Abstract
Using a conditional mutagenesis strategy we demonstrate here that a gene cluster encoding putative aminoarabinose (Ara4N) biosynthesis enzymes is essential for the viability of Burkholderia cenocepacia. Loss of viability is associated with dramatic changes in bacterial cell morphology and ultrastructure, increased permeability to propidium iodide, and sensitivity to sodium dodecyl sulfate, suggesting a general cell envelope defect caused by the lack of Ara4N.
Cationic antimicrobial peptides (APs), a group of structurally diverse molecules found in all eukaryotes (17, 34), can kill gram-positive and gram-negative bacteria, fungi, and viruses and also modulate innate immune responses (30). In gram-negative bacteria, APs interact with lipopolysaccharide (LPS) and via the self-promoted uptake pathway reach targets in the plasma membrane (16). LPS is a major surface component of gram-negative bacteria, consisting of O antigen polysaccharide, core oligosaccharide, and lipid A (29). Phosphate groups covalently attached to residues on the lipid A and core oligosaccharide provide sites for electrostatic interactions with APs (18). Bacteria can resist APs by modifying their lipid A with 4-amino-4-deoxy-l-arabinose (Ara4N), effectively reducing the net negative charge of LPS molecules. In all species examined to date, this is a highly regulated process in response to environmental Mg2+ limitation, excess Fe3+, or the presence of APs (1, 8, 9, 12, 15, 24). However, LPS modification with Ara4N is dispensable for growth in vitro under routine laboratory conditions.
Burkholderia cenocepacia belongs to the B. cepacia complex, a group of closely related environmental bacteria that are emerging opportunistic pathogens of cystic fibrosis patients and other immunocompromised individuals (23). They are highly resistant to the majority of clinically useful antimicrobials and also resistant to APs even at concentrations that kill other bacteria with Ara4N-modified LPS (21). Very little is known about the mechanism of resistance to APs in Burkholderia spp. LPS from various Burkholderia species, such as B. cepacia, B. caryophylli, and B. cenocepacia, contains Ara4N residues in the lipid A and also the unusual glycero-talo-octulosonic acid (Ko) residue in the inner-core oligosaccharide (10, 13, 19, 20, 25, 26, 31, 32). Here, we show that a putative gene cluster for Ara4N biosynthesis and LPS modification is essential for the viability of B. cenocepacia.
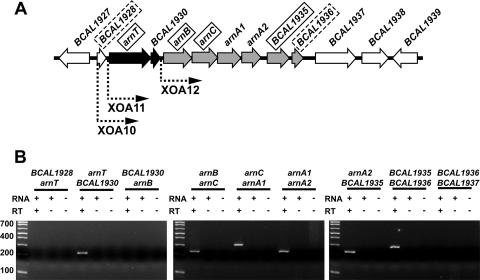
A putative gene cluster for Ara4N biosynthesis in B. cenocepacia.
