Abstract
Gene conversion mediates the variation of virulence-associated surface structures on pathogenic microorganisms, which prevents host humoral immune responses from being effective. One of the best-studied gene conversion systems is antigenic variation (Av) of the pilin subunit of the Neisseria gonorrhoeae type IV pilus. To identify cis-acting DNA sequences that facilitate Av, the 700-bp region upstream of the pilin gene pilE was targeted for transposon mutagenesis. Four classes of transposon-associated mutations were isolated, distinguishable by their pilus-associated phenotypes: (i) insertions that did not alter Av or piliation, (ii) insertions that blocked Av, (iii) insertions that interfered with Av, and (iv) insertions that interfered with pilus expression and Av. Mutagenesis of the pilE promoter did not affect the frequency of Av, directly demonstrating that pilin Av is independent of pilE transcription. Two stretches of sequence upstream of pilE were devoid of transposon insertions, and some deletions in these regions were not recoverable, suggesting that they are essential for gonococcal viability. Insertions that blocked pilin Av were located downstream of the RS1 repeat sequence, and deletion of the region surrounding these insertions completely abrogated pilin Av, confirming that specific sequences 5′ to pilE are essential for the recombination events underlying pilin Av.
Many pathogens vary their surface structures in order to successfully colonize and cause disease in the host. This variation can occur via phase or antigenic variation (Av). Phase variation typically involves a heritable and reversible switch between two states (either on/off or alpha/beta) and often alters the expression state of a surface structure, such as capsule, pili, or flagella, within a clonal population (62). In contrast, Av refers to the expression of multiple antigenically distinct forms of a single gene product within a clonal population. Av is often achieved by gene conversion, the unidirectional recombination of variant DNA into a homologous locus, and underlies yeast mating-type switching (16), chicken and rabbit immunoglobulin rearrangements (28, 35), and variation of virulence-associated surface determinants in pathogens such as Plasmodium, Trypanosoma, Borrelia, Mycoplasma, and Neisseria (62).
Neisseria gonorrhoeae (the gonococcus) is the causative agent of the disease gonorrhea. The bacterium attaches to the human urogenital epithelium via its type IV pilus, which is primarily composed of pilin monomers encoded by the pilE gene. The pilin proteins that compose pili undergo Av at rates greater than 4 × 10−3 per cell per generation, the highest reported for any pathogenic gene conversion system to date (6). High-frequency Av of pilin contributes to gonococcal immune evasion by thwarting the development of protective host immunity that would otherwise prevent reinfection, as evidenced by the failure of a pilus-based gonococcal vaccine (4). In addition to pilE, N. gonorrhoeae strain FA1090 possesses five transcriptionally silent pilS loci found at multiple sites throughout the chromosome, and each locus contains from one to six variable silent copies of pilin genetic information (17). These 19 silent copies lack a promoter sequence, a ribosome binding site, and the 5′ portion of conserved pilin information and are therefore not expressed (39). The 3′ two-thirds of the pilin information found in all pilin copies contains regions of variable sequences flanked by conserved sequences, including cys1 and cys2. Comparison of the relative sequence homology among all pilS copies has led to the designation of the central portion of pilE as the semivariable region, while the 3′ end of pilE can be divided into a hypervariable loop and tail (22, 50). Pilin Av occurs when sequence from a silent pilS copy unidirectionally recombines with the expressed pilE gene at short regions of shared DNA sequence identity (15, 48).
Pilin Av requires RecA and therefore is a homologous recombination-mediated process (30). Pilin Av also requires a RecF-like pathway (RecQ, RecO, RecR, and RecJ), the RecA modulator RecX, the growth regulator RdgC, the Rep helicase, and the Holliday junction-processing enzymes RecG and RuvABC (27, 36-38, 46, 54, 56). Several cis-acting elements have been proposed to facilitate pilin Av, but their exact roles remain poorly defined. These include an ∼30-bp conserved cys2 element found within all pilin copies and a 66-bp Sma/Cla repeat located at the 3′ end of pilE and the five pilS loci (21, 23, 63, 64). There is also evidence that sequences upstream of the pilE gene participate in pilin Av. The original sequence analysis of the pilE and pilS loci from strain MS11 indicated that they were often associated with the repetitive sequences RS1, RS2, and RS3 (15), but the importance of these sequences in pilin Av has never been directly tested. Recently, a cis-acting transposon insertion that inhibited pilin Av but not pilus expression was isolated in the region upstream of the pilE promoter (46). This transposon suppressed the survival defect of a gonococcal strain deficient in the ability to process Holliday junctions, suggesting this transposon insertion blocks an event occurring prior to the RecA-dependent formation of Holliday junction recombination intermediates (46).
pilE recombination proceeds at a high frequency during exponential bacterial growth, and iron availability is the only environmental cue known to regulate the frequency of recombination at pilE (53). Since DNA sequence from any of the 19 silent gene copies can be directed to pilE during pilin Av, it is possible that the transcription of pilE allows the donor and recipient genes to be differentiated from one another in this gene conversion process (18, 19). Transcription of pilE proceeds at a high level from a single promoter (10) and is not known to involve specific regulatory factors but is facilitated by the binding of integration host factor (IHF) upstream of pilE (19) and by the presence of two UP-like elements flanking the IHF binding site (11). PilA, the gonococcal FtsY homolog (1), also binds to two sites flanking the IHF binding site upstream of the pilE promoter (2), but PilA is no longer considered to be involved in the transcription of pilE (1). FtsY is a functional homologue of the eukaryotic docking protein (34) and is the receptor for the signal recognition particle, which is required for the biogenesis of some inner membrane proteins (51, 61). It has been proposed that the binding of DNA upstream of pilE by the gonococcal signal recognition particle components Ffh and 4.5S RNA plays a role in targeting nascent pilin transcripts to the inner membrane (9). Since both IHF and PilA are essential proteins (19, 59), the role of these factors in pilin Av has never been directly tested.
In this study, we used transposon mutagenesis to determine which sequences upstream of the pilE gene are important for pilin Av in N. gonorrhoeae. We establish that transposon insertions between the upstream silent pilS copy and the RS1 repeat have no effect on pilin Av. We show that the region previously identified by a transposon insertion that abrogates pilin Av is limited to a relatively small stretch of DNA sequence and that insertions throughout the region between the RS1 repeat and the pilE promoter can inhibit pilin Av. Finally, we show that the elimination of pilE transcription has no effect on the process of pilin Av. These data are consistent with a model that proposes a specific initiation event occurring in the pilE upstream region that targets homologous recombination factors to pilE, thus allowing the gene conversion reactions that define pilin Av.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli One Shot TOP10 competent cells (Invitrogen) were grown on Luria-Bertani broth or agar at 37°C and used to propagate plasmids. Plate media contained 15 g of agar per liter. Gonococcal strains were grown on Gc medium base (Difco) plus Kellogg supplements (GCB) [22.2 mM glucose, 0.68 mM glutamine, 0.45 mM cocarboxylase, and 1.23 mM Fe(NO3)3, all from Sigma] (25) at 37°C in 5% CO2 or in gonococcal liquid medium (1.5% protease peptone no. 3 [Difco], 0.4% K2HPO4, 0.1% KH2PO4, and 0.1% NaCl) with Kellogg supplements and 0.042% sodium bicarbonate. Kanamycin (Km) was added to E. coli at 50 mg/liter and to N. gonorrhoeae strain FA1090 at 40 mg/liter.
