Abstract
A Tn5 mutant strain of Sinorhizobium meliloti with an insertion in tpiA (systematic identifier SMc01023), a putative triose phosphate isomerase (TPI)-encoding gene, was isolated. The tpiA mutant grew more slowly than the wild type on rhamnose and did not grow with glycerol as a sole carbon source. The genome of S. meliloti wild-type Rm1021 contains a second predicted TPI-encoding gene, tpiB (SMc01614). We have constructed mutations and confirmed that both genes encode functional TPI enzymes. tpiA appears to be constitutively expressed and provides the primary TPI activity for central metabolism. tpiB has been shown to be required for growth with erythritol. TpiB activity is induced by growth with erythritol; however, basal levels of TpiB activity present in tpiA mutants allow for growth with gluconeogenic carbon sources. Although tpiA mutants can be complemented by tpiB, tpiA cannot substitute for mutations in tpiB with respect to erythritol catabolism. Mutations in tpiA or tpiB alone do not cause symbiotic defects; however, mutations in both tpiA and tpiB caused reduced symbiotic nitrogen fixation.
Triose phosphate isomerase (TPI) catalyzes the reversible interconversion of glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). This activity makes TPI a key enzyme of central carbon metabolism, allowing it to play a role in the glycolysis (Embden-Meyerhof-Parnas [EMP]), gluconeogenesis, pentose phosphate (PP), and Entner-Doudoroff (ED) pathways. Past research on the symbiotic soil bacterium Sinorhizobium meliloti and other rhizobia has shown that hexose catabolism proceeds through the ED and PP pathways, while the EMP pathway functions at very low levels, if at all (49). Gluconeogenesis, however, is functional, and mutations in gluconeogenic enzymes have been shown to cause complex symbiotic phenotypes, including reduced or abolished nitrogen-fixing activity (6, 15, 16, 19, 24).
Recent work has confirmed the roles of the ED and PP pathways as catabolic pathways in S. meliloti. Analyses of carbon flux carried out using labeled carbon compounds and gas chromatography-mass spectrometry have confirmed the absence of the EMP pathway during growth with glucose (17). Another set of experiments done using labeled carbon and nuclear magnetic resonance is in agreement, confirming that glucose is degraded primarily through the ED pathway (22, 40). The gas chromatography-mass spectrometry and nuclear magnetic resonance experiments also confirm that some of the G3P produced by the catabolism of hexoses through the ED pathway is converted back to higher-molecular-weight compounds through TPI and fructose bisphosphate aldolase (FBA) in a cyclic pathway. Because FBA requires G3P and DHAP as substrates, the interconversion of G3P and DHAP is necessary for the cyclic metabolism of hexoses as well as gluconeogenesis. An S. meliloti strain missing TPI activity would therefore be compromised metabolically.
Based on gene homology, S. meliloti has two putative chromosomal genes predicted to encode TPI enzymes (18). The gene tpiA (SMc01023) appears to be transcribed independently, while tpiB (SMc01614) appears to be transcribed along with two other genes: SMc01615 and rpiB. A putative operon upstream of tpiB includes homologs of genes involved in the catabolism of erythritol in Rhizobium leguminosarum and Brucella abortus (45, 50). The tpiB gene and its proximity to erythritol catabolic genes are conserved in a number of organisms, which led us to hypothesize that tpiB is involved in erythritol catabolism. In this work we describe the isolation and characterization of TPI mutants in S. meliloti. We have found that both tpiA and tpiB encode functional TPI enzymes and that tpiB is induced by and specifically required for erythritol catabolism. Mutations in both TPI genes cause a loss of the ability to use gluconeogenic carbon sources, but viability and growth with other carbon sources or combinations of nonpermissive carbon sources remain possible. Mutations in either tpiA or tpiB alone did not affect symbiosis, while mutations in both TPI-encoding genes were found to cause reduced levels of symbiotic nitrogen fixation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used and generated in this work are listed in Table 1. Bacteria were grown at 30°C, using either Luria-Bertani broth (LB) or Vincent's minimal medium (VMM), as previously described (43, 44). All carbon sources used in VMM were filter sterilized and used at a concentration of 15 mM, unless otherwise stated. All sugars were in the d configuration, except l-arabinose, l-fucose, l-rhamnose, and l-lyxose. When required, antibiotics were used at the following concentrations for Escherichia coli: tetracycline (Tc), 5 μg ml−1; neomycin (Nm), 200 μg ml−1; kanamycin, 20 μg ml−1; streptomycin, 200 μg ml−1; rifampin (Rf), 50 μg ml−1; chloramphenicol (Cm), 20 μg ml−1; and gentamicin (Gm), 20 μg ml−1. Gm was used at a concentration of 50 μg ml−1 for S. meliloti.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Strains | ||
