre box reduced CcpA binding in vitro and relieved repression in vivo. These data are consistent with previous data implicating CcpA in the negative regulation of a number of components involved in aerobic respiration (18, 20, 21) and with the observation that cydA was induced during carbon-limiting aerobic growth (37). However, our data differ from those of studies (17, 21) which suggest that CcpA activates the cydA promoter. The reason for the differences between in vivo cydA expression observed previously (17) and our data are not clear. Culture conditions and strain differences may be factors. A role for CcpA in activation of the cydA promoter was suggested by the results of microarray analysis (21). That analysis compared gene expression in a WT versus a ccpA mutant strain cultured in LB medium during exponential phase, either with or without glucose. Previous work from both our lab and others (17, 28, 36), as well as data presented here (Fig. 2, 3, and 5), establishes that the cydA promoter is expressed during stationary phase, with only low levels of expression during exponential growth. The fact that the microarray analysis was performed on cells harvested during exponential growth most likely led to the differences in the data that we observed. Interestingly, a second array analysis of cells grown to exponential phase in C minimal medium supplemented with succinate and glutamate showed no role for CcpA in the regulation of the cydABCD operon (1), which is consistent with our in vivo cydA-lacZ fusion data during exponential growth (Fig. 5A).
One reason for the weak similarity between the cre box found in the cydA promoter and the consensus cre site may be the location of the binding site (over the −10 and −35 promoter elements). The fact that the cre box in the cydA promoter is a weak match is probably a contributing factor to the somewhat lower affinity of CcpA for this promoter, as previous work in our laboratory with a cre box that was a perfect match to the consensus had a very high affinity for CcpA (26). The addition of G-6-P to the EMSA reaction buffer enhanced CcpA binding to the cydA promoter. The importance of the requirement of a cofactor is underscored by the observation that the gel shift observed in the absence of G-6-P not only required a higher concentration to bind but also exhibited a smear instead of a discrete band. A smear is usually observed when a DNA-protein complex tends to dissociate during its movement through the gel, suggesting a weak DNA-protein interaction. The addition of G-6-P replaced the smear with discrete bands via increased affinity of CcpA for cydA.
Hierarchy among cydA regulators.
ResD is required for expression from the cydAFL promoter fusion at the onset of stationary-phase growth in the WT or a ccpA mutant strain. Even though cydAFL expression was elevated and prolonged in the absence of CcpA repression in a ccpA mutant strain, the dependency on ResD prevailed. A logical explanation for the timing of expression is that when ResD induction occurs (31), cydA is induced. Interestingly, cydA expression in a WT or ccpA mutant strain (Fig. 3B and 5B) was no longer entirely dependent on ResD when the downstream Rex binding sites were removed from the promoter fusion or when rex was deleted (28), since low-level induction was observed in a resD or resD ccpA strain, but the timing of induction did not change. While this low-level induction suggests that the cydA promoter is capable of low-level function without any of the three known regulators, it raises another question. Is there an additional cydA regulator(s) that functions to halt the expression of this presumed σA-responsive promoter during exponential growth, perhaps a transition state regulator? The answer awaits further investigation.
Regulation by multiple regulators ensures redox balance.
cydABCD codes for a cytochrome bd-type terminal oxidase, and Rex is a major regulator of this operon. Rex responds to the NADH/NAD+ ratio in the cell (11). The requirement of ResD for the activation of this operon affirms its importance in the respiratory scheme. ResD was shown to be required for the expression of ctaA (24, 31, 38), and therefore heme A (32, 33), which is required for production of the other two terminal oxidases present in B. subtilis, i.e., cytochromes aa3 and caa3. During aerobic respiration, electrons from reducing equivalents are transferred to molecular oxygen by terminal oxidases, whose production can be need-based depending on the physiological state of the cell. cydA was derepressed in a rex mutant, similar to what would be expected under physiological conditions when the NADH/NAD+ ratio is high. A lower binding affinity of Rex for cydA was shown in the presence of NADH than in the presence of NAD+ (11). A similar condition can be envisioned during respiration using a poor carbon source when CcpA is inactive (for a review, see reference 30), a condition which sharply decreases overflow metabolism (4) via pta, ackA, and alsSD pathways (19, 25, 34) and leads to increased energy flow via the Krebs cycle (4) that requires higher levels of terminal oxidases to maintain the NADH/NAD+ ratio. The higher NADH/NAD+ ratio is accompanied by derepression of cydA (37), which we now understand occurs via Rex, thus coordinating derepression of cydA by Rex and CcpA. Recent microarray data suggested that Rex may also repress Kreb cycle enzymes (11), as does CcpA (15, 16). If this is proven correct, then under carbon starvation conditions, Kreb cycle enzymes would be relieved of repression by both Rex and CcpA repression.