Gonococcal strains in this study were derivatives of strain FA1090 variant 1-81-S2 (50) with matched pilE sequences and matched opacity colony types. Mutations were created in a recA6 background, which contains an isopropyl β-d-thiogalactopyranoside (IPTG)-regulatable gonococcal recA allele and allows for control of recA expression and recA-dependent processes (49). IPTG (Diagnostic Chemicals Limited) was used at 1 mM to provide maximal induction of recA transcription (49), which yields RecA expression levels and RecA-dependent phenotypes similar to those of strains with a wild-type recA gene (55).
DNA manipulations and analysis.
Standard procedures were performed as described previously (44). Plasmid DNA was isolated from strains of E. coli with plasmid isolation kits (QIAGEN Inc.). Enzymes were used as specified by the manufacturer (New England Biolabs Inc.). Southern blot analysis was performed by transfer of DNA to a Magnagraph nylon membrane (Micro Separations Inc.) and hybridization with digoxigenin-labeled dUTP probes and chemiluminescent substrate from the DIG system (Boehringer Mannheim), according to the manufacturer's instructions. pilE sequences were determined by amplifying pilE from the chromosome of lysed cells with primers PILRBS and SP3A (50) using Taq polymerase (Promega). The resulting PCR product was sequenced with PILRBS and/or SP3A.
Sequencing reactions were performed with the CEQ Dye Terminator Cycle Sequencing Quick Start kit and a CEQ 2000XL automated sequencer (Beckman Coulter), according to the manufacturer's instructions, or were performed commercially (Seqwright). DNA analysis was performed with Lasergene software (DNASTAR, Inc.) and VectorNTI software (Informax, Inc.). BLAST searches were performed, and the N. gonorrhoeae strain FA1090 Gonococcal Genome Sequencing Project (http://www.genome.ou.edu/gono.html) was accessed to locate transposon insertion sites.
Plasmid construction.
Plasmid pPilEc2 contains a 3.4-kb ClaI fragment spanning the gonococcal DNA sequence containing the upstream silent sequence (uss) and pilE (17). The ClaI fragment from pPilEc2 was subcloned into pCRBlunt (Invitrogen) to create pPilEc2pb. Plasmid pΔuss::ermCUP was created by digesting pPilEc2 sequentially with AccI (691 bp upstream of the uss) and NdeI (116 bp downstream of the uss) to release the 1,271-bp uss-containing DNA fragment. The remaining Δuss DNA fragment was gel isolated, the ends were blunted, and the fragment was ligated with ermCUP, the blunt-ended ermC gene amplified from pHSS24 (64) with primers ErmAInUP (5′-GCCGTCTGAATCTTTTATTCAATAATCGCATCAG-3′ [the gonococcal uptake sequence is underlined]) and ErmBIn (5′-ACAAAAAATAGGTACACGAAAAACAAG-3′). Attempts to create gonococcal strain FA1090 Δuss::ermCUP by spot transformation of N. gonorrhoeae strain FA1090 variant 1-81-S2 recA6 with pΔuss::ermCUP resulted in transformants that carried both the deleted and wild-type versions of the DNA.
Plasmid pUSS1 was created by amplifying the FA1090 chromosome fragment with primers pildelete4 (5′-GGCTTGCGCTATCCCGATGAG) and upstreamrev1 (5′-GCAACCGGCAGATGAACG). Plasmid pUSS1 contains the FA1090 chromosomal fragment spanning 1,586 to 428 bp upstream of the pilE start codon (∼300 bp upstream of the uss to the 5′ end of RS1). Plasmid pPilERev3 was created by cloning the intergenic region upstream of pilE with primers USS1 (5′-CGGCACGGAAACTTATCGGG) and pilERev3 (5′-GCCGTCTGAAGGAAGGGCGACTGCCGCC [the gonococcal uptake sequence is underlined]) into pCRBlunt (13). Plasmid pPilERev3 contains the FA1090 chromosomal fragment spanning from 584 bp upstream of the pilE start codon to 82 bp downstream of the pilE start codon (roughly from the middle of RS2 into the pilE constant region). Plasmid pPilERev3 was designed to limit pilin expression in E. coli and therefore contains only the constant region of pilE.
Transposon mutagenesis.
In vitro transposition was performed with the EZ::TN transposition system (Epicentre) on plasmids pPilERev3 and pUSS1. These plasmids contain two separate fragments of FA1090 chromosomal DNA totaling 1,668 bp, overlapping by 156 bp. The EZ::TN transposon and plasmid DNA were combined at a 1:1 molar ratio with transposition buffer, transposase, and water to a final volume of 15 μl and incubated at 37°C for 2 h. Stop solution (1% sodium dodecyl sulfate) was added to the transposition reaction and incubated at 70°C for 10 min. The DNA was then ethanol precipitated and resuspended in 10 μl of H2O. The gapped transposon insertion was repaired with T4 DNA polymerase and T4 DNA ligase (43). The insertion was then directly introduced into the gonococcal genome by DNA spot transformation, as previously described (23).
Transposon insertions were identified by PCR and subsequent sequencing with either primer KANFOR (5′-TTGATGCTCGATGAGTTTTTCTAA) or primer KANREV (5′-GTTTCCCGTTGCCTATGGCTCATA), which anneal with their 3′ ends directed out of the mini-Tn5 transposon (mTn), in combination with one or more N. gonorrhoeae-specific primers, including pildelete4, USS1, upstreamrev1, or pilERev3, depending on the location of the transposon insertion.
Mutagenesis upstream of pilE.
The method described by Datsenko and Wanner (7) was adapted for the creation of deletions upstream of pilE. Deletions were linked to the kan-2 gene from the mTn inserted at the position of pilE::mTn#9 (which does not affect pilin Av; see Fig. 1A), to provide a selectable Km resistance marker for identification of these deletions. PCR products were generated with primer UpstreamRS1 Kan-2ForB (5′-TGCCGTCAACCTGCCGCGACGCTTCATCTGCCGGTTGCATAGAAACTGTCTCTTATACACATCTC) in combination with ΔRS1 Kan-2Rev (5′-GGTGGGTTGGGTGGGGAATTTTTTATTTTTTAAAAAGCTCCGTTTCCCTGAAGCTTGCATGCC) or ΔRS1-region 2 Kan-2Rev (5′-TTCGTTACTTTTTTATTGGCATGGGGTATCGGGTGTGTTGATTGGCCCTGAAGCTTGCATGCC) for the ΔRS1 and ΔRS1-region 2 deletions, respectively. A PCR product was generated with primersUpstreamRS1 Kan-2ForA (5′-TGCCGTCAACCTGCCGCGACGCTTCATCTGCCGGTTGCATAGAAAATGAGCCATATTCAACGGGAAAC) and ΔRS1-PilA Kan-2Rev (5′-CGGAAGTAGGGGGCGGCAGTGTCGAAAATTGTCAGTTTTAGTGCCTTAGAAAAACTCATCGAGCATCAA) for the ΔRS1-pilA deletion. These PCR products contain the kan-2 marker flanked by short regions of homology to the region upstream of pilE at the 5′ and 3′ ends (underlined in the primer sequences). E. coli strain AB1157 (pKD46) was cotransformed with plasmid pPilERev3 and each of the PCR products. Kmr colonies were selected to identify the Km insertion and accompanied deletion upstream of pilE and verified to contain the desired deletion by sequence analysis, yielding strains AB1157 pPilERev3 KanTn9ΔRS1, AB1157 pPilERev3 KanTn9ΔRS1-region 2, and AB1157 pPilERev3 KanTn9ΔRS1-PilA. The gonococcal strains FA1090 KanTn9ΔRS1 and FA1090 KanTn9ΔRS1-PilA (ΔRS1 and ΔRS1-PilA) were created by spot transformation of N. gonorrhoeae strain FA1090 variant 1-81-S2 recA6 with plasmids pPilERev3 KanTn9ΔRS1 and pPilERev3 KanTn9ΔRS1-PilA, respectively. Transformation with pPilERev3 KanTn9ΔRS1-region 2 never yielded Kmr colonies bearing the correct deletion.