| S. meliloti | ||
| Rm1021 | SU47 str-21, Smr | 30 |
| Rm5000 | SU47 rif-5, Rfr | 12 |
| Rm5439 | Rm1021 pckA1::TnV | 16 |
| SRmA185 | Rm1021 tpiA1::Tn5 | This work |
| SRmA327 | tpiA1, Φ(SRmA185)→Rm1021, Nmra | This work |
| SRmA355 | Rm5000 tpiB1::pKNOCK-Gm | This work |
| SRmA366 | tpiA1 tpiB1, Φ(SRmA185)→SRmA355, Nmr | This work |
| SRmA449 | Rm1021 ΔtpiA | This work |
| SRmA515 | ΔtpiA tpiB1, Φ(SRmA355)→SRmA449, Gmr | This work |
| SRmA584 | Rm1021 tpiB2::Tn5-B20 | This work |
| SRmA585 | ΔtpiA tpiB2, Φ(SRmA584)→SRmA449, Nmr | This work |
| E. coli | ||
| MM294A | pro-82 thi-1 hsdR17 supE44 | 14 |
| MT607 | MM294A recA56 | 14 |
| MT616 | MT607(pRK600) | 14 |
| S17-1 | recA derivative of MM294A with integrated RP4-2 (Tc::Mu::Km::Tn7) | 46 |
| DH5α | gyrA96, Nalr | Invitrogen |
| EcA100 | MT607 Rifr derivative | Lab collection |
| EcA101 | MT607 Tn5-B20 Kanr | 9 |
| Plasmids | ||
| pRK600 | pRK2013 npt::Tn9, Cmr | 14 |
| pRK602 | pRK600W Tn5, Cmr Nmr | 13 |
| pBluescriptII SK+ | Cloning vector, Apr | Stratagene |
| pKNOCK-Gm | Suicide vector, Gmr | 1 |
| pRK7813 | Broad-host-range vector, Tcr | 25 |
| pJQ200SK | Gene replacement suicide vector, Gmr | 42 |
| pPH1JI | IncP plasmid, Gmr Tcr | 5 |
| pNP150 | pKNOCK-Gm with 350-bp internal tpiB fragment | This work |
| pNP152 | pBluescriptII SK+ with 700-bp tpiA 5′ flanking region | This work |
| pNP154 | pBluescriptII SK+ with 660-bp tpiA 3′ flanking region | This work |
| pNP155 | pJQ200SK with tpiA flanking regions | This work |
| pNP163 | tpiB-complementing cosmid | This work |
| pNP166 | Constitutive tpiA | This work |
| pNP167 | Constitutive tpiB | This work |
Notation indicates that SRmA327 was constructed by ΦM12 transduction of SRmA185 into Rm1021 with selection for Nmr.
DNA manipulations and plasmid constructions.
Standard techniques were used for DNA isolation, restriction enzyme digests, ligations, transformations, and agarose gel electrophoresis (44).
To construct pNP150, a 350-bp internal fragment of tpiB was PCR amplified using primers 1 and 2 (Table 2) and genomic Rm1021 DNA as a template. The PCR product was gel isolated and cloned into pKNOCK-Gm by use of the BamHI and KpnI sites provided by primers 1 and 2. The resulting construct was named pNP150 and was transformed into competent E. coli S17-1.
TABLE 2.
Primers used in this work
| Primer no. | Nucleotide sequence (5′ → 3′) |
|---|---|
| 1 | ATATGATCCAGAACATGCACTGGGACGAT |
| 2 | TATAGGTACCGCTCGTAAGCCAGAAGAACG |
| 3 | AAGCGGCCGCCATGGGCGAGGGCCCA |
| 4 | AAGGATCCGGGATCTCCTCCAGATCA |
| 5 | AAGGATCCTTGCCGCGGGACTTGGA |
| 6 | AAGTCGACACCGCTCGGAACCTGCG |
| 7 | ATGGATCCAGGAGAAATAATTATGAGAGGATCGCATCATCATCATCATCATACGCCCGATATCCGCCC |
| 8 | ATGAATTCTCAGGCAGTCAATTCTTCATA |
| 9 | ATGGATCCAGGAGAAATAATTATGAGAGGATCGCATCATCATCATCATCATAGCAAGTCTGGTCTCTGGG |
| 10 | ATGAATTCTCAGATCGCCCGGGCTAC |
| 11 | CACGATGAAGAGCAGAAG |
| 12 | GGCCACGCGTCGACTAGTCAGNNNNNNNNNNACGCC |
| 13 | TAGGAGGTCACATGGAAGTCAGAT |
| 14 | GGCCACGCGTCGACTAGTCAG |
The plasmid pNP155 was constructed to create a chromosomal tpiA deletion in the strain Rm1021. A 700-bp region upstream of tpiA was PCR amplified from Rm1021 genomic DNA by use of primers 3 and 4 (Table 2), which add the restriction sites NotI and BamHI to the 5′ and 3′ ends of the fragment, respectively. This region was cloned into pBluescriptII with NotI and BamHI, yielding pNP152. Similarly, a 660-bp region downstream of tpiA was amplified with primers 5 and 6 (Table 2), which add the restriction sites BamHI and SalI to the 5′ and 3′ ends of the fragment, respectively. The 660-bp fragment was cloned into pNP152 by use of BamHI and SalI, yielding pNP154. A 1,360-bp NotI/SalI fragment was then subcloned from pNP154 into pJQ200SK (42), and this construct was named pNP155.
In order to constitutively express tpiA and tpiB, both open reading frames were cloned into a broad-host-range vector, pRK7813 (25). Primers 7 and 8 (Table 2) were used to amplify tpiA from Rm1021, which adds a ribosome binding site (GGAG), an N-terminal RGS(His)6 tag, and BamHI and EcoRI restriction sites to the gene. Primers 9 and 10 (Table 2) were used to amplify tpiB, adding the same features as tpiA. Both tpiA and tpiB were cloned into pRK7813, yielding pNP166 and pNP167, respectively. Both constructs were sequenced entirely, showing that neither had any deviation in sequence from the published Rm1021 genome (data not shown).
Genetic techniques and mutant construction.
Mutagenesis of Rm1021 with Tn5 was carried out using pRK602 as previously described (13). Conjugations between E. coli and S. meliloti were carried out using the mobilizing strain MT616 as previously described (16). Transduction with phage ΦM12 (12) was routinely used to move transposon markers from mutant strains into wild-type parent strains, and 50 to 100 colonies were screened to ensure that phenotypes were 100% linked in transduction and therefore associated solely with the transposon insertion.