In summary, our analysis of the cydA promoter has led to the identification of three proteins that regulate promoter activity, with ResD acting as an activator and CcpA and Rex acting as repressors. The fact that at least three regulatory proteins bind the cydA promoter region to control the level of transcription of this operon suggests that this operon is subject to complex regulation to allow responses to different environmental conditions.
Acknowledgments
This work was supported by National Institutes of Health grant GM-33471 to F.M.H.
We thank Elena Sharkova for pES67. We thank Xiaohui Zhang for pXH3 and the resD mutant. We thank Tina Henkin for providing the plasmid used for the overproduction and isolation of CcpA. We thank Jörg Stülke for providing the ccpA mutant strain.
Footnotes
Published ahead of print on 23 February 2007.
REFERENCES
- 1.Blencke, H. M., G. Homuth, H. Ludwig, U. Mader, M. Hecker, and J. Stulke. 2003. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5:133-149. [DOI] [PubMed] [Google Scholar]
- 2.Brekasis, D., and M. S. Paget. 2003. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2). EMBO J. 22:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 24-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Inc., New York, NY.
- 4.Dauner, M., T. Storni, and U. Sauer. 2001. Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J. Bacteriol. 183:7308-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari, E., S. M. Howard, and J. A. Hoch. 1986. Effect of stage 0 sporulation mutations on subtilisin expression. J. Bacteriol. 166:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortnagel, P., and E. Freese. 1968. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J. Bacteriol. 95:1431-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita, Y., and Y. Miwa. 1994. Catabolite repression of the Bacillus subtilis gnt operon mediated by the CcpA protein. J. Bacteriol. 176:511-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng, H., S. Nakano, and M. M. Nakano. 2004. Transcriptional activation by Bacillus subtilis ResD: tandem binding to target elements and phosphorylation-dependent and -independent transcriptional activation. J. Bacteriol. 186:2028-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng, H., Y. Zhu, K. Mullen, C. S. Zuber, and M. M. Nakano. 2007. Characterization of ResDE-dependent fnr transcription in Bacillus subtilis. J. Bacteriol. 189:1745-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosseringer, R., E. Kuster, A. Galinier, J. Deutscher, and W. Hillen. 1997. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J. Mol. Biol. 266:665-676. [DOI] [PubMed] [Google Scholar]
- 11.Gyan, S., Y. Shiohira, I. Sato, M. Takeuchi, and T. Sato. 2006. Regulatory loop between redox sensing of the NADH/NAD+ ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J. Bacteriol. 188:7062-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning II: a practical approach. IRL Press, Washington, DC.
