Abstract
Sinorhizobium meliloti participates in a nitrogen-fixing symbiosis with legume plant host species of the genera Medicago, Melilotus, and Trigonella. We recently identified an S. meliloti two-component sensory histidine kinase, CbrA, which is absolutely required to establish a successful symbiosis with Medicago sativa (K. E. Gibson, G. R. Campbell, J. Lloret, and G. C. Walker, J. Bacteriol. 188:4508-4521, 2006). In addition to having a symbiotic defect, the cbrA::Tn5 mutant also has free-living phenotypes that suggest a cell envelope perturbation. Because the bases for these phenotypes are not well understood, we undertook an identification of CbrA-regulated genes. We performed a microarray analysis and compared the transcriptome of the cbrA::Tn5 mutant to that of the wild type. Our global analysis of gene expression identified 162 genes that are differentially expressed in the cbrA::Tn5 mutant, including those encoding proteins involved in motility and chemotaxis, metabolism, and cell envelope function. With regard to those genes with a known role in symbiosis, we observed increased expression of nine genes with overlapping functions in bacterial invasion of its host, which suggests that the mutant could be competent for invasion. Since these CbrA-repressed genes are vital to the invasion process, it appears that down-regulation of CbrA activity is important at this stage of nodule development. In contrast, our previous work showed that CbrA is required for bacteria to establish themselves within the host as nitrogen-fixing symbionts. Therefore, we propose a model in which CbrA functions as a developmental switch during symbiosis.
As a model system for studying methods that invasive bacteria employ to transition from a free-living environment to their niche within the host, we explored how the gram-negative bacterium Sinorhizobium meliloti establishes a nitrogen-fixing symbiosis within the roots of leguminous plants such as Medicago sativa. These bacteria elicit formation of a specialized plant organ, called a nodule, within which they establish themselves as chronic intracellular symbionts. Once intimately associated with their host, these bacteria reduce dinitrogen to biologically accessible ammonia, which is provided to the host in exchange for carbon derived from photosynthesis. Thus, these symbiotic bacteria play a crucial role in the global nitrogen cycle and can reduce the need for synthetic nitrogen fertilizers (83).
Nodule formation is a highly regulated and complex developmental process (11). Nodulation is initiated by a highly specific chemical dialog involving the bacterial perception of plant flavonoids and the responding synthesis of Nod factor, which elicits nodule organogenesis (26, 30, 36, 45). Bacteria subsequently invade the newly formed nodule through a plant-derived structure called the infection thread (27). There are a number of complex polysaccharides secreted by S. meliloti that are required to promote infection thread formation and growth (26, 36). For instance, low-molecular-weight (LMW) forms of the exopolysaccharide succinoglycan (EPS I) promote infection thread invasion, and they provide an overlapping function with the exopolysaccharide EPS II and K antigen (18, 58, 59). Cyclic β-(1,2)-glucan production is also required for infection thread invasion, although this requirement can be bypassed through the overproduction of succinoglycan (23, 52).
Once bacteria gain entry into host tissues via the infection thread, they are individually taken up into a membrane-bound compartment within the host cell cytoplasm where they differentiate into physiologically and morphologically distinct bacteroid forms capable of nitrogen fixation (56, 62). As part of this developmental process, both the bacterium and its plant host undergo profound changes in gene expression (3). In comparison to the requirements for infection thread invasion, the bacterial requirements for intracellular adaptation and differentiation are less well understood. These events are complicated by physiological challenges imposed by the plant host, which include the release of reactive oxygen species and an acidic intracellular host compartment (6, 9, 63, 70). It is now well established that certain molecular mechanisms these chronic endosymbionts use to establish a beneficial relationship with the plant host are shared with the chronic pathogen species Agrobacterium and Brucella (5, 82). Therefore, any insights gained into the Sinorhizobium-Medicago symbiosis have potential to further our understanding of these phylogenetically related pathogens.
We recently identified a new regulatory protein, CbrA, whose activity is crucial to S. meliloti for establishing an effective symbiosis with the host legume M. sativa (32). CbrA is a putative two-component histidine kinase with a sensory PAS domain, a ligand-binding motif commonly involved in sensing redox status (80). In contrast to a successful symbiosis that produces pink nodules, the cbrA::Tn5 mutant primarily elicits the formation of immature nodules from which fewer bacteria can be recovered, suggesting that the mutant either is unable to colonize its host efficiently or is compromised for survival within the host environment. Moreover, the cbrA::Tn5 mutant is unable to compete successfully with the wild type for nodule colonization during a mixed infection.