Construction of the tpiB single crossover mutant SRmA355 was accomplished by conjugating pNP150 into Rm5000. Single crossovers were selected for by plating on VMM agar containing Rf and Gm. The pKNOCK tpiB insertion was confirmed by transductional linkage analysis with a nearby marker (data not shown). The tpiA tpiB mutant SRmA366 was constructed by transducing an SRmA185 ΦM12 lysate into an SRmA355 background. The chromosomal deletion of tpiA in SRmA449 was constructed by mating pNP155 into Rm1021 and selecting for single crossover events by use of Gm. Several of these were plated onto LB containing 5% sucrose to select for double crossover events. A sucrose-sensitive, Gms colony was purified, and the tpiA deletion was confirmed by PCR amplifying the deleted region with primers 3 and 6 (Table 2), sequencing the gel-isolated PCR product, and comparing the result with the expected nucleotide sequence (data not shown). Strain SRmA515 was created by moving the Gm marker from SRmA355 into SRmA449 by use of transduction. The tpiB-complementing cosmid pNP163 was isolated by conjugating an S. meliloti cosmid bank with SRmA515 and plating on a defined medium containing erythritol as a sole carbon source. The cosmid pNP163 was then mutagenized by passage through the Tn5-B20-carrying strain EcA101, selecting for cotransfer of the plasmid and Tn5-B20 into EcA100. Individual cosmids carrying Tn5-B20 were then mated into SRmA515 and screened for loss of the ability to complement growth on erythritol. One such mutagenized cosmid was isolated and shown to contain a mutation in tpiB by use of a protocol described below (data not shown). Marker exchange of the cosmid-borne tpiB mutation into the Rm1021 chromosome was performed as described previously (20), yielding strain SRmA584. The SRmA584 transposon marker was subsequently transferred into SRmA449 by transduction, yielding SRmA585.
Mutant identification.
To identify the point of insertion for individual Tn5 mutations, a modification of an arbitrary PCR protocol was utilized, as previously described (32, 41). Briefly, strains containing Tn5 or Tn5-B20 inserts were purified and the genomic DNA was used as a template. Two sequential PCRs were performed, the first with primers 11 and 12 (Table 2) and the second with primers 13 and 14, with the products of the first reaction as the template. The final PCR product was gel isolated and sequenced with primer 13 (Table 2) to locate the point of insertion in the genome.
TPI assays.
Enzyme assays were performed on cells grown in rich medium or in defined medium containing succinate, glucose, or erythritol. Cells were grown overnight and resuspended in an extraction buffer containing 100 mM Tris, 5 mM β-mercaptoethanol, and 1 mM MgCl2 at pH 7.6. A French pressure cell (16,000 lb/in2) was used to disrupt cells, with subsequent centrifugation to remove insoluble debris. TPI activity was assayed essentially as described previously (27). Volumes of cell extract were assayed such that measured rates of activity increased linearly with increasing volume of extract. Specific activities are expressed as nmol NADH oxidized per minute per milligram protein. Protein concentration was measured as described by Bradford, using a protein determination reagent (Sigma) and bovine serum albumin as a standard (7).
Symbiotic competence assays.
Plant tests were carried out as described previously (33, 41). Briefly, surface-sterilized alfalfa seeds (Medicago sativa cv. Rangelander) were sown onto 1.5% water agar plates and left to germinate. Sprouted seeds were then aseptically transferred to pots containing sterile sand and vermiculite with nitrogen-free Jensen's plant nutrient solution (20). Plants were inoculated with 104 to 105 bacteria per plant. Plants were harvested 28 to 35 days after inoculation, and dry weights of shoots cut at the cotyledons were used to assess symbiotic competence. Bacteria were routinely isolated from nodules and tested for expected phenotypes. Competition for nodule occupancy was assessed by inoculating plants with a combination of the wild type and the strain to be tested, followed by enumeration of nodule occupants, as previously described (41).
RESULTS
Analysis of tpiA and tpiB.
As part of our ongoing interest in rhamnose catabolism and associated symbiotic phenotypes (34, 43), we have screened many S. meliloti mutants for the ability to catabolize rhamnose as a sole carbon source. A number of Tn5-induced Rm1021 mutants unable to use rhamnose were isolated, including one strain, SRmA185, which was able to grow slowly only on plates containing rhamnose and unable to grow on glycerol plates. All mutations except for that of SRmA185 were situated in a contiguous chromosomal locus (systematic identifiers SMc02321 to SMc02325 and SMc03000 to SMc03003) (data not shown). This locus was found to be homologous to a rhamnose utilization locus previously reported for R. leguminosarum (43). An SRmA185 genomic DNA fragment spanning the Tn5 insertion and flanking DNA was amplified with an arbitrary PCR protocol. Sequencing the fragment revealed that the Tn5 insertion was interrupting the putative TPI-encoding gene tpiA (data not shown). The genome annotation of S. meliloti predicts that there are two chromosomal TPI-encoding genes, which have been annotated tpiA1 and tpiA2 (systematic identifiers SMc01023 and SMc01614, respectively) (18). In this study, we have shown that the two genes encode TPIs but that there is a significant difference in function between them. We are therefore suggesting the names tpiA and tpiB to replace tpiA1 and tpiA2, respectively, as the new names conform more to the standard genetic nomenclature (11). Our suggested gene names are used throughout the paper.