FIG. 1.
Transposon and site-directed mutants upstream of pilE. (A) Map of transposon insertions (gray inverted triangles) recovered upstream of pilE. The figure is drawn to scale. Sites of single transposon insertions are dark gray inverted triangles, and sites where multiple insertions were isolated are light gray inverted triangles. The uss and pilin-associated repeats RS1 and RS2 are indicated. Dark gray boxes indicate sequences identical to the pilE constant region, and checkered boxes indicate sequences identical to the cys2 coding sequence in pilE. Diagonally striped boxes indicate PilA binding sites (2), the white triangle indicates the partial Sma/Cla repeat (17), and the open circle denotes the IHF binding site (19). The line from the arrow indicates the direction of pilE transcription. Lines across the top indicate regions in which transposon insertions have different phenotypes. Region 1 contains transposon insertions that have no effect on pilin Av. Regions 2 and 3 contains transposon insertions that retain a piliated phenotype; region 2 insertions completely abrogate pilin Av, while region 3 insertions interfere with Av. Region 4 contains transposon insertions that disrupt pilin Av and also display a piliation defect. Solid lines across the bottom, marked “A” and “B,” indicate spaces in which no transposon insertions were detected. Dashed lines across the bottom indicate the overlapping cloned fragments targeted for transposition. Arrows indicate the borders of the uss deletion. Transposon insertions with numbers above them indicate mutants that have been further characterized and are referred to in the text and subsequent figures. (B) DNA sequence of the region between the cys2 sequence at the 3′ end of RS1 and the pilE start codon. The locations of the upstream cys2 element, RS1, the two PilA binding sites, the IHF binding site, and the pilE start codon are labeled and indicated by the underlined and boldface sequence. The location of each Tn5 insertion is denoted by the dotted boxes containing the 9 bp of sequence that is duplicated as a result of mTn insertion and flanks the mTn insertion site (capitalized sequence). The −10 promoter sequence targeted for mutagenesis is indicated, and the nucleotide changes introduced in the promoter mutant are underlined. Arrows indicate the 5′ and 3′ borders of the deletions.
Overlap extension PCR was performed to mutate the −10 box of the pilE promoter according to the method of Fyfe et al. (12). Primers R2 (5′-GCATAGAAACACCACGCGC) and FM2 (5′-CGACAACTGCTAGCTAGCAAGCAAGATTCG) or F2 (5′-GGAAGGGCGACTGCCGC) and RM2 (5′-CGAATCTTGCTTGCTAGCACGCAGTTGTCG) were used to amplify partial fragments of the pilE promoter region and introduce the NheI restriction site (underlined in the primer sequences) into the −10 box. Equimolar amounts of each PCR product were annealed and used as templates for primers R2 and F2 to amplify the full promoter region containing the NheI site. The resulting PCR product was cloned into pCR-Blunt (Invitrogen), sequenced to verify the proper mutation, and then transformed into FA1090 1-81-S2 recA6 by spot transformation to create FA1090pilE-10::NheI (FA1090 promoter mutant). Gonococcal mutants were verified by PCR using primers FM2 and RM2 with subsequent digestion by NheI.
Kinetic Av assay.
The kinetic assay of colony variation was performed as previously described (46, 54). Briefly, N. gonorrhoeae strains were passaged for isolated colonies on GCB plates containing 1 mM IPTG and incubated at 37°C with 5% CO2. Ten individual colonies per strain were visually inspected at 20, 22, 24, 26, and 28 h for the presence of sectors of P− colony morphology, indicative of pilus-dependent variation. Colony variation scores ranging from 0 to 4 were assigned to each isolated colony based on the number of sectors of variation present per colony. A score of 0 indicates zero sectors and 1 indicates one sector, etc. Colonies that contained more than four sectors of variation were given a score of 4. Each assay was performed at least twice, in triplicate or quadruplicate. Results are reported as the mean colony variation score ± the standard error of the mean at each time point. Differences between strains were considered significant if P was <0.05 by Student's two-tailed t test.
Sequencing Av assay.
The sequencing assay of pilin Av was performed as previously described with modifications to provide additional statistical power for reduced overall cost (6, 26). N. gonorrhoeae strains were streaked for isolated colonies on plates containing 1 mM IPTG and incubated at 37°C in 5% CO2 for 20 h. Seven isolated piliated “starter” colonies per strain were selected and passaged onto separate plates without IPTG to turn off Av and lock in place any sequence changes that occurred at pilE. Twenty-eight colonies that arose from each starter colony were passaged two more times to ensure clonal populations, and the pilE gene of each colony was sequenced as described above.
Since the pilE::mTn#15 and ΔRS1-PilA mutants had undetectable levels of pilus-dependent colony morphology changes (see Fig. 2), we modified the DNA sequencing assay to maximize detection of very small numbers of pilE recombination events by isolating two starter colonies grown on IPTG and analyzing ∼180 progeny from each by pilE DNA sequencing (pilE::mTn#15 starter 1, 161 progeny; starter 2, 184 progeny; ΔRS1-PilA starters 1 and 2 both had 177 progeny). No pilE recombinants were detected, resulting in a calculated limit of detection for each of the two starter colonies for each mutant. This averaged value is reported as the mean frequency of pilin Av (see Table 2).
FIG. 2.
Transposon insertions upstream of pilE display a range of pilin Av defects. Kinetic variation assay measuring the average number of pilus-dependent colony morphology changes occurring over time. A representative piliated transposon mutant from each phenotypic region of transposon insertions is shown. Mutant pilE::mTn#9 represents region 1, pilE::mTn#15 represents region 2, and pilE::mTn#4 represents region 3. The parental strain FA1090 1-81-S2 recA6, grown in the presence of IPTG to activate RecA expression, is also depicted. Error bars represent the standard error of the mean of two experiments done in triplicate or quadruplicate (n = 7 or 8). Mutant pilE::mTn#15 does not antigenically vary, similar to a recA null strain (data not shown). Statistically significant differences (P < 0.05), as determined by Student's t test, are indicated by asterisks for comparison to the isogenic parental strain.
TABLE 2.
Sequence analysis of Av at pilE
| Genotype | Frequency of pilin Ava
|
Fold difference vs parental (comparison of means) | |
|---|---|---|---|
| Median (25th-75th percentile) | Mean (SEM) | ||
| Parental | 0.11 (0.071-0.13) | 0.11 (0.012) | |
| Region 1 | |||
| pilE::mTn#9 | 0.11 (0.019-0.13) | 0.077 (0.024) | −1.3 |
| Region 2 | |||
| pilE::mTn#15 | 0* | <0.0058† | >(−17.6) |
| Region 3 | |||
| pilE::mTn#3 | 0* (0-0.038) | 0.021† (0.011) | −4.8 |
| pilE::mTn#4 | 0.036* (0-0.071) | 0.046† (0.022) | −2.2 |
| Region 4 | |||
| pilE::mTn#7 | 0.042* (0.031-0.046) | 0.038† (0.0089) | −2.7 |
| pilE::mTn#2 | 0.065* (0.043-0.084) | 0.063 (0.023) | −1.6 |
| pilE::mTn#6 | 0.021* (0.010-0.052) | 0.035 (0.025) | −2.9 |
| Promoter mutant | 0.071 (0.071-0.13) | 0.11 (0.021) | +1.1 |
| Deletions | |||
| ΔRS1 | 0.11 (0.073-0.11) | 0.12 (0.034) | +1.2 |
| ΔRS1-PilA | 0* | <0.0057† | >(−18.1) |
The frequency of pilin Av at pilE was determined for selected mutants in each region upstream of pilE by the DNA sequencing assay for pilin Av, with modifications as described in Results and Materials and Methods (6). The parental strain is FA1090 1-81-S2 recA6, grown in 1 mM IPTG for full induction of recA and, therefore, pilin Av. Medians and means of the frequency of pilin Av are not significantly different from those of the parental strain unless indicated as follows: *, P < 0.05 (Wilcoxon rank-sum test of medians); †, P < 0.05 (Student's t test of means).