We originally identified the cbrA::Tn5 mutant in a screen based on its succinoglycan overproduction phenotype (32). Succinoglycan is an exopolysaccharide that plays a critical role in infection thread development and hence in nodule invasion (18). Importantly, our identification of the genes exoH, exoK, and exoT as CbrA-regulated genes suggests that the cbrA::Tn5 mutant is biased toward production of succinylated LMW forms of this exopolysaccharide, the structural forms most effective at promoting bacterial invasion via the infection thread (58). It therefore seems unlikely that succinoglycan overproduction is responsible for the severe symbiotic defects of the cbrA::Tn5 mutant. However, the cbrA::Tn5 mutant also exhibits a severe sensitivity to a variety of membrane-disrupting agents, including the bile salt deoxycholate and the hydrophobic dye crystal violet (32), indicating that the mutant has a cell envelope defect that may account for the symbiotic phenotype of the mutant.
The physiology of the gram-negative cell envelope determines how these bacteria interact physically with their environment. In addition to its key role in regulating the flow of molecules into and out of the cell (54), the envelope modulates host cell adhesion and host responses to bacterial invasion through a variety of mechanisms (50, 53, 60). The well-characterized S. meliloti lpsB and bacA mutants have cell envelope defects which result from distinct alterations to the lipopolysaccharide (LPS) that are associated with bacterial sensitivity to a variety of toxic agents (13, 24). These mutants fail to establish an effective symbiosis due to aborted infections that allow bacterial uptake into the host cell cytoplasm but are blocked for bacteroid differentiation (13, 34). In fact, these mutants appear to degrade rapidly upon uptake into the host membrane-bound compartment, suggesting they are either unable to survive within this intracellular environment or they are subject to a lethal host innate immune response. In contrast with the LPS, little is known regarding the requirement for S. meliloti outer membrane proteins (OMPs) in either cell envelope integrity or symbiosis.
The cbrA::Tn5 mutation affects gene expression primarily during the stationary phase growth, indicating that CbrA activity is responsive to the growth phase (32). We therefore examined global gene expression in the cbrA::Tn5 mutant to address the role of CbrA in both stationary phase regulation and cell envelope physiology and anticipated that we would gain potentially novel insights into bacterial requirements for host colonization. The set of differentially expressed genes identified in this study is significantly enriched for those encoding OMPs, secreted extracellular proteins, and proteins involved in motility and chemotaxis. Our results suggest CbrA has a broad regulatory role in modulating the physiology of the bacterial outer membrane and cell surface and thereby regulates the expression of proteins that could be involved in direct contact with the host during symbiosis.
MATERIALS AND METHODS
Bacterial strains, phage, plasmids, and growth conditions.
Bacterial strains and a generalized transducing phage are listed in Table 1. Sinorhizobium meliloti strains were grown in LB/MC (LB medium supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2). Unless otherwise noted, S. meliloti strains were cultured at 30°C in LB/MC into stationary phase (approximate optical density at 600 nm [OD600] of 5.0) for physiological and gene expression assays. Escherichia coli was grown in LB medium supplemented with appropriate antibiotics at 37°C. Antibiotic concentrations used for S. meliloti selection are as follows: streptomycin, 500 μg/ml; neomycin, 200 μg/ml; gentamicin, 12.5 μg/ml; and spectinomycin, 100 μg/ml. Antibiotic concentrations used for E. coli selection were as follows: spectinomycin, 50 μg/ml; chloramphenicol, 34 μg/ml; and kanamycin, 50 μg/ml. Symbiosis assays were performed as previously described (31), except that buffered nodulation medium was buffered at pH 6.5, and 1 mM aminoethoxyvinyl glycine (Sigma) was added for Medicago truncatula assays.
TABLE 1.