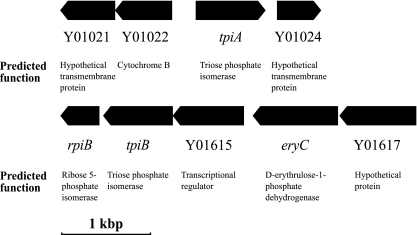
The gene tpiA is on the S. meliloti chromosome and appears to be isolated rather than transcribed within an operon (Fig. 1). The gene tpiB is also located on the chromosome and appears to be in a small operon downstream of a large operon which contains homologs of the erythritol utilization genes eryA, eryB, and eryC from B. abortus and R. leguminosarum (45, 50). Over the course of our characterization of TPI in S. meliloti, strains carrying several independent tpiA and/or tpiB alleles were constructed (Table 1).
FIG. 1.
Physical maps of two S. meliloti Rm1021 chromosomal regions carrying either (top) tpiA or (bottom) tpiB. Boxes represent predicted open reading frames, with the pointed end indicating the direction of transcription.
tpiA and tpiB are needed for gluconeogenesis.
Wild-type strain Rm1021 and the constructed tpiA and tpiB mutant strains were tested for the ability to grow on a number of types of defined media containing single carbon sources (Table 3). Mutations in tpiA caused a loss of the ability to utilize glycerol for growth as well as slow growth on rhamnose compared with that of the wild-type parent strain. Mutations in tpiB caused only a loss of the ability to use erythritol as a carbon source. This correlates with the fact that tpiB is situated downstream of a predicted erythritol catabolism operon (Fig. 1) and that tpiB-dependent TPI activity is induced by erythritol (Table 4). Not only does the tpiA tpiB strain have a combination of the phenotypes of tpiA and tpiB strains, but having no TPI genes causes a loss of the ability to utilize fucose, succinate, γ-aminobutyric acid (GABA), acetate, arabinose, glutamate, and lyxose. Strains with both tpiA and tpiB mutations were able to grow with combinations of nonpermissive carbon sources, such as glycerol and succinate or glycerol and arabinose. This is consistent with the hypothesis that gluconeogenesis occurs through FBA, as the permissive combinations of carbon sources supply both DHAP from glycerol and G3P from arabinose or succinate (3, 16, 49).
TABLE 3.
Relevant growth phenotypes of strains used in this work on defined media
| Strain | Relevant genotype | Growth with carbon sourcea
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glc | Suc | Gly | Rha | Ery | Fuc | GABA | Ace | Ara | Glu | Rib | Xyl | Lyx | ||
| Rm1021 | Wild type | + | + | + | + | + | + | + | + | + | + | + | + | + |
| SRmA449 | tpiA | + | + | − | ± | + | + | + | + | + | + | + | + | + |
| SRmA584 | tpiB | + | + | + | + | − | + | + | + | + | + | + | + | + |
| SRmA585 | tpiA tpiB | + | − | − | ± | − | − | − | − | − | − | + | + | − |
Grown on VMM media with 15 mM of a given carbon source. Abbreviations: Glc, glucose; Suc, succinate; Gly, glycerol; Rha, rhamnose; Ery, erythritol; Fuc, fucose; Ace, acetate; Ara, arabinose; Glu, glutamate; Rib, ribose; Xyl, xylose; Lyx, lyxose. The symbol indicates the growth phenotype as follows: +, growth same as that of the wild type; ±, slow growth compared to that of the wild type; −, no visible growth.
TABLE 4.
TPI activities measured for different strains
| Strain | Relevant genotype | Activity (nmol min−1 mg−1)a
|
|||
|---|---|---|---|---|---|
| LB | Glc | Suc | Ery | ||
| Rm1021 | Wild type | 879 ± 29 | 990 ± 55 | 942 ± 27 | 1,569 ± 117 |
| SRmA327 | tpiA | 69 ± 4 | 85 ± 9 | 92 ± 4 | 415 ± 2 |
| SRmA355 | tpiB | 615 ± 14 | 816 ± 67 | 899 ± 44 | ND |
| SRmA366 | tpiA tpiB | 6 ± 1 | 1 ± 1 | ND | ND |
Activities reported are the averages of three enzyme assays. Cell extracts are from cultures grown with the following: LB, Luria-Bertani broth; Glc, VMM with 15 mM glucose; Suc, VMM with 15 mM succinate; Ery, VMM broth with 15 mM erythritol. ND, not determined, due to inability to grow with the carbon source listed.
Both tpiA and tpiB encode TPIs.
To confirm the predicted TPI functions encoded by tpiA and tpiB, cell extracts were prepared and assayed for enzyme activity. Cells were grown in flasks containing a rich medium or a defined medium with glucose, succinate, or erythritol as the sole carbon source. Due to their inability to grow, extracts were not prepared for SRmA355 (tpiB) with erythritol or SRmA366 (tpiA tpiB) with either erythritol or succinate as the sole carbon source.
Assays for TPI activity indicate that tpiA mutants had less than 10% of wild-type activity when grown in rich medium or defined medium with either glucose or succinate, as shown in Table 4. However, activity levels were approximately 25% of wild-type activity levels when grown with erythritol. SRmA355 cell extract activity levels were similar to those of the wild type for all growth media tested, while SRmA366 extracts had background levels of TPI activity. Taken together, these data show that both tpiA and tpiB encode TPI enzymes and suggest that tpiA may be constitutively expressed, providing most of the cell's TPI activity, whereas tpiB is induced by erythritol and expressed at low levels under the other conditions tested.
In vivo complementation of tpiA.