Previously we have reported results of the DNA sequencing assay as the mean frequency of pilin Av ± the standard error of the mean, and differences between strains were considered significant if P was <0.05 by Student's two-tailed t test. Because pilE recombinants arise stochastically under RecA-inducing conditions and because a recombination event early after RecA induction will result in an abnormally high reported frequency of pilin Av, it is more appropriate to report the frequency of pilin Av as a median from the seven starter colonies along with the 25th and 75th percentiles of the data set (L. Welty, personal communication). Therefore, both the mean and median frequencies of pilin Av are reported (see Table 2). Differences between the median frequency of pilin Av of a mutant of interest and that of its parental strain were considered significant if P was <0.05 by the Wilcoxon rank-sum test.
RESULTS
Saturation transposon mutagenesis of the pilE upstream region.
The hybrid intermediate model of pilin Av proposes that sequences upstream of pilE play a crucial role in pilin Av (22). In silico analysis of the sequences upstream of pilE revealed that they are unique to the pilin coding region and include one pilin copy, termed the uss, spanning the region from 1,213 to 698 bp upstream of the pilE start codon. The intergenic region between the uss and pilE contains a 51-bp partial Sma/Cla repeat, as well as a 71-bp region identical to nucleotides 102 to 180 of the pilE constant region, a 48-bp region homologous to the pilE hypervariable loop region and containing a cys2 element, and two conserved pilin-associated repeats (RS1 and RS2) (Fig. 1A) (15, 17). To investigate the involvement of sequences upstream of pilE in pilin Av, transposon mutagenesis was performed on a 1,668-bp region spanning the uss through the 5′ constant region of pilE (Fig. 1A). Gonococcal mutants were generated by in vitro transposition of an mTn derivative (EZ::TN; Epicentre Technologies [14]) into two different plasmids containing cloned, overlapping fragments of the 1,668-bp sequence (Fig. 1A). Transposon-mutagenized plasmids were transformed into the chromosome of gonococcal strain FA1090 encoding the pilin variant 1-81-S2 (50) and harboring a recA allele under the control of lac regulatory sequences (49). The locations of 73 independent insertions were mapped by DNA sequencing and are shown schematically in Fig. 1A.
Random transposition of 1,668 bp (albeit on two target fragments) and subsequent isolation of 73 mutants would be predicted to give rise to approximately one transposon insertion every 23 bp. This prediction was not observed. Instead, more than one mTn insertion was isolated at 20 different sites, and two extended areas (areas A and B) were found to contain no mTn insertions (Fig. 1A). Area A contains no mTn insertions and spans 100 bp in the uss, including the cys2 element of that pilin copy. Area B spans 150 bp and includes the majority of the RS2 repeat, as well as the orphan cys2 element found in this intergenic region. These data suggest that the region extending from uss to pilE carries “hot” and “cold” spots for Tn5 insertion (3, 33). Although the mTn does not target specific sequences for insertion, the probability of obtaining 73 mTn insertions at only 37 sites is very low (Poisson distribution P value of 1.6e−5) and argues that these sites represent bona fide hot spots for insertion. Conversely, the cold spots could be due to inhibition of transposition or selections against isolation after transformation of gonococci. As evidence for the latter, we were unable to replace the uss (between the inverted arrows in Fig. 1A), which contains area A, with an Ermr gene without also retaining a wild-type uss allele (data not shown). These data suggest that the region upstream of pilE is important for gonococcal viability, although the mechanism behind this is presently unknown.
Transposon insertions upstream of pilE show differing effects on pilin Av.
During pilin Av, recombination events between a pilS copy and pilE can give rise to new pilin variants that differ in the ability to be expressed or assembled into pili. As a result, pilin Av results in a variety of pilus-dependent colony morphologies, ranging from highly piliated (P+) to under- or nonpiliated (P−) (40, 47). To determine whether mTn insertions upstream of pilE affect pilin Av, mutant strains were examined for changes in pilus-dependent colony morphology. In this assay, gonococci are grown on solid media containing IPTG to turn on recA expression and thus pilin Av, and the outgrowth of P− sectors from P+ progenitor colonies is monitored over time (46). Using the pilus-dependent colony morphology assay to assess pilin Av, four categories of mTn insertion effects on piliation state and pilin Av were identified, with the causative insertions falling into four regions of the pilE upstream region (Fig. 1A and Table 1). Three of the four phenotypic categories consisted of mTn insertion mutants that retained parental levels of piliation (i.e., retained the P+ colony morphology) but exhibited different levels of pilin Av. The fourth category carried mTn insertions near the pilE promoter that reduced pilus expression (i.e., the colonies exhibited a P− phenotype). Since the pilus-dependent colony variation assay could not be applied to the P− colonies, we assessed the frequency of pilin Av with the pilE sequencing assay, which measures pilin Av independently of colony morphology changes (see below).
TABLE 1.
Locations and pilus-associated phenotypes of transposon insertion mutants identified upstream of pilE
| Region | mTn mutant | Position of mTn insertion (bp upstream of pilE start codon) | Piliation statea | Antigenic variation phenotypeb |
|---|---|---|---|---|
| 1 | pilE::mTn#9 | 423 | + | + |
| 2 | pilE::mTn#1 | 340 | + | − |
| pilE::mTn#15 | 335 | + | − | |
| 3 | pilE::mTn#3 | 297 | + | +/− |
| pilE::mTn#12 | 280 | + | +/− | |
| pilE::mTn#4 | 204 | + | +/− | |
| 4 | pilE::mTn#7 | 165 | +/− | +/− |
| pilE::mTn#2 | 110 | +/− | +/− | |
| pilE::mTn#6 | 70 | − | +/− |
Piliation state, compared to the isogenic parental strain, was determined visually by inspection of colony morphology under a stereomicroscope. Symbols: +, piliation equivalent to that of the parental strain; +/−, less piliation than that of the parental strain; −, nonpiliated colony morphology.
Antigenic variation phenotype was assessed for mTn insertions in regions 1 through 3 by the pilus-dependent colony morphology assay and for region 4 by the DNA sequencing assay for pilin Av. Symbols: +, wild-type level of pilin Av; +/−, reduced levels of pilin Av; −, no measurable pilin Av.
Region 1 carried mTn insertions that had no effect on pilin Av and also encompassed the two “cold” areas (Fig. 1A). This region spanned the uss through the RS1 repeat. A representative mutant from this region, pilE::mTn#9, has an mTn insertion 423 bp upstream of the pilE start codon, after the third nucleotide of RS1, which marks the 3′ border of region 1 (Fig. 1). This mTn mutant displays no Av defect compared to the parental strain, as measured by pilus-dependent colony morphology changes (Fig. 2); therefore, we conclude that the DNA sequences between the uss and the RS1 repeat, with the possible exception of the cold spots, have no direct role in pilin Av.