A region spanning nucleotides 2128443 to 2135736 in chromosome 1 of B. cenocepacia strain J2315 (http://www.sanger.ac.uk/Projects/B_cenocepacia/) contains genes encoding proteins that are highly similar to those required for Ara4N biosynthesis and lipid A modification in Salmonella and other bacteria (14) (Fig. 1A). These genes correspond to homologues of arnT (pmrK, Ara4N transferase), BCAL1930 (pmrLM, unknown function), arnB (pmrH, UDP-4′-ketopentose aminotransferase), arnC (pmrF, Ara4N Und-P transferase), and BCAL1935 (pmrJ, unknown function) (Fig. 1A). Also, unlike other bacteria in which arnA (pmrI) is a single gene encoding a bifunctional enzyme (3, 4), two distinct genes are found in B. cenocepacia which we have designated arnA1 (UDP-Ara4N formyltransferase) and arnA2 (UDP-4′-keto-5′-carboxypentose decarboxylase) (Fig. 1A). The transcriptional organization of the putative B. cenocepacia arn gene cluster was experimentally determined by nested reverse transcriptase PCR (RT-PCR) amplifications, as previously described (27). We prepared cDNA from total RNA isolated from strain J2315 grown to logarithmic phase in Luria-Bertani broth with or without polymyxin B (100 μg/ml) and amplified it by PCR using primer pairs that would allow detection of cotranscription. All PCRs were performed alongside appropriate controls, including a non-RT control to assay for genomic DNA contamination. Primers used for RT-PCR analysis are available from the authors upon request. These experiments demonstrated that the putative arn genes are organized into two transcriptional units (Fig. 1B). One unit comprises arnT and BCAL1930, while the other includes the arn gene loci BCA1A2, BCAL1935, and BCAL1936 (Fig. 1A). This genetic organization suggests that the B. cenocepacia genes for the synthesis of UDP-Ara4N (arn BCA1A2) are transcribed independently from those for transferring Ara4N to the lipid A (arnT and possibly BCAL1930). The same genetic organization of the arn cluster was demonstrated for B. cenocepacia strain K56-2 (data not shown), which is clonally related to J2315 (22) but is more amenable to genetic manipulations. RT-PCR analysis also revealed that the arn cluster in both strains was expressed regardless of polymyxin B challenge (data not shown), indicating that it is not subjected to the same transcriptional regulation as has been observed in all other bacteria examined to date (1, 24). To facilitate genetic manipulations the remaining studies were performed using strain K56-2, which is also highly resistant to APs (21).
FIG. 1.
(A) Genetic organization of the putative arn gene cluster and flanking regions on chromosome 1 of B. cenocepacia strains J2315 and K56-2. Solid boxes around gene and locus names indicate the genes for which polar mutations failed; dotted boxes indicate genes for which the creation of polar mutations was successful. Mutagenesis of the remaining genes was not attempted. The dotted arrows indicate the locations of the inserted rhamnose-inducible promoter in strains XOA10, XOA11, and XOA12. Black and gray arrows denote the genes within the two transcriptional units of the putative arn cluster that were identified by RT-PCR. (B) RT-PCR analysis of the arn cluster, performed on strain J2315 in the absence of polymyxin B challenge.
The putative Ara4N biosynthesis cluster is essential for viability of B. cenocepacia.
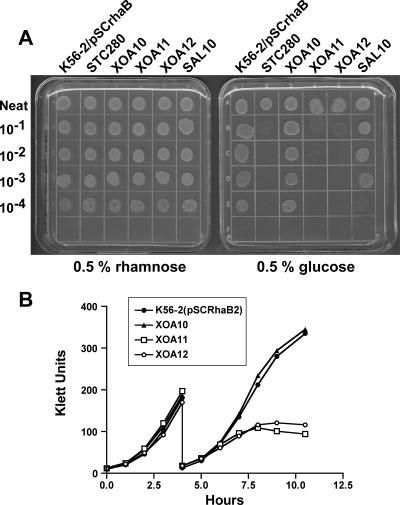
Repeated attempts to mutagenize arnT, arnB, arnC, and BCAL1935 by insertional inactivation using the pGPΩTp suicide plasmid (11) containing internal fragments from each target gene consistently failed. In contrast, the genes BCAL1928 and BCAL1936 that flank each end of the putative arn cluster were readily mutated. We hypothesized that the putative arn genes perform one or more functions that are essential for the viability of B. cenocepacia. To confirm the essentiality of the arn genes, we constructed plasmid pSC200, which enables the delivery of the rhamnose-inducible PrhaB promoter into the chromosome to drive the expression of a targeted gene. The construction of pSC200 was done by combining the multiple-cloning site, oriR6K, and mob genes from pGpΩTp (11) with the PrhaB rhamnose-inducible promoter, rhaR, rhaS, and the dhfr cassette from pSCrhaB2 (7). Details on the construction of this plasmid are available from the authors upon request. Several 300-bp fragments spanning the 5′ region of each targeted gene were cloned into pSC200. The resulting plasmids were transferred into B. cenocepacia K56-2 by triparental mating, and the exconjugants were plated on LB agar plates supplemented with trimethoprim (Tp) (100 μg/ml), gentamicin (50 μg/ml), and 0.5% (wt/vol) rhamnose. The correct insertion in the B. cenocepacia chromosome was confirmed by colony PCR and Southern blot hybridization. This strategy created conditional mutants in which the expression of the targeted gene depended on the rhamnose concentration in the medium. We have shown that the PrhaB promoter is useful for characterizing essential operons in B. cenocepacia (6). Thus, we constructed conditional mutants by insertion of pSC200 derivatives upstream of arnT (strain XOA11) and arnB (strain XOA12) as well as a mutant in which this plasmid interrupts the gene locus BCAL1928 (strain XOA10) and which served as a negative control (Fig. 1A). Also, as a positive control for essentiality, a pSC200 derivative was used to target the conserved essential gene dxs encoding a key enzyme for the synthesis of undecaprenyl phosphate (2, 28), resulting in strain STC280.