Because tpiB was identified as a locus necessary for erythritol catabolism and tpiB-dependent TPI activity was induced by erythritol, it was hypothesized that induction of tpiB in a tpiA strain could rescue the ability to grow on glycerol. To test this, SRmA327 was streaked onto a number of plates of defined medium containing 15 mM glycerol and various amounts of erythritol (from 0.1 mM to 5 mM) to determine the lowest concentration of erythritol that could rescue the ability to grow on glycerol. SRmA327 was found to be able to grow as well as Rm1021 with glycerol when supplemented with at least 0.4 mM erythritol. This amount of erythritol alone does not support the growth of S. meliloti on agar plates, and the tpiA tpiB mutant SRmA366 was not able to grow with glycerol supplemented with erythritol. This indicates that tpiB is inducible by erythritol and has in vivo TPI activity with the ability to complement tpiA. Based on these results, it was hypothesized that suppressors to tpiA would easily be isolated with an up-regulated tpiB. Although more than 1010 SRmA327 cells were plated on media with glycerol as a sole carbon source, we were unable to isolate any suppressors over the course of several independent experiments.
Constitutively expressed tpiA cannot complement tpiB.
As both tpiA and tpiB were found to have in vitro TPI activity, we were interested in why tpiB mutants were unable to grow with erythritol as a sole carbon source. To determine if tpiB was directly required for erythritol catabolism, we complemented tpiA and tpiB mutations with constitutively expressed TPI genes. Both tpiA and tpiB were cloned into a broad-host-range vector, pRK7813, such that the genes were constitutively expressed (25). The resulting plasmids, pNP166 and pNP167, respectively, were mated into various strains and tested for the ability to use glycerol, succinate, arabinose, glutamate, or erythritol as the sole carbon source (Table 5). Constitutively expressed tpiB was able to complement tpiA, tpiB, and tpiA tpiB mutants for growth on all of the carbon sources tested. Constitutively expressed tpiA was able to complement the inability of tpiA mutant strains to grow on glycerol and the inability of tpiA tpiB mutants to grow on glycerol, succinate, arabinose, and glutamate. In contrast to tpiB, tpiA did not complement the inability to use erythritol caused by tpiB mutations. This indicates that although tpiA and tpiB have the same enzyme activity, tpiB is specifically required for erythritol catabolism in S. meliloti. We note that when the same experiment was performed with SRmA584 (tpiB) and SRmA585 (tpiA tpiB), pNP167 (tpiB+) complementation resulted in growth on erythritol that was slower than that of the wild type. We are attributing this to the Tn5-B20 insertion in SRmA584 and SRmA585 being polar on rpiB (Fig. 1), which has been shown to be necessary for erythritol catabolism in R. leguminosarum (50). The complementing cosmid containing the genomic DNA surrounding tpiB, pNP163, was able to complement each strain, restoring wild-type growth.
TABLE 5.
Complementation of TPI mutant strains by plasmid-borne tpiA and tpiB
| Strain | Relevant chromosomal genotype (plasmid genotype) | Growth with carbon sourcea
|
|||
|---|---|---|---|---|---|
| Glc | Gly | Ery | Suc | ||
| Rm1021 | tpiA+tpiB+ | + | + | + | + |
| Rm1021(pNP166) | tpiA+tpiB+ (tpiA+) | + | + | + | + |
| Rm1021(pNP167) | tpiA+tpiB+ (tpiB+) | + | + | + | + |
| SRmA327 | tpiA tpiB+ | + | − | + | + |
| SRmA327(pNP166) | tpiA tpiB+ (tpiA+) | + | + | + | + |
| SRmA327(pNP167) | tpiA tpiB+ (tpiB+) | + | + | + | + |
| SRmA355 | tpiA+tpiB | + | + | − | + |
| SRmA355(pNP166) | tpiA+tpiB (tpiA+) | + | + | − | + |
| SRmA355(pNP167) | tpiA+tpiB (tpiB+) | + | + | + | + |
| SRmA366 | tpiA tpiB | + | − | − | − |
| SRmA366(pNP166) | tpiA tpiB (tpiA+) | + | + | − | + |
| SRmA366(pNP167) | tpiA tpiB (tpiB+) | + | + | + | + |
Grown on VMM media with the given carbon source. Abbreviations: Glc, glucose; Gly, glycerol; Ery, erythritol; Suc, succinate. The symbol indicates the growth phenotype as follows: +, growth same as that of the wild type; −, no visible growth.
Symbiotic competence phenotypes.
R. leguminosarum rhamnose catabolism mutants have previously been shown to have a deficiency in competition for nodule occupancy (34). It was hypothesized that tpiA mutants would have a similar phenotype, based on their inability to grow with rhamnose at the same rate as wild-type S. meliloti. In addition, there have been many reports of defective symbiosis associated with the inability to use succinate as a sole carbon source (6, 15, 19, 24), suggesting that tpiA tpiB strains may be unable to symbiotically fix nitrogen or may have only partial activity.
To test the hypothesis that tpiA mutants would be compromised in the ability to compete with the wild type for nodule occupancy, alfalfa were coinoculated with SRmA327 and Rm1021. The relative amount of each strain recovered from nodules was not significantly different from the amount of each strain found in the inoculum (data not shown). Therefore, the ability to compete for nodule occupancy is not compromised in a tpiA strain, under the conditions tested.
Plants inoculated with tpiB strains did not show any significant difference in dry weight compared to that of the wild type. However, plants inoculated with tpiA tpiB strains reached dry weights of approximately 50% that of wild-type-inoculated plants. This reduced nitrogen fixation phenotype is very similar to the phenotype described for the succinate mutant pckA (15, 35). Dry weights of plants inoculated with Rm5439 (pckA) were not significantly different from those of the tpiA tpiB mutant SRmA585 (data not shown). In order to test whether the reduced nitrogen-fixing phenotype was due to bacteria that had undergone a suppression or reversion event, SRmA585 was isolated from the nodules of plants from one experiment and used as an inoculum in another experiment. SRmA585 isolated from nodules had all of the expected genetic markers and phenotypes intact and resulted in similar plant dry weights for the second experiment, confirming that tpiA tpiB has a reproducible symbiotic phenotype.