Region 2 contains two mTn insertions, pilE::mTn#1 and pilE::mTn#15 (Fig. 1), that completely block pilin Av (Fig. 2 and data not shown). These mutants have mTn insertions 5 bp apart and are 340 bp and 335 bp upstream of the pilin start codon, respectively. Mutant pilE::mTn#1 is 83 bp downstream of pilE::mTn#9 and 44 bp downstream from the 3′ end of RS1. The insertion site of pilE::mTn#15 is identical to that of a previously isolated mTn insertion that was also reported to block pilin Av (46) and to another mTn insertion arising in this screen (pilE::mTn#33 [data not shown]). We conclude that region 2 carries sequences essential for pilin Av.
Region 3 carried transposon insertion mutants that lowered the frequency of, but did not completely block, pilin Av. These mTn insertions span a 133-bp region extending downstream of the insertion in pilE::mTn#15 to the 5′ PilA binding site (Fig. 1). Mutant strains pilE::mTn#3, pilE::mTn#12, and pilE::mTn#4 retained visually piliated phenotypes (data not shown) and displayed intermediate defects in pilin Av as measured by pilus-dependent colony morphology changes (Fig. 2 and data not shown). These data indicate that sequences in region 3 contribute to pilin Av.
A fourth group of mTn insertions displayed significant defects in piliation (Fig. 1). This region, termed region 4, contains the putative pilE promoter (10, 32) and the IHF and PilA binding sites (1, 2, 11, 19); mTn insertions in this region presumably disrupt pilE transcription. Another method, independent of colony morphology, was necessary in order to measure pilin Av in these insertion mutants. Therefore, we adapted a DNA sequencing assay developed to measure pilin Av regardless of colony morphology changes (6).
Optimizing a sequencing assay for the detection of pilin Av.
To perform the sequencing assay to measure pilin Av, single starter colonies of the parental or isogenic mTn mutant strains were grown on solid medium in the presence of IPTG to allow RecA-dependent pilin Av to occur. Colonies were then dispersed and grown on solid medium in the absence of IPTG, and the pilE genes of progeny arising from each starter colony were examined for the presence of nonparental pilE sequence, indicative of pilin Av having occurred. The frequency of pilin Av is expressed as the number of recombination events detected per CFU examined for each of the starter colonies, and the overall frequency is reported as both the mean and the median from all of the starter colonies. Although we have previously reported the frequency of pilin Av as a mean of the starter colonies, the median is more statistically appropriate since it minimizes the effect a recombination event in one of the first generations of bacterial growth would have on the frequency of pilin Av for a particular starter colony.
Previously, we had found that examination of five starter colonies with 48 associated progeny was sufficient for measuring a reproducible frequency of pilin Av for variant 1-81-S2. Given the significant cost associated with sequencing these large numbers of pilE genes, statistical modeling was used to determine whether these numbers could be reduced while maintaining the sensitivity of the assay. This analysis predicted that seven starter colonies, each with 28 to 34 associated progeny, would be sufficient to distinguish a fivefold difference in pilin Av with 90% power. We experimentally tested this prediction by comparing the frequency of the 1-81-S2 parental pilE variant to pilE::mTn#4 (see below) and were able to measure a statistically significant difference in pilin Av by using seven starter colonies and 28 progeny. We have therefore amended the pilin Av sequencing assay to incorporate these numbers of starter and progeny colonies.
We first validated that the sequencing assay yielded results similar to those of the pilus-dependent colony morphology assay for the mTn insertion mutants that retained the parental P+ colony morphology (Tables 1 and 2). The region 1 mutant pilE::mTn#9 exhibited a slight decrease in the mean frequency of pilin Av that was not statistically different from that of the parental strain and was identical to that of the parental strain when expressed as the median frequency. In contrast, no pilE variants were detected in ∼200 progeny per starter colony arising from the two region 2 mutants, pilE::mTn#1 and pilE::mTn#15, similar to a recA mutant strain (data not shown). The region 2 mutants yielded a frequency of pilin Av of <0.005 recombination events per CFU, at least an 18-fold reduction relative to the isogenic parent. pilE recombination events were detectable in the progeny arising from the two region 3 mutants, pilE::mTn#3 and pilE::mTn#4, but the frequency of pilin Av in both mutants was significantly lower than that of the parental strain. pilE::mTn#3 exhibited a slightly higher frequency of pilin Av than did pilE::mTn#4, but this difference was not statistically significant. Importantly, the pilin Av sequencing assay was able to detect significant differences in pilin Av between strains differing by <2.5-fold in measured frequency, demonstrating the power of this assay for detecting the recombination events underlying pilin Av. Taken together, the results of the DNA sequencing assay confirm the observations made with the pilus-dependent colony morphology assay for pilin Av and show that the transposon insertions in the pilE upstream region that do not interfere with pilus expression have differential effects on pilin Av.
Transcription of pilE is not required for pilin Av.
The DNA sequencing assay was applied to the region 4 mTn mutants, whose reduced piliation state precluded the use of the pilus-dependent colony morphology assay. The median frequencies of pilin Av in strains pilE::mTn#2, pilE::mTn#6, and pilE::mTn#7 were 1.6- to 2.9-fold less than that of the parental strain. These differences are statistically significant compared to the parent (Table 2). These insertions have a pilin Av phenotype similar to that of the region 3 mutants that interfere with, but do not block, pilin Av. This phenotype could be due either to direct effects on pilin Av or to an indirect effect of reduced transcription on pilin variation. Since the effect of transcription on pilin Av has never been directly tested, we therefore asked whether transcription of pilE was required for this process.
It has been proposed that transcription of pilE may be linked to Av by providing an open DNA structure that is more readily accessible to recombining molecules (18, 19). Transposon mutants isolated in region 4 display a piliation defect, presumably due to altered transcription of pilE, as well as an Av defect. To test whether transcription and recombination at pilE are linked, site-directed mutagenesis of the pilE promoter mutant was performed to mutate the −10 box, as previously described (12) (Fig. 1B). Since the promoter mutant was nonpiliated, as previously reported (12), its frequency of pilin Av was analyzed by the DNA sequencing assay. Disruption of the pilE promoter had no significant effect on the frequency of pilin Av compared to that of the parental strain (Table 2). Therefore, unlike other recombination systems, such as V(D)J rearrangement in B cells or direct repeat recombination in Saccharomyces cerevisiae (42, 60), pilE transcription is not required for recombination at pilE. We conclude that the region 4 transposon mutants interfere with pilin Av through a mechanism independent of transcription and most likely act via a mechanism similar to that underlying the effects of the region 3 mTn mutants.
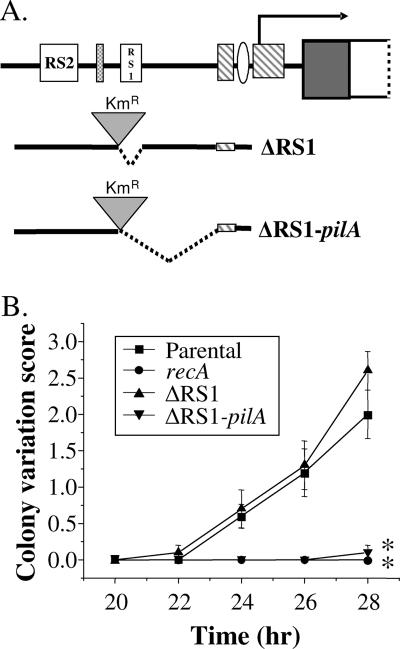
A deletion upstream of pilE disrupts Av.