Bacterial strains, phage, plasmids, and PCR primers used in this study
| Strain, phage, plasmid, primer | Relevant characteristics | Source/reference |
|---|---|---|
| Strain | ||
| DH5α | E. coli [endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argG)U169 deoR] | BRL Corp. |
| MT616 | E. coli MM294 pRK600, Cmr | T. Finan |
| Rm1021 | SU47 Smr | F. Ausubel |
| KEG1002 | Rm1021 ropB1′-uidA+ | This study |
| KEG1003 | KEG1002 cbrA::Tn5-233 | This study |
| KEG2016 | Rm1021 cbrA::Tn5 | 32 |
| KEG2141 | MB669 cbrA::Tn5 | This study |
| KEG2143 | MB670 cbrA::Tn5 | This study |
| KEG2145 | MB671 cbrA::Tn5 | This study |
| KEG2147 | MB672 cbrA::Tn5 | This study |
| KEG2149 | MB673 cbrA::Tn5 | This study |
| KEG2150 | Rm1021 bioS-uidA+ | This study |
| KEG2151 | KEG2150 cbrA::Tn5 | This study |
| KEG2152 | Rm1021 SMc00489-uidA+ | This study |
| KEG2153 | KEG2152 cbrA::Tn5 | This study |
| KEG2155 | VO2674 cbrA::Tn5 | This study |
| MB669 | Rm1021 flaC′-uidA+ | This study |
| MB670 | Rm1021 mcpX′-uidA+ | This study |
| MB671 | Rm1021 mcpU′-uidA+ | This study |
| MB672 | Rm1021 SMc00888′-uidA+ | This study |
| MB673 | Rm1021 SMc00887′-uidA+ | This study |
| VO2674 | Rm1021 nex18′-bacA+-uidA+ | 55 |
| Phage | ||
| φM12 | Generalized transducing phage | T. Finan |
| Plasmid | ||
| pCRBluntII-TOPO | Blunt PCR cloning vector | Invitrogen |
| pK18mobsacB | Allele exchange vector | 71 |
| pWM3 | Vector with nonpolar uidA+ cassette | 49 |
| pVO155 | Suicide vector with uidA+ cassette, Nmr | V. Oke |
| pVO345 | Suicide vector with uidA+ cassette, Spr | V. Oke |
| pKEG11 | pVO155 ropB1′-uidA+ | This study |
| pKEG15 | pK18mobsacB bioS′-uidA+ | This study |
| pKEG16 | pK18mobsacB SMc00489′-uidA+ | This study |
| pMB694 | pVO345 flaC′-uidA+ | This study |
| pMB695 | pVO345 mcpX′-uidA+ | This study |
| pMB696 | pVO345 mcpU′-uidA+ | This study |
| pMB697 | pVO345 SMc00888′-uidA+ | This study |
| pMB698 | pVO345 SMc00887′-uidA+ | This study |
| PCR primer | ||
| bioS-Pst′1 | CTGCAGGTCCCACAGGTAGCGTC | This study |
| bioS-BamHI′1 | CGTAGGCGGATCCGGCAC | This study |
| bioS-BamHI′2 | GTGCCGGATCCGCCTACG | This study |
| bioS-Pst′2 | CTGCAGGGTCATCGGTCTCGGCTC | This study |
| flaC-Age | CTTGCGTCAGACTTGCCGGAT | This study |
| flaC-Xba | GCGCGCAATGTCTGCAACGCC | This study |
| mcpU-Age | AGCTCCCGCTAGCGTCCAGCC | This study |
| mcpU-Xba | ACGCGGCAACGAGAATGCGCG | This study |
| mcpX-Age | CTAGGGCACTTCCAGGAAAAG | This study |
| mcpX-Avr | GCCAGCGATCTGGCTTGGAAC | This study |
| ropB1-up | GCTCTAGAGCCGCAAACTCAATG | This study |
| ropB1-dn | CCTTCATCGTTTCGAGCAGGTTCC | This study |
| C00489-Pst′1 | CTGCAGGAGGGCGTTC | This study |
| C00489-BamHI′1 | CGTCGCGGATCCAGGTCGTAG | This study |
| C00489-BamHI′2 | CTACGACCTGGATCCGCGACG | This study |
| C00489-Pst′2 | CTGCAGGAACGTGTAGCCGAGCAT | This study |
| C00887-Age | GAACTCGGCAATGCACGCCAC | This study |
| C00887-Xba | CCAGCGGCAGGACGATGGCGA | This study |
| C00888-Age | CACTTCTCCATAGCTGTCGGC | This study |
| C00888-Xba | GATGTCTGCGCCGCCCGACGC | This study |
Genetic techniques and DNA manipulations.
Bacteriophage transductions with φM12 (25) and triparental matings (44) were performed as previously described. S. meliloti genomic DNA was isolated as described previously (2). Plasmids and primer sequences are listed in Table 1. To construct the pK18mobsacB derivatives, genomic DNA was used as a template for PCRs with Thermalase DNA polymerase (Invitrogen). The PCR products were initially cloned and sequenced in pCRBluntII-TOPO (Invitrogen) and then subcloned into pK18mobsacB (71). The suicide vector pK18mobsacB was used for allelic replacement so that a gene of interest could be disrupted with the uidA+ transcription fusion cassette. The uidA+ cassette was obtained by BamHI digestion of pWM3 (49). The suicide vectors pVO155 and pVO345 were used to create uidA+ transcription fusions at native gene loci. Derivatives of the suicide vectors pVO155, pVO345, and pK18mobsacB were mated into strain Rm1021 by selecting for Smr Nmr or Smr Spr, and the correct genomic location of the plasmid was verified through PCR. Rm1021 strains containing pK18mobsacB derivatives were cultured overnight in LB/MC medium, and an aliquot of the culture was plated onto LB medium containing 10% sucrose to promote the loss of the pK18mobsacB vector. The resulting colonies were screened for lack of growth on LB medium containing Nm. Nms Sucr colonies were further screened by PCR to determine whether the native allele was interrupted with the uidA+ cassette. Those Nms Sucr colonies with the uidA+ cassette properly integrated into the genome were retained for further study.