DISCUSSION
Despite the central role of TPI in carbon metabolism, very few tpi mutants have been characterized for prokaryotic organisms. In fact, to the best of our knowledge, mutations in tpi have only been described previously for three organisms (2, 31, 37, 52). Of these, two are relatively closely related (2, 37, 52), and the other is associated with autotrophic growth (31). Moreover, well-characterized mutations have been studied in only a single case (52). Given that the number of genome sequences predicting multiple metabolic enzyme activities is increasing, it is imperative that a functional characterization of central metabolic pathways is carried out and not assumed. Our analysis of the two putative S. meliloti genes is only the second study to show two functional tpi genes within any organism and is the first within the Rhizobiaceae. As many organisms do not possess two TPI-encoding genes and their functions were initially unclear, we decided to investigate the physiological roles of tpiA and tpiB.
The enzyme activities we have reported are consistent with tpiA being constitutively expressed, and we hypothesize that tpiA plays primarily a gluconeogenic role (Table 4). S. meliloti microarray data are consistent with this and have shown that tpiA is expressed at similar levels during growth with glucose, succinate, and rich media and in the bacteroid (4). Our work indicates that tpiB is induced by growth with erythritol and is required for erythritol catabolism. S. meliloti contains homologs of the ery operon, which suggests that erythritol is catabolized to DHAP as it is in B. abortus (47). We have shown that constitutively expressed copies of tpiA cannot complement the inability of tpiB strains to grow with erythritol (Table 5). Interestingly, while characterizing erythritol catabolism in B. abortus, Sperry and Robertson (47) noted that TPI activity could not be completely uncoupled from 3-keto-l-erythronate-4-phosphate decarboxylase activity. This is consistent with the possibility of TpiB and the enzyme(s) of erythritol catabolism forming a metabolic complex. The existence of metabolic complexes and sequential reactions has been proposed for a number of pathways, including glycolysis (48).
Each of the genomes of B. abortus (8, 23), Brucella melitensis (10), Brucella suis (38), Mesorhizobium loti (26), Rhizobium etli (21), R. leguminosarum (51), and S. meliloti (18) has homologs of tpiA and tpiB. In each case, tpiB is followed by rpiB directly downstream, and in all but the genome of R. etli, tpiB and rpiB are directly downstream of operons containing homologs of the erythritol catabolism genes eryA, eryB, eryC, and eryD. This leads us to hypothesize that tpiB may be necessary for erythritol catabolism in each of these organisms, except for R. etli, which does not grow with erythritol and does not carry the erythritol genes (21, 50). In addition to our report that tpiB is necessary for growth on erythritol by S. meliloti, this has been confirmed for R. leguminosarum (50).
The fact that the S. meliloti genome annotation predicts that there are no TPI-encoding genes other than tpiA and tpiB, combined with the observation that TPI activity was essentially depleted in SRmA366 (Table 4), suggests that tpiA tpiB strains are unable to perform gluconeogenesis. This is consistent with the fact that tpiA tpiB strains are unable to grow with carbon sources that require gluconeogenesis (e.g., succinate, GABA, glutamate, arabinose, acetate, erythritol, and glycerol). That the low levels of TPI activity measured in tpiA mutants (Table 4) are able to allow gluconeogenic growth was somewhat surprising but is consistent with the report of E. coli tpi revertants growing gluconeogenically with only 5% of wild-type TPI activity (2). Although gluconeogenic growth is possible in a tpiA strain, there does not seem to be enough TPI activity to allow catabolism of glycerol, which is thought to proceed through glycerol kinase and glycerolphosphate dehydrogenase forming DHAP, requiring TPI for further catabolism (3, 49).
We hypothesized that tpiB would be regulated negatively by the adjacent deoR-type regulator SMc01615. Our inability to isolate spontaneous mutations leading to up-regulation of tpiB by plating on minimal media with glycerol was surprising. The fact that over 1010 cells were plated and no suppressors were isolated suggests that the regulation of tpiB may not be solely due to negative regulation by SMc01615. We note that the erythritol catabolism operon upstream of tpiB has been shown to be induced by xylitol, adonitol, sorbitol, and erythritol (28). As tpiB function is required for erythritol catabolism, these two operons may be coordinately regulated, suggesting a regulatory mechanism involving more than one event.
The first mutation in tpiA was isolated by screening for mutants with an impaired ability to utilize rhamnose as a sole carbon source. This led us to test the tpiA mutant for a symbiotic competition defect, as has been reported for rha mutants. Data from three independent experiments showed that there is no difference in nodulation competition between the two strains (data not shown). However, tpiA does not cause a complete loss of the ability to utilize rhamnose as a sole carbon source, so whether rhamnose catabolism mutants of S. meliloti are less competitive than the wild type remains an open question.
Consistent with the reported literature for S. meliloti mutants unable to grow on succinate, the tpiA tpiB mutant was tested and shown to have a compromised symbiotic phenotype. When inoculated on alfalfa, plant dry weights reached less than 50% that of wild-type-inoculated plants, similar to the phenotype of S. meliloti gluconeogenic pckA mutants (15, 35). As tpiA tpiB strains are unable to perform gluconeogenesis, the fact that symbiotic nitrogen fixation is not abolished suggests that gluconeogenesis is beneficial but not required in symbiosis with alfalfa, possibly due to small amounts of plant-supplied sugar reaching the bacteroid. Consistent with this hypothesis, the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK), encoded by pckA, is not found at detectable levels in S. meliloti bacteroids, although mutations in pckA cause reduced nitrogen fixation (15). On the other hand, R. leguminosarum bacteroids were found to have PEPCK activity, and a PEPCK-deficient mutant was found to have no difference in symbiosis (29). Mutations in pckA of the broad-host-range Rhizobium sp. strain NGR234 were found to cause several phenotypes, ranging from Fix− to low rates of nitrogen fixation, depending on the host species (36). It has been suggested previously that the strain- and host-dependent differences in severity of symbiotic phenotypes of bacterial gluconeogenesis mutants may be due to differences in peribacteroid membrane sugar permeability (39).