- 13.Hartig, E., H. Geng, A. Hartmann, A. Hubacek, R. Munch, R. W. Ye, D. Jahn, and M. M. Nakano. 2004. Bacillus subtilis ResD induces expression of the potential regulatory genes yclJK upon oxygen limitation. J. Bacteriol. 186:6477-6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homuth, G., A. Rompf, W. Schumann, and D. Jahn. 1999. Transcriptional control of Bacillus subtilis hemN and hemZ. J. Bacteriol. 181:5922-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, H. J., C. Jourlin-Castelli, S. I. Kim, and A. L. Sonenshein. 2002. Regulation of the Bacillus subtilis ccpC gene by ccpA and ccpC. Mol. Microbiol. 43:399-410. [DOI] [PubMed] [Google Scholar]
- 16.Kim, H. J., A. Roux, and A. L. Sonenshein. 2002. Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol. Microbiol. 45:179-190. [DOI] [PubMed] [Google Scholar]
- 17.Larsson, J. T., A. Rogstam, and C. von Wachenfeldt. 2005. Coordinated patterns of cytochrome bd and lactate dehydrogenase expression in Bacillus subtilis. Microbiology 151:3323-3335. [DOI] [PubMed] [Google Scholar]
- 18.Liu, X., and H. W. Taber. 1998. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J. Bacteriol. 180:6154-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moir-Blais, T. R., F. J. Grundy, and T. M. Henkin. 2001. Transcriptional activation of the Bacillus subtilis ackA promoter requires sequences upstream of the CcpA binding site. J. Bacteriol. 183:2389-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monedero, V., G. Boel, and J. Deutscher. 2001. Catabolite regulation of the cytochrome c550-encoding Bacillus subtilis cccA gene. J. Mol. Microbiol. Biotechnol. 3:433-438. [PubMed] [Google Scholar]
- 21.Moreno, M. S., B. L. Schneider, R. R. Maile, W. Weyler, and M. H. Saier, Jr. 2001. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 39:1366-1381. [DOI] [PubMed] [Google Scholar]
- 22.Nakano, M. M., G. Zheng, and P. Zuber. 2000. Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis. J. Bacteriol. 182:3274-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano, M. M., Y. Zhu, M. Lacelle, X. Zhang, and F. M. Hulett. 2000. Interaction of ResD with regulatory regions of anaerobically induced genes in Bacillus subtilis. Mol. Microbiol. 37:1198-1207. [DOI] [PubMed] [Google Scholar]
- 24.Paul, S., X. Zhang, and F. M. Hulett. 2001. Two ResD-controlled promoters regulate ctaA expression in Bacillus subtilis. J. Bacteriol. 183:3237-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Presecan-Siedel, E., A. Galinier, R. Longin, J. Deutscher, A. Danchin, P. Glaser, and I. Martin-Verstraete. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J. Bacteriol. 181:6889-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri-Taneja, A., S. Paul, Y. Chen, and F. M. Hulett. 2006. CcpA causes repression of the phoPR promoter through a novel transcription start site, PA6. J. Bacteriol. 188:1266-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., D. W. Russell, N. Irwin, and K. A. Janssen. 2001. Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Schau, M., Y. Chen, and F. M. Hulett. 2004. Bacillus subtilis YdiH is a direct negative regulator of the cydABCD operon. J. Bacteriol. 186:4585-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimotsu, H., and D. J. Henner. 1986. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene 43:85-94. [DOI] [PubMed] [Google Scholar]
- 30.Stulke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54:849-880. [DOI] [PubMed] [Google Scholar]
- 31.Sun, G., E. Sharkova, R. Chesnut, S. Birkey, M. F. Duggan, A. Sorokin, P. Pujic, S. D. Ehrlich, and F. M. Hulett. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson, B., K. K. Andersson, and L. Hederstedt. 1996. Low-spin heme A in the heme A biosynthetic protein CtaA from Bacillus subtilis. Eur. J. Biochem. 238:287-295. [DOI] [PubMed] [Google Scholar]
- 33.Svensson, B., and L. Hederstedt. 1994. Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J. Bacteriol. 176:6663-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turinsky, A. J., T. R. Moir-Blais, F. J. Grundy, and T. M. Henkin. 2000. Bacillus subtilis ccpA gene mutants specifically defective in activation of acetoin biosynthesis. J. Bacteriol. 182:5611-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weickert, M. J., and S. Adhya. 1992. A family of bacterial regulators homologous to Gal and Lac repressors. J. Biol. Chem. 267:15869-15874. [PubMed] [Google Scholar]
- 36.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamboni, N., N. Mouncey, H. P. Hohmann, and U. Sauer. 2003. Reducing maintenance metabolism by metabolic engineering of respiration improves riboflavin production by Bacillus subtilis. Metab. Eng. 5:49-55. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, X., and F. M. Hulett. 2000. ResD signal transduction regulator of aerobic respiration in Bacillus subtilis: ctaA promoter regulation. Mol. Microbiol. 37:1208-1219. [DOI] [PubMed] [Google Scholar]