Since mTn insertions upstream of pilE did not act on pilin Av through transcriptional effects, we postulated that sequences upstream of pilE were directly required for Av. We focused our attention on the RS1 pilin-associated repeat, which falls between region 1 and region 2, at the boundary where mTn insertions begin to interfere with pilin Av (Fig. 1A). There are two possible explanations for the block in pilin Av observed in the two region 2 mutants: (i) the mTn insertions displace or otherwise interfere with the DNA sequence of the RS1 repeat, or (ii) the sequences found within region 2, downstream of RS1, are required for pilin Av.
To differentiate between these possibilities, mutants were constructed in which RS1 alone (ΔRS1) or RS1 plus the adjacent 3′ 135 bp were deleted, resulting in deletion through the 5′ PilA binding site (ΔRS1-PilA) (Fig. 1B and 3A). These deletion mutants retained parental levels of piliation, as determined by visual inspection of the colonies, allowing for the use of the pilus-dependent colony morphology assay to assess pilin Av. The retention of the P+ phenotype in the ΔRS1-PilA strain confirms that the PilA binding site is not required for pilus expression (1). We observed that the ΔRS1 strain underwent pilin Av as well as the parental strain (Fig. 3B). However, the ΔRS1-PilA mutant was completely abrogated for pilin Av, similar to a phenotypically RecA-null strain (Fig. 3B) and to the region 2 mutants pilE::mTn#1 and pilE::mTn#15 (Fig. 2). These results were validated with the DNA sequencing assay for pilin Av, which showed that deletion of RS1 alone had no effect on the measured frequency of pilE recombination, whereas no pilE recombinants were recovered after passage of the ΔRS1-PilA mutant (Table 2). These data show that the RS1 repeat upstream of pilE is not required for pilin Av, leaving open the question of the function of this and other conserved pilin-associated repeats. More importantly, these results indicate that sequences within the 135 bp between RS1 and the 5′ PilA binding site are essential for pilin Av.
FIG. 3.
Deletion upstream of pilE disrupts pilin Av. (A) Schematic diagram of deletions upstream of pilE. The locations of RS2, RS1, and the pilE promoter and transcriptional start site (arrow) are indicated. The dashed line denotes the region of deletion. Deletion constructs were linked to the Kmr marker to provide a selectable marker linked to the deletion for transformation. (B) The kinetic variation assay was used to measure the average number of pilus-dependent colony morphology changes that occurred over time in the ΔRS1 and ΔRS1-PilA strains. The deletion strains are compared to the parental strain FA1090 1-81-S2 recA6 grown in the presence (Parental) and absence (recA) of IPTG to turn on and off RecA expression, respectively. Error bars represent the standard error of the mean of one representative experiment of three performed, each in triplicate. Statistically significant differences (P < 0.001), as determined by Student's t test, are indicated by asterisks for comparison to the isogenic parental strain.
We attempted to narrow down the sequences in this 135-bp region that were necessary for pilin Av by introducing an 87-bp deletion construct spanning RS1 and including the sequences where the region 2 mTn insertions were located. Although this technique was successful in creating the ΔRS1 and ΔRS1-PilA deletions, we were unable to recover gonococcal Kmr mutants that carried the ΔRS1-region 2 deletion. This observation strongly suggests that this deletion is selected against during transformation, unfortunately preventing direct testing of the extent of sequences lying between RS1 and the PilA binding site that are required for pilin Av. However, we are able to conclude that sequences within the 135 bp downstream of RS1 and upstream of the pilE promoter are absolutely required not only for pilin Av but also for gonococcal viability under conditions in which pilin Av is induced.
DISCUSSION
To investigate the role of sequence elements upstream of pilE that mediate pilin Av, an mTn mutagenesis screen of the ∼1.7-kb fragment of the pilE upstream region was undertaken. Several phenotypic classes of mTn insertions were identified, including those that abrogate Av, those that diminish Av, and those that have no effect on Av. The majority of the mTn insertions identified in this work had no effect on pilin Av and were located in region 1, spanning the uss to RS1. All insertions downstream of the RS1 repeat blocked or diminished the frequency of pilin Av. However, deletion of the RS1 repeat by itself had no effect on pilin Av. Thus, while from one to three copies of the RS1 repeat are found at each multicopy pilin locus (15, 17), the observation that deletion of RS1 had no effect on pilin Av indicates that this pilin-associated repeat has been conserved for another, as-yet-unidentified, reason. Since there is no effect of the RS1 deletion on pilus expression or pilin Av, we propose that the 5′ border of the pilE locus be redefined as the end of the RS1 repeat.
A group of mTn insertion mutants (strains pilE::mTn#3, pilE::mTn#12, pilE::mTn#4, pilE::mTn#7, pilE::mTn#2, and pilE::mTn#6) had variable effects on pilus-dependent colony morphology, but all exhibited a decrease in pilin Av relative to the parental strain, as measured by the DNA sequencing assay. All of these insertions fall in the 297 bp immediately upstream of the pilE start codon, and we speculate that they act to modulate pilin Av for the same reason. We explored the possibility that the Av defects in strains pilE::mTn#2, pilE::mTn#7, and pilE::mTn#6, which display underpiliated colony morphologies, were due to a link between Av and pilE transcription. Although active transcription enhances some recombination systems (5, 24, 42, 52, 60), the data presented here show that pilE transcription from the conventional −10 promoter is not required for pilin Av. While it is possible that low levels of transcription of pilE are provided by cryptic promoter sequences, the fact that there is no change in the frequency of pilin Av when normal transcription is blocked by mutation of the −10 sequence (Table 2) shows that these processes are not linked. In other recombination systems, such as V(D)J rearrangement, recombination is not directly controlled by transcription but is modulated by the action of transcription factors and changes in chromatin structure mediated by histone modifications and chromatin remodeling (8). As a bacterium, N. gonorrhoeae is not thought to carry nucleosomes or other well-ordered structures found in eukaryotic chromatin, but it does possess genes predicted to be involved in chromosome structure, including genes encoding a histone deacetylase-like protein (hda), the DNA binding protein HU-beta (dbhB), and the heterodimeric bacterial histone-like protein IHF (ihfA and ihfB) (58). Whether or not the chromosome structure affects Av remains to be tested, but we conclude that pilE transcription is not required for pilin Av.
Transposon insertions with the most-severe effects on Av, pilE::mTn#1 and pilE::mTn#15, caused a complete loss of Av. The pilE::mTn#15 insertion mutant has been previously shown to block pilin Av by acting upstream of the effects of recO, recG, ruvB, recJ, recQ, and recA (45). Since this mTn insertion blocks Av upstream of all of the known factors involved in this recombination process, we have postulated that this mTn insertion disrupts recombination initiation and that the pilE::mTn#1 insertion is likely to act identically since it is only 5 bp away and shows identical phenotypes. Studies are under way to specifically define which sequences are required for pilin Av in the region defined by the pilE::mTn#1 and pilE::mTn#15 insertions and to ask whether a specific endonuclease acts on those sequences.
The observation that initiation of pilin Av without resolution is toxic to gonococci undergoing pilin Av (45) may explain the existence of two “cold” regions upstream of pilE, where no transposon insertions were found (Fig. 1A). Our findings are consistent with previous reports in which insertions in the cys2 of either pilE or a silent copy disrupted pilin Av (20, 23). Furthermore, our attempts to allelically replace uss with an erythromycin resistance cassette were unsuccessful, resulting instead in a duplication of the uss (one uss containing the selected antibiotic resistance cassette and the other retaining a wild-type copy). It is likely that these transformants arose by a rare single-crossover event or by another mechanism that leads to duplication, but these duplications strongly suggest that the simple deletion provides a selective disadvantage. These observations are consistent with an important mechanistic role for conserved repeats and the uss in modulating pilin Av, although the reasons these mutations are selected against are not known at this time.