In this study, we have shown that there are two TPI-encoding genes in the S. meliloti genome with different functions, as tpiA cannot substitute for tpiB. The gene tpiA is hypothesized to be constitutively active in a gluconeogenic role, while tpiB is required for erythritol catabolism by an unknown mechanism. In addition, tpiA tpiB mutants lose the ability to perform gluconeogenesis but retain the ability to symbiotically fix nitrogen at a lower level than the wild type. We are directing our efforts towards understanding the functional difference between tpiA and tpiB which leads to the specificity of tpiB for erythritol catabolism.
Acknowledgments
We are grateful to D. Court for critical reading and discussion of the manuscript.
Funding was provided by the Natural Sciences and Engineering Research Council of Canada in the form of a scholarship to N.J.P. and a grant to I.J.O.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram negative bacteria. BioTechniques 26:824-826, 828. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, A., and R. Cooper. 1969. Gluconeogenesis in Escherichia coli—the role of triose phosphate isomerase. FEBS Lett. 4:19-20. [DOI] [PubMed] [Google Scholar]
- 3.Arias, A., and G. Martinez-Drets. 1976. Glycerol metabolism in Rhizobium. Can. J. Microbiol. 22:150-153. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, M. J., C. J. Toman, R. F. Fisher, and S. R. Long. 2004. A dual genome symbiosis chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc. Natl. Acad. Sci. USA 101:16636-16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beringer, J. E., J. L. Beynon, A. V. Buchanan-Wollaston, and A. W. B. Johnston. 1978. Transfer of the drug-resistance transposon Tn5 to Rhizobium. Nature 276:633-634. [Google Scholar]
- 6.Bolton, E., B. Higgisson, A. Harrington, and F. O'Gara. 1986. Dicarboxylic acid transport in Rhizobium meliloti: isolation of mutants and cloning of dicarboxylic acid transport genes. Arch. Microbiol. 144:142-146. [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Chain, P. S. G., D. J. Comerci, M. E. Tolmasky, F. W. Larimer, S. A. Malfatti, L. M. Vergez, F. Aguero, M. L. Land, R. A. Ugalde, and E. Garcia. 2005. Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 73:8353-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, S. R., I. J. Oresnik, and M. F. Hynes. 2001. RpoN of Rhizobium leguminosarum bv. viciae strain VF39SM plays a central role in FnrN-dependent microaerobic regulation of genes involved in nitrogen fixation. Mol. Gen. Genet. 264:623-633. [DOI] [PubMed] [Google Scholar]
- 10.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J.-J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demerec, M., E. A. Adelberg, A. J. Clark, and P. E. Hartman. 1966. A proposal for a uniform nomenclature in bacterial genetics. Genetics 54:61-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan, T. M., E. Hartweig, K. LeMieux, K. Bergman, G. C. Walker, and E. R. Signer. 1984. General transduction in Rhizobium meliloti. J. Bacteriol. 159:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finan, T. M., A. M. Hirsch, J. A. Leigh, E. Johansen, G. A. Kuldau, S. Deegan, G. C. Walker, and E. R. Signer. 1985. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell 40:869-877. [DOI] [PubMed] [Google Scholar]
- 14.Finan, T. M., B. Kunkel, G. F. De Vos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finan, T. M., E. McWhinnie, B. Driscoll, and R. J. Watson. 1991. Complex symbiotic phenotypes result from gluconeogenic mutations in Rhizobium meliloti. Mol. Plant-Microbe Interact. 4:386-392. [Google Scholar]
- 16.Finan, T. M., I. Oresnik, and A. Bottacin. 1988. Mutants of Rhizobium meliloti defective in succinate metabolism. J. Bacteriol. 170:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuhrer, T., E. Fischer, and U. Sauer. 2005. Experimental identification and quantification of glucose metabolism in seven bacterial species. J. Bacteriol. 187:1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dréano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thébault, M. Vandenbol, F. J. Vorhölter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 19.Gardiol, A., A. Arias, C. Cerveñansky, and G. Martínez-Drets. 1982. Succinate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 151:1621-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazebrook, J., and G. C. Walker. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398-418. [DOI] [PubMed] [Google Scholar]
- 21.González, V., R. I. Santamaría, P. Bustos, I. Hernández-González, A. Medrano-Soto, G. Moreno-Hagelsieb, S. C. Janga, M. A. Ramírez, V. Jiménez-Jacinto, J. Collado-Vides, and G. Dávila. 2006. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. USA 103:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin, I., O. Wattraint, D. Riboul, J. Barbotin, and J. Portais. 2001. A deeper investigation on carbohydrate cycling in Sinorhizobium meliloti. FEBS Lett. 499:45-49. [DOI] [PubMed] [Google Scholar]
- 23.Halling, S. M., B. D. Peterson-Burch, B. J. Bricker, R. L. Zuerner, Z. Qing, L.-L. Li, V. Kapur, D. P. Alt, and S. C. Olsen. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187:2715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornez, J. P., M. El Guezzar, and J. C. Derieux. 1989. Succinate transport in Rhizobium meliloti: characteristics and impact on symbiosis. Curr. Microbiol. 19:207-212. [Google Scholar]