The conditional mutants were grown at 37°C in M9 minimal medium (42 mM Na2HPO4, 22 mM KH2PO4, 8 mM NaCl, 10 mM NH4Cl) supplemented with (final concentrations) yeast extract (5 mg/ml), Casamino Acids (2 mg/ml), vitamin B1 (2 μg/ml), tryptophan (20 μg/ml), CaCl2 (1 μM), and 0.5% (wt/vol) glycerol plus 0.5% (wt/vol) glucose or 0.5% (wt/vol) rhamnose and Tp (100 μg/ml) when required. An aliquot of an overnight culture in M9 medium with rhamnose was spun down and washed three times with sterile phosphate-buffered saline (PBS), resuspended in PBS, and adjusted to an optical density at 600 nm (OD600) of 1. Drops (10 μl) of undiluted solution and 10−1, 10−2, 10−3, and 10−4 dilutions were plated onto M9 agar plates supplemented with 0.5% (wt/vol) glucose or 0.5% (wt/vol) rhamnose and incubated at 37°C. Strain STC280 grew in the presence of rhamnose (permissive condition) but failed to grow in the presence of glucose (nonpermissive condition), as expected for a mutant with an essential gene under the control of PrhaB. A similar growth phenotype was found for XOA11 and XOA12, but XOA10 grew equally well in the presence of rhamnose or glucose (Fig. 2A). We therefore concluded that expression of one or more genes in the two transcriptional units of the putative arn cluster is indispensable in B. cenocepacia K56-2.
FIG. 2.
(A) Conditional lethal phenotype of strains XOA11 and XOA12 on M9 agar plates supplemented with 0.5% (wt/vol) rhamnose or 0.5% (wt/vol) glucose. XOA11 and XOA12 grow only in the presence of rhamnose, while XOA10 and SAL10 grow equally well in either media. STC280 is shown as a control mutant for a known essential gene. (B) Depletion experiments using strains K56-2(pSCrhaB2), XOA10, XOA11, and XOA12 in nonpermissive conditions. Growth was monitored every hour using a Klett-Summerson colorimeter. The figure is representative of two independent experiments with similar results.
The first biochemical reaction for the biosynthesis of Ara4N is the conversion of UDP-glucose into UDP-glucuronic acid by the enzyme UDP-glucose 6-dehydrogenase (Ugd or PmrE). In previous work, we characterized a gene operon containing a ugd homologue (BCAL2946) and genes encoding modification of glycero-manno-heptose, which are part of the biosynthesis of the ADP-glycero-manno-heptose precursor for core oligosaccharide biosynthesis (21). To assess whether or not ugdBCAL2946 is also essential, we constructed a conditional ugdBCAL2946 mutant (SAL10). This mutant did not show a conditional lethal phenotype in medium with glucose (Fig. 2A), indicating that ugdBCAL2946 is not essential for bacterial viability. A BLAST analysis of the B. cenocepacia J2315 genome revealed the presence of two additional ugd homologues in chromosome 2 (BCAM0855 and BCAM2034), which we assume provide redundant UDP-glucose dehydrogenase activities.