Models have been proposed for the molecular rearrangements underlying bacterial gene conversion, including the half-crossing-over model (29). Since these models have not sufficed to explain all the experimental observations of the behavior of N. gonorrhoeae when pilin Av is inhibited (23), an alternative model called the hybrid intermediate model was developed (22). In the first step of the hybrid intermediate model, RecA-independent recombination between the pilE locus and a pilS silent copy results in the formation of a hybrid locus carrying pilE sequences fused to a pilS copy at a short region of shared sequence identity. In the second step of this model, extensive stretches of upstream or downstream sequence homology between the pil loci target the hybrid donor for subsequent RecA-dependent recombination with the recipient pilE of a second chromosome. Consistent with this model, insertions in region 2 (i.e., pilE::mTn#1 and pilE::mTn#15) could abrogate pilin Av by interfering with the second step and preventing targeting of the hybrid intermediate to the recipient pilE.
Numerous Av systems in prokaryotes such as Mycoplasma and Borrelia species have been described (62), but no mechanism for initiating these specialized recombination processes has been elucidated. The recombination system for which initiation has been best characterized is the gene conversion leading to mating-type switching that occurs in Saccharomyces cerevisiae. In this yeast system, recombination is initiated by a double-strand break within the MAT locus made by the Ho endonuclease (31, 57). The double-strand break generated by Ho cleavage creates two 3′ overhangs that are 4 nucleotides long (41). A minimum sequence element of 16 bp is required for recognition by Ho in vitro, while 24 bp is required for cleavage in vivo (41). It has been proposed that additional proteins, such as YZbp (a binding protein that binds the Y/Z junction of the MAT locus), which binds sequences flanking the minimal 16-bp sequence and is required for Ho cleavage in vivo (65), may positively enhance Ho activity. Intriguing parallels can be drawn between the yeast MAT switching and the pilin Av gene conversion systems, including a minimal sequence element required for initiation and flanking sequences that enhance the initiating event. Although the absolute minimal sequence element required for the initiation of pilin Av is unknown, it is likely to be greater than 5 bp (the sequence element identified in which mTn insertions block Av) and less than 255 bp (the length of regions 3 and 4, in which insertions result in an intermediate defect in pilin Av). However, significant differences, such as a limited donor DNA repertoire and a reliance on chromatin structure in yeast, suggest that these diversity-generating systems may possess divergent mechanistic details. Therefore, an understanding of the initiating mechanism of pilin Av may reveal novel aspects of recombination initiation, some specific and some broadly applicable to the Av systems of both prokaryotes and eukaryotes.
Acknowledgments
We thank Leah Welty (Biostatistical Consulting Center, Northwestern University) for assistance in statistical analysis of the frequency of pilin Av.
This work was supported by Public Health Service grants R01 AI044239 and R01 AI033493 to H.S.S. and Individual Kirschstein NRSA training grant AI056681 to A.K.C. K.A.K. was partially supported by Public Health Service training grant T32 GM08061.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Arvidson, C. G., T. Powers, P. Walter, and M. So. 1999. Neisseria gonorrhoeae PilA is an FtsY homolog. J. Bacteriol. 181:731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidson, C. G., and M. So. 1995. Interaction of the Neisseria gonorrhoeae PilA protein with the pilE promoter involves multiple sites on the DNA. J. Bacteriol. 177:2497-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ason, B., and W. S. Reznikoff. 2004. DNA sequence bias during Tn5 transposition. J. Mol. Biol. 335:1213-1225. [DOI] [PubMed] [Google Scholar]
- 4.Boslego, J. W., E. C. Tramont, R. C. Chung, D. G. McChesney, J. Ciak, J. C. Sadoff, M. V. Piziak, J. D. Brown, C. C. Brinton, Jr., S. W. Wood, and J. R. Bryan. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154-162. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri, J., and F. W. Alt. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 4:541-552. [DOI] [PubMed] [Google Scholar]
- 6.Criss, A. K., K. A. Kline, and H. S. Seifert. 2005. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 58:510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feeney, A. J., P. Goebel, and C. R. Espinoza. 2004. Many levels of control of V gene rearrangement frequency. Immunol. Rev. 200:44-56. [DOI] [PubMed] [Google Scholar]
- 9.Frasz, C., and C. G. Arvidson. 2003. Role for both DNA and RNA in GTP hydrolysis by the Neisseria gonorrhoeae signal recognition particle receptor. J. Bacteriol. 185:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fyfe, J. A., C. S. Carrick, and J. K. Davies. 1995. The pilE gene of Neisseria gonorrhoeae MS11 is transcribed from a σ70 promoter during growth in vitro. J. Bacteriol. 177:3781-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyfe, J. A., and J. K. Davies. 1998. An AT-rich tract containing an integration host factor-binding domain and two UP-like elements enhances transcription from the pilEp1 promoter of Neisseria gonorrhoeae. J. Bacteriol. 180:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyfe, J. A., R. A. Strugnell, and J. K. Davies. 1993. Control of gonococcal pilin-encoding gene expression in Escherichia coli. Gene 123:45-50. [DOI] [PubMed] [Google Scholar]
- 13.Goodman, S. D., and J. J. Scocca. 1988. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 85:6982-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goryshin, I. Y., and W. S. Reznikoff. 1998. Tn5 in vitro transposition. J. Biol. Chem. 273:7367-7374. [DOI] [PubMed] [Google Scholar]
- 15.Haas, R., and T. F. Meyer. 1986. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell 44:107-115. [DOI] [PubMed] [Google Scholar]
- 16.Haber, J. E. 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32:561-599. [DOI] [PubMed] [Google Scholar]
- 17.Hamrick, T. S., J. A. Dempsey, M. S. Cohen, and J. G. Cannon. 2001. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology 147:839-849. [DOI] [PubMed] [Google Scholar]
- 18.Hill, S. A., S. G. Morrison, and J. Swanson. 1990. The role of direct oligonucleotide repeats in gonococcal pilin gene variation. Mol. Microbiol. 4:1341-1352. [DOI] [PubMed] [Google Scholar]
- 19.Hill, S. A., D. S. Samuels, J. H. Carlson, J. Wilson, D. Hogan, L. Lubke, and R. J. Belland. 1997. Integration host factor is a transcriptional cofactor of pilE in Neisseria gonorrhoeae. Mol. Microbiol. 23:649-656. [DOI] [PubMed] [Google Scholar]
- 20.Howell-Adams, B. 1998. Ph.D. thesis. Northwestern University, Evanston, IL.