- 25.Jones, J. D., and N. Gutterson. 1987. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene 61:299-306. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shrimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 27.Lynch, W. H., J. MacLeod, and M. Franklin. 1975. Effect of temperature on the activity and synthesis of glucose-catabolizing enzymes in Pseudomonas fluorescens. Can. J. Microbiol. 21:1560-1572. [DOI] [PubMed] [Google Scholar]
- 28.Mauchline, T. H., J. E. Fowler, A. K. East, A. L. Sartor, R. Zaheer, A. H. F. Hosie, P. S. Poole, and T. M. Finan. 2006. Mapping the Sinorhizobium meliloti 1021 solute-binding protein-dependent transportome. Proc. Natl. Acad. Sci. USA 103:17933-17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay, I. A., A. R. Glenn, and M. J. Dilworth. 1985. Gluconeogenesis in Rhizobium leguminosarum MNF3841. J. Gen. Microbiol. 131:2067-2073. [Google Scholar]
- 30.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer, W. G., P. de Boer, and G. van Keulen. 1997. Xanthobacter flavus employs a single triosephosphate isomerase for heterotrophic and autotrophic metabolism. Microbiology 143:1925-1931. [DOI] [PubMed] [Google Scholar]
- 32.Miller-Williams, M., P. C. Loewen, and I. J. Oresnik. 2006. Isolation of salt-sensitive mutants of Sinorhizobium meliloti strain Rm1021. Microbiology 152:2049-2059. [DOI] [PubMed] [Google Scholar]
- 33.Oresnik, I. J., T. C. Charles, and T. M. Finan. 1994. Second site mutations specifically suppress the Fix− phenotype of Rhizobium meliloti ndvF mutations on alfalfa: identification of a conditional ndvF-dependent mucoid colony phenotype. Genetics 136:1233-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oresnik, I. J., L. A. Pacarynuk, S. A. P. O'Brien, C. K. Yost, and M. F. Hynes. 1998. Plasmid-encoded catabolic genes in Rhizobium leguminosarum bv. trifolii: evidence for a plant-inducible rhamnose locus involved in competition for nodulation. Mol. Plant-Microbe Interact. 11:1175-1185. [Google Scholar]
- 35.Østeräs, M., B. T. Driscoll, and T. M. Finan. 1995. Molecular and expression analysis of the Rhizobium meliloti phosphoenolpyruvate carboxykinase (pckA) gene. J. Bacteriol. 177:1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Østeräs, M., T. M. Finan, and J. Stanley. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257-269. [DOI] [PubMed] [Google Scholar]
- 37.Pahel, G., F. R. Bloom, and B. Tyler. 1979. Deletion mapping of the polA-metB region of the Escherichia coli chromosome. J. Bacteriol. 138:653-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. V. Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole, P., and D. Allaway. 2000. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 43:117-163. [DOI] [PubMed] [Google Scholar]
- 40.Portais, J. C., P. Tavernier, I. Gosselin, and J. N. Barbotin. 1999. Cyclic organization of the carbohydrate metabolism in Sinorhizobium meliloti. Eur. J. Biochem. 265:473-480. [DOI] [PubMed] [Google Scholar]
- 41.Poysti, N. J., E. D. M. Loewen, Z. Wang, and I. J. Oresnik. 2007. Sinorhizobium meliloti pSymB encodes genes necessary for arabinose transport and catabolism. Microbiology 153:727-736. [DOI] [PubMed] [Google Scholar]
- 42.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 43.Richardson, J. S., M. F. Hynes, and I. J. Oresnik. 2004. A genetic locus necessary for rhamnose uptake and catabolism in Rhizobium leguminosarum bv. trifolii. J. Bacteriol. 186:8433-8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. L. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 45.Sangari, F. J., J. Aguero, and J. M. Garcia-Lobo. 2000. The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology 146:487-495. [DOI] [PubMed] [Google Scholar]
- 46.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic-engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 47.Sperry, J. F., and D. C. Robertson. 1975. Erythritol catabolism by Brucella abortus. J. Bacteriol. 121:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srere, P. A. 1987. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 56:89-124. [DOI] [PubMed] [Google Scholar]
- 49.Stowers, M. D. 1985. Carbon metabolism in Rhizobium species. Annu. Rev. Microbiol. 39:89-108. [DOI] [PubMed] [Google Scholar]
- 50.Yost, C. K., A. M. Rath, T. C. Noel, and M. F. Hynes. 2006. Characterization of genes involved in erythritol catabolism in Rhizobium leguminosarum bv. viciae. Microbiology 152:2061-2074. [DOI] [PubMed] [Google Scholar]
- 51.Young, J. P. W., L. C. Crossman, A. W. B. Johnston, N. R. Thomson, Z. F. Ghazoui, K. H. Hull, M. Wexler, A. R. J. Curson, J. D. Todd, P. S. Poole, T. H. Mauchline, A. K. East, M. A. Quail, C. Churcher, C. Arrowsmith, I. Cherevach, T. Chillingworth, K. Clarke, A. Cronin, P. Davis, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, K. Mungall, H. Norbertczak, E. Rabbinowitsch, M. Sanders, M. Simmonds, S. Whitehead, and J. Parkhill. 2006. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 7:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng, P., J. Sun, J. van den Heuvel, and A.-P. Zeng. 2006. Discovery and investigation of a new, second triose phosphate isomerase in Klebsiella pneumoniae. J. Biotechnol. 125:462-473. [DOI] [PubMed] [Google Scholar]