Loss of viability is associated with morphological changes in the bacterial cells and defects in cell envelope permeability.
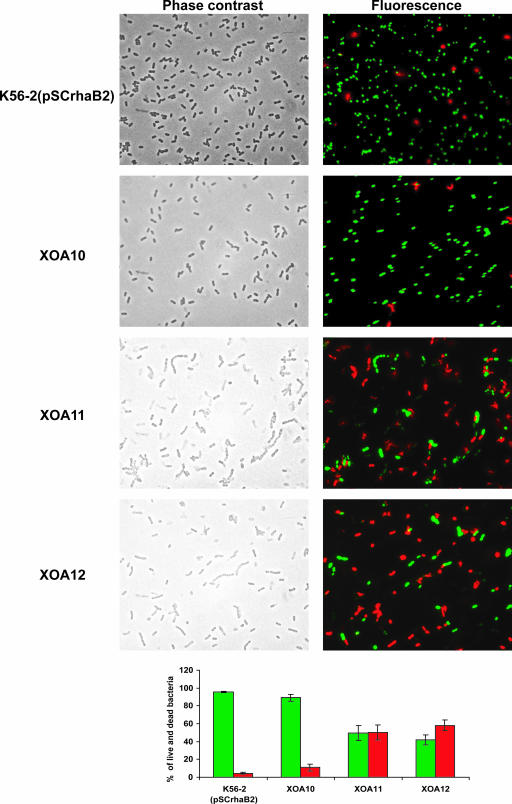
The essentiality of Ara4N synthesis was further demonstrated by depletion experiments performed using liquid medium (Fig. 2B). Eight-hour cultures were diluted to an OD600 of 0.001 in M9 medium supplemented with 5% (wt/vol) yeast extract and 0.5% (wt/vol) rhamnose and incubated at 37°C for 11 h. Cells from these cultures were spun down, washed three times with sterile PBS, and diluted with fresh M9 medium supplemented with 5% (wt/vol) yeast extract and 0.5% (wt/vol) glucose at a final OD600 of 0.13. Growing cells were subcultured with fresh medium containing 0.5% (wt/vol) glucose at 4 h and monitored for growth for another 6 h. Growth curves of the wild-type strain and all the mutants in permissive conditions were identical (data not shown). After serial passages in medium with glucose, the wild type and the XOA10 mutant also grew to similar levels (Fig. 2B). In contrast, after serial passage in medium with glucose of XOA11 and XOA12 cultures, the turbidity of the cultures rapidly reached a plateau compared to wild-type and XOA10 control strain results. The results indicate that XOA11 and XOA12 had stopped growing after 6 h (roughly corresponding to six generations). Phase-contrast microscopy and fluorescent staining with Syto9 and propidium iodide (LIVE/DEAD BacLight bacterial viability kit; Molecular Probes, Invitrogen Detection Technologies, Eugene, OR) were carried out to investigate the bacterial cell morphology and to assess whether or not the cell envelope was compromised. After 8 h in nonpermissive conditions, strains XOA11 and XOA12, but not K56-2 and XOA10, formed chains of cells and the cultures contained flocculent particulate material indicative of cell lysis. Also, 50% of XOA11 and 58% of XOA12 bacterial cells were permeable to propidium iodide in contrast to 5% to 10% of the control strains, suggesting a loss of viability and a compromised cell envelope in the arn conditional mutants (Fig. 3). No differences in morphology or viability were observed between these same strains grown overnight under permissive conditions before being transferred to the nonpermissive medium for the depletion experiments (data not shown).
FIG. 3.
Microscopy and live-dead staining of strains K56-2, XOA10, XOA11, and XOA12 at 8 h of growth during the depletion experiments. Live bacteria appear fluorescent green, while dead bacteria and bacteria with compromised membranes appear fluorescent red. These experiments were carried out twice with similar results.