- 21.Howell-Adams, B., and H. S. Seifert. 1999. Insertion mutations in pilE differentially alter gonococcal pilin antigenic variation. J. Bacteriol. 181:6133-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell-Adams, B., and H. S. Seifert. 2000. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol. Microbiol. 37:1146-1159. [DOI] [PubMed] [Google Scholar]
- 23.Howell-Adams, B., L. A. Wainwright, and H. S. Seifert. 1996. The size and position of heterologous insertions in a silent locus differentially affect pilin recombination in Neisseria gonorrhoeae. Mol. Microbiol. 22:509-522. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda, H., and T. Matsumoto. 1979. Transcription promotes recA-independent recombination mediated by DNA-dependent RNA polymerase in Escherichia coli. Proc. Natl. Acad. Sci. USA 76:4571-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellogg, D. S., Jr., W. L. Peacock, W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline, K. A., and H. S. Seifert. 2005. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J. Bacteriol. 187:5347-5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline, K. A., and H. S. Seifert. 2005. Role of the Rep helicase gene in homologous recombination in Neisseria gonorrhoeae. J. Bacteriol. 187:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight, K. L. 1992. Restricted VH gene usage and generation of antibody diversity in rabbit. Annu. Rev. Immunol. 10:593-616. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi, I. 1992. Mechanisms for gene conversion and homologous recombination: the double-strand break repair model and the successive half crossing-over model. Adv. Biophys. 28:81-133. [DOI] [PubMed] [Google Scholar]
- 30.Koomey, M., E. C. Gotschlich, K. Robbins, S. Bergstrom, and J. Swanson. 1987. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics 117:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostriken, R., J. N. Strathern, A. J. Klar, J. B. Hicks, and F. Heffron. 1983. A site-specific endonuclease essential for mating-type switching in Saccharomyces cerevisiae. Cell 35:167-174. [DOI] [PubMed] [Google Scholar]
- 32.Laskos, L., C. S. Ryan, J. A. Fyfe, and J. K. Davies. 2004. The RpoH-mediated stress response in Neisseria gonorrhoeae is regulated at the level of activity. J. Bacteriol. 186:8443-8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodge, J. K., K. Weston-Hafer, and D. E. Berg. 1991. Tn5 insertion specificity is not influenced by IS50 end sequences in target DNA. Mol. Gen. Genet. 228:312-315. [DOI] [PubMed] [Google Scholar]
- 34.Luirink, J., C. M. ten Hagen-Jongman, C. C. van der Weijden, B. Oudega, S. High, B. Dobberstein, and R. Kusters. 1994. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 13:2289-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack, W. T., L. W. Tjoelker, and C. B. Thompson. 1993. Immunoglobulin gene diversification by gene conversion. Prog. Nucl. Acid Res. Mol. Biol. 45:27-45. [DOI] [PubMed] [Google Scholar]
- 36.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehr, I. J., and H. S. Seifert. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation, and DNA repair. Mol. Microbiol. 30:697-710. [DOI] [PubMed] [Google Scholar]
- 38.Mehr, I. J., and H. S. Seifert. 1997. Random shuttle mutagenesis: gonococcal mutants deficient in pilin antigenic variation. Mol. Microbiol. 23:1121-1131. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, T. F., E. Billyard, R. Haas, S. Storzbach, and M. So. 1984. Pilus genes of Neisseria gonorrhoeae: chromosomal organization and DNA sequence. Proc. Natl. Acad. Sci. USA 81:6110-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, T. F., N. Mlawer, and M. So. 1982. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell 30:45-52. [DOI] [PubMed] [Google Scholar]
- 41.Nickoloff, J. A., E. Y. Chen, and F. Heffron. 1986. A 24-base-pair DNA sequence from the MAT locus stimulates intergenic recombination in yeast. Proc. Natl. Acad. Sci. USA 83:7831-7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oltz, E. M., F. W. Alt, W. C. Lin, J. Chen, G. Taccioli, S. Desiderio, and G. Rathbun. 1993. A V(D)J recombinase-inducible B-cell line: role of transcriptional enhancer elements in directing V(D)J recombination. Mol. Cell. Biol. 13:6223-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelicic, V., S. Morelle, D. Lampe, and X. Nassif. 2000. Mutagenesis of Neisseria meningitidis by in vitro transposition of Himar1 mariner. J. Bacteriol. 182:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 45.Sechman, E. V., K. A. Kline, and H. S. Seifert. 2006. Loss of both Holliday junction processing pathways is synthetically lethal in the presence of gonococcal pilin antigenic variation. Mol. Microbiol. 61:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sechman, E. V., M. S. Rohrer, and H. S. Seifert. 2005. A genetic screen identifies genes and sites involved in pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 57:468-483. [DOI] [PubMed] [Google Scholar]
- 47.Segal, E., E. Billyard, M. So, S. Storzbach, and T. F. Meyer. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293-300. [DOI] [PubMed] [Google Scholar]
- 48.Segal, E., P. Hagblom, H. S. Seifert, and M. So. 1986. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc. Natl. Acad. Sci. USA 83:2177-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifert, H. S. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215-220. [DOI] [PubMed] [Google Scholar]
- 50.Seifert, H. S., C. J. Wright, A. E. Jerse, M. S. Cohen, and J. G. Cannon. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 93:2744-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seluanov, A., and E. Bibi. 1997. FtsY, the prokaryotic signal recognition particle receptor homologue, is essential for biogenesis of membrane proteins. J. Biol. Chem. 272:2053-2055. [DOI] [PubMed] [Google Scholar]
- 52.Serizawa, N., T. Horiuchi, and T. Kobayashi. 2004. Transcription-mediated hyper-recombination in HOT1. Genes Cells 9:305-315. [DOI] [PubMed] [Google Scholar]
- 53.Serkin, C. D., and H. S. Seifert. 2000. Iron availability regulates DNA recombination in Neisseria gonorrhoeae. Mol. Microbiol. 37:1075-1086. [DOI] [PubMed] [Google Scholar]
- 54.Skaar, E. P., M. P. Lazio, and H. S. Seifert. 2002. Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J. Bacteriol. 184:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stohl, E. A., and H. S. Seifert. 2006. Neisseria gonorrhoeae DNA recombination and repair enzymes protect against oxidative damage caused by hydrogen peroxide. J. Bacteriol. 188:7645-7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stohl, E. A., and H. S. Seifert. 2001. The recX gene potentiates homologous recombination in Neisseria gonorrhoeae. Mol. Microbiol. 40:1301-1310. [DOI] [PubMed] [Google Scholar]
- 57.Strathern, J. N., A. J. Klar, J. B. Hicks, J. A. Abraham, J. M. Ivy, K. A. Nasmyth, and C. McGill. 1982. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell 31:183-192. [DOI] [PubMed] [Google Scholar]
- 58.Swinger, K. K., and P. A. Rice. 2004. IHF and HU: flexible architects of bent DNA. Curr. Opin. Struct. Biol. 14:28-35. [DOI] [PubMed] [Google Scholar]
- 59.Taha, M. K., B. Dupuy, W. Saurin, M. So, and C. Marchal. 1991. Control of pilus expression in Neisseria gonorrhoeae as an original system in the family of two-component regulators. Mol. Microbiol. 5:137-148. [DOI] [PubMed] [Google Scholar]
- 60.Thomas, B. J., and R. Rothstein. 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56:619-630. [DOI] [PubMed] [Google Scholar]
- 61.Ulbrandt, N. D., J. A. Newitt, and H. D. Bernstein. 1997. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88:187-196. [DOI] [PubMed] [Google Scholar]
- 62.van der Woude, M. W., and A. J. Baumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainwright, L. A., J. V. Frangipane, and H. S. Seifert. 1997. Analysis of protein binding to the Sma/Cla DNA repeat in pathogenic Neisseria. Nucleic Acids Res. 25:1362-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wainwright, L. A., K. H. Pritchard, and H. S. Seifert. 1994. A conserved DNA sequence is required for efficient gonococcal pilin antigenic variation. Mol. Microbiol. 13:75-87. [DOI] [PubMed] [Google Scholar]
- 65.Wang, R., Y. Jin, and D. Norris. 1997. Identification of a protein that binds to the Ho endonuclease recognition sequence at the yeast mating type locus. Mol. Cell. Biol. 17:770-777. [DOI] [PMC free article] [PubMed] [Google Scholar]