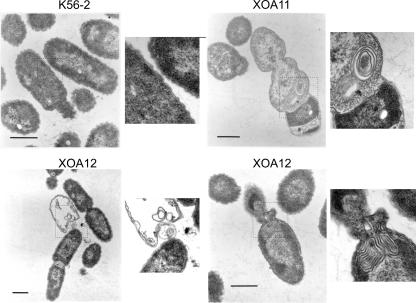
Ultrastructural analysis of bacterial cells after 8 h following depletion revealed accumulations of membranous material in strains XOA11 and XOA12 (Fig. 4), suggesting that Ara4N is required for the proper assembly of the outer membrane. Furthermore, empty cells and cells with cell division defects could be observed in the mutant cultures (Fig. 4). To obtain further evidence that the envelope was compromised in XOA11 and XOA12 under nonpermissive conditions, bacteria were grown in the presence of 0.05% (wt/vol) sodium dodecyl sulfate (SDS). For these experiments, cells were diluted to an OD600 of 0.1 and dilutions were plated for colony counts on M9 agar plates supplemented with 5% (wt/vol) yeast extract, 0.5% (wt/vol) rhamnose, and Tp (100 μg/ml) with or without 0.05% (wt/vol) SDS. The survival rate of K56-2 cells in the presence of SDS was 1.2% of the initial inoculum, while the survival rates of XOA11 and XOA12 cells were 0.09% and 0.08%, respectively. Together, these experiments demonstrate that the putative arn gene cluster is essential for B. cenocepacia viability and that its function is required for an intact cell envelope. It is possible that LPS modifications with Ara4N are an essential requirement for the stability of the outer membrane in this group of bacteria, which may explain at least in part their extreme resistance to APs. Recent structural studies of the lipid A moiety of B. cepacia (formerly genomovar I) and B. mallei revealed that only a proportion of lipid A species are nonstoichiometrically replaced with Ara4N (5, 31), although we do not know whether this is also the case for the lipid A from B. cenocepacia. Perhaps not all the lipid A molecules are required to be replaced with Ara4N to maintain the stability of the outer membrane. Furthermore, our results did not conclusively establish whether or not Ara4N is essential in the lipid A and/or the inner core oligosaccharide. The morphological defects of the mutants under nonpermissive conditions are strikingly similar to those found in Escherichia coli mutants with defects in the Imp/RlpB complex, which is essential for proper assembly of LPS in the outer membrane (33). Therefore, it is possible that the products encoded by the putative arn gene cluster in B. cenocepacia are also required for the assembly of LPS in this bacterium. A detailed biochemical analysis of the lipid A biosynthesis in B. cenocepacia, currently under way in our laboratories, will help to elucidate the role of these genes and will also confirm their predicted function in the synthesis of Ara4N.
FIG. 4.
Transmission electron microscopy of strains K56-2, XOA11, and XOA12 after 8 h in nonpermissive conditions. The inserts in each panel are digital magnifications of selected regions of the electron micrographs (indicated by the dotted-line squares) showing the accumulation of membranous material, the presence of empty cells, and cell division abnormalities in more detail. Bars, 0.5 μm.
Acknowledgments
We thank J. Bartholdson and J. Schmitt for technical assistance and M. Holden and J. Parkhill for giving us access to the preliminary annotation of the strain J2315. The electron microscopy was conducted in the Electron Microscopy Research Facility of the Schulich School of Medicine and Dentistry, University of Western Ontario, Canada, with the expert assistance of J. Sholdice.
A.R.B. is supported jointly by the Big Lottery Fund of the United Kingdom and the Cystic Fibrosis Trust. R.S.F. and S.A.L. are supported by graduate scholarships from the Canadian Cystic Fibrosis Foundation and Canadian Institutes of Health Research, respectively. This work was supported by grants from the Canadian Cystic Fibrosis Foundation and the Canadian Institutes of Health Research (to M.A.V.). D.J.C. thanks the Royal Society of Edinburgh. M.A.V. holds a Canada Research Chair in Infectious Diseases and Microbial Pathogenesis.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461-472. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, A. M., S. Mahapatra, P. J. Brennan, and D. C. Crick. 2002. Identification, cloning, purification, and enzymatic characterization of Mycobacterium tuberculosis 1-deoxy-d-xylulose 5-phosphate synthase. Glycobiology 12:813-820. [DOI] [PubMed] [Google Scholar]
- 3.Breazeale, S. D., A. A. Ribeiro, and C. R. Raetz. 2002. Oxidative decarboxylation of UDP-glucuronic acid in extracts of polymyxin-resistant Escherichia coli. Origin of lipid a species modified with 4-amino-4-deoxy-l-arabinose. J. Biol. Chem. 277:2886-2896. [DOI] [PubMed] [Google Scholar]
- 4.Breazeale, S. D., A. A. Ribeiro, and C. R. H. Raetz. 2003. Origin of lipid A species modified with 4-amino-4-deoxy-l-arabinose in polymyxin-resistant mutants of Escherichia coli: an aminotransferase (arnB) that generates UDP-4-amino-4-deoxy-l-arabinose. J. Biol. Chem. 278:24731-24739. [DOI] [PubMed] [Google Scholar]
- 5.Brett, P. J., M. N. Burtnick, D. S. Snyder, J. G. Shannon, P. Azadi, and F. C. Gherardini. 2007. Burkholderia mallei expresses a unique lipopolysaccharide mixture that is a potent activator of human Toll-like receptor 4 complexes. Mol. Microbiol. 63:379-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardona, S. T., C. L. Mueller, and M. A. Valvano. 2006. Identification of essential operons with a rhamnose-inducible promoter in Burkholderia cenocepacia. Appl. Environ. Microbiol. 72:2547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardona, S. T., and M. A. Valvano. 2005. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54:219-228. [DOI] [PubMed] [Google Scholar]
- 8.Chamnongpol, S., W. Dodson, M. J. Cromie, Z. L. Harris, and E. A. Groisman. 2002. Fe(III)-mediated cellular toxicity. Mol. Microbiol. 45:711-719. [DOI] [PubMed] [Google Scholar]
- 9.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44:561-571. [DOI] [PubMed] [Google Scholar]
- 10.Cox, A. D., and S. G. Wilkinson. 1991. Ionizing groups in lipopolysaccharides of Pseudomonas cepacia in relation to antibiotic resistance. Mol. Microbiol. 5:641-646. [DOI] [PubMed] [Google Scholar]
- 11.Flannagan, R. S., D. Aubert, C. Kooi, P. A. Sokol, and M. A. Valvano. 12 January 2007. Burkholderia cenocepacia requires a periplasmic HtrA protease for growth under thermal and osmotic stress and for survival in vivo. Infect. Immun. doi: 10.1128/IAI.01581-06. [DOI] [PMC free article] [PubMed]
- 12.Groisman, E. A., J. Kayser, and F. C. Soncini. 1997. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol. 179:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronow, S., C. Noah, A. Blumenthal, B. Lindner, and H. Brade. 2003. Construction of a deep-rough mutant of Burkholderia cepacia ATCC 25416 and characterization of its chemical and biological properties. J. Biol. Chem. 278:1647-1655. [DOI] [PubMed] [Google Scholar]
- 14.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 15.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helander, I. M., I. Kilpelainen, and M. Vaara. 1994. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol. Microbiol. 11:481-487. [DOI] [PubMed] [Google Scholar]
- 19.Isshiki, Y., K. Kawahara, and U. Zahringer. 1998. Isolation and characterisation of disodium (4-amino-4-deoxy-beta-l-arabinopyranosyl)-(1→8)-(d-glycero-alpha-d-talo-oct-2-ulopyranosylonate)-(2→4)-(methyl 3-deoxy-d-manno-oct-2-ulopyranosid)onate from the lipopolysaccharide of Burkholderia cepacia. Carbohydr. Res. 313:21-27. [DOI] [PubMed] [Google Scholar]
- 20.Isshiki, Y., U. Zahringer, and K. Kawahara. 2003. Structure of the core-oligosaccharide with a characteristic d-glycero-α-d-talo-oct-2-ulosylonate-(2→4)-3-deoxy-d-manno-oct-2-ulosonate [α-Ko-(2→4)-Kdo] disaccharide in the lipopolysaccharide from Burkholderia cepacia. Carbohydr. Res. 338:2659-2666. [DOI] [PubMed] [Google Scholar]
- 21.Loutet, S. A., R. S. Flannagan, C. Kooi, P. A. Sokol, and M. A. Valvano. 2006. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to polymyxin B and bacterial survival in vivo. J. Bacteriol. 188:2073-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 24.McPhee, J. B., S. Lewenza, and R. E. Hancock. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50:205-217. [DOI] [PubMed] [Google Scholar]
- 25.Molinaro, A., C. De Castro, R. Lanzetta, A. Evidente, M. Parrilli, and O. Holst. 2002. Lipopolysaccharides possessing two l-glycero-d-manno-heptopyranosyl-α-(1→5)-3-deoxy-d-manno-oct-2-ulopyranosonic acid moieties in the core region. The structure of the core region of the lipopolysaccharides from Burkholderia caryophylli. J. Biol. Chem. 277:10058-10063. [DOI] [PubMed] [Google Scholar]
- 26.Molinaro, A., B. Lindner, C. De Castro, B. Nolting, A. Silipo, R. Lanzetta, M. Parrilli, and O. Holst. 2003. The structure of lipid A of the lipopolysaccharide from Burkholderia caryophylli with a 4-amino-4-deoxy-l-arabinopyranose 1-phosphate residue exclusively in glycosidic linkage. Chem. Eur. J. 9:1542-1548. [DOI] [PubMed] [Google Scholar]
- 27.Ortega, X., T. A. Hunt, S. Loutet, A. D. Vinion-Dubiel, A. Datta, B. Choudhury, J. B. Goldberg, R. Carlson, and M. A. Valvano. 2005. Reconstitution of O-specific lipopolysaccharide expression in the Burkholderia cenocepacia strain J2315 that is associated with transmissible infections in patients with cystic fibrosis. J. Bacteriol. 187:1324-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Querol, J., M. Rodriguez-Concepcion, A. Boronat, and S. Imperial. 2001. Essential role of residue H49 for activity of Escherichia coli 1-deoxy-d-xylulose 5-phosphate synthase, the enzyme catalyzing the first step of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid synthesis. Biochem. Biophys. Res. Commun. 289:155-160. [DOI] [PubMed] [Google Scholar]
- 29.Raetz, C. R. H., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott, M. G., A. C. Vreugdenhil, W. A. Buurman, R. E. Hancock, and M. R. Gold. 2000. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164:549-553. [DOI] [PubMed] [Google Scholar]
- 31.Silipo, A., A. Molinaro, P. Cescutti, E. Bedini, R. Rizzo, M. Parrilli, and R. Lanzetta. 2005. Complete structural characterization of the lipid A fraction of a clinical strain of B. cepacia genomovar I lipopolysaccharide. Glycobiology 15:561-570. [DOI] [PubMed] [Google Scholar]
- 32.Silipo, A., A. Molinaro, T. Ierano, A. De Soyza, L. Sturiale, D. Garozzo, C. Aldridge, P. A. Corris, C. M. Khan, R. Lanzetta, and M. Parrilli. 12 January 2007, posting date. The complete structure and pro-inflammatory activity of the lipooligosaccharide of the highly epidemic and virulent Gram-negative bacterium Burkholderia cenocepacia ET-12 (strain J2315). Chemistry. doi: 10.1002/chem.200601406. [DOI] [PubMed]
- 33.Wu, T., A. C. McCandlish, L. S. Gronenberg, S. S. Chng, T. J. Silhavy, and D. Kahne. 2006. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 103:11754-11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zasloff, M. 1992. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 4:3-7. [DOI] [PubMed] [Google Scholar]