Affymetrix GeneChip analysis.
Design of the Symbiosis GeneChip, RNA isolation, cDNA synthesis, labeling, and hybridization were as described previously (4). Three biological replicates, 20-ml cultures grown in separate 250-ml flasks from single colonies, were used for each experimental strain (KEG2016) or control (Rm1021) strain data set. Experimental and control cultures were grown in LB/MC medium with streptomycin selection to similar OD600 values ranging from 4.1 to 4.5. For data mining, we used Affymetrix software (GCOS and DMT) as described previously (4). Briefly, we normalized using all probe sets with the target signal value set to 500 and used comparison, also known as relative, expression analysis: one array is designated as experimental and the other as baseline. Hence, this analysis with three control and three experimental arrays would yield nine pairwise comparisons. We considered an average signal log ratio (SLR) of ≥1.0 for the increase or decrease change calls to be significant only if eight or nine of the nine pairwise comparisons were deemed by the software to be significantly changed (a P value of ≤0.05). Note that SLR is expressed as the log2 ratio of the change, i.e., an SLR of 1 equals a twofold change. For data shown in Table 2, SLR values were converted to change (n = fold) values.
TABLE 2.
Functional grouping for differentially expressed genes identified by microarray
| Gene | Gene product (reference) | SLR of cbrA::Tn5/WT converted to fold change values |
|---|---|---|
| Regulators | ||
| sinI (SMc00168) | Quorum-sensing autoinducer synthase (47) | +2.59 |
| cspA5 (SMc00289) | Putative CspD family cold shock protein (43) | −2.44 |
| SMc00887a,b | Putative c-di-GMP GGDFF/EAL enzyme | −2.58 |
| SMc00888a,b | Putative hybrid histidine kinase with an N-terminal PAS domain and a C-terminal response regulator domain | −2.70 |
| bioS (SMc02061)a,b | LysR family biotin-responsive transcription factor (37) | −3.74 |
| SMc02369 | Putative PleC-like histidine kinase with a PAS/PAC domain | −2.34 |
| visN (SMc03015) | LuxR family transcriptional regulator of fla genes (75) | −2.44 |
| rem (SMc03046) | OmpR family response regulator of fla genes (68) | −3.24 |
| Exopolysaccharide biosynthesis | ||
| Succinoglycan biosynthesis and modification | ||
| exoY (SMb20946) | Galactosyltransferase (51) | +2.42 |
| exoH (SMb20954) | Succinyltransferase (35) | +3.44 |
| exoK (SMb20955) | Endo-β-1,3-1,4-glycanase (7) | +2.65 |
| exoL (SMb20956) | Glucosyltransferase (64) | +2.20 |
| exoA (SMb20957) | Glucosyltransferase (64) | +2.04 |
| exoN (SMb20960) | Glucosyltransferase (8) | +2.45 |
| exoW (SMb21690) | Glucosyltransferase (64) | +2.78 |
| Additional polysaccharide biosynthesis and export | ||
| pssF (SMb20748) | Putative Wca-like type 2 glycosyltransferase | +2.82 |
| SMc01793 | Putative RfaG-like type 1 glycosyltransferase | −2.28 |
| SMc01794 | Putative Wza-like periplasmic polysaccharide transporter | −2.04 |
| ndvA (SMc03900) | Inner membrane ABC-family transporter of cyclic β-(1,2)-glucan (78) | +3.28 |
| Flagellar biosynthesis and motility (representative subset of 56 genes) | ||
| mcpU (SMc00975)a | Chemoreceptor (48) | −4.12 |
| mcpX (SMc01104)a | Chemoreceptor (48) | −4.73 |
| cheY1 (SMc03006) | Chemotaxis regulator protein (76) | −5.00 |
| fliI (SMc03025) | Flagellum-specific ATP synthase (76) | −10.40 |
| flaC (SMc03040)a | Flagellin protein C (76) | −3.98 |
| motC (SMc03043) | Chemotaxis motility protein C (76) | −10.54 |
| Histidine uptake and metabolism | ||
| SMa0387 | Putative HisC-like histidinol-phosphate aminotransferase | +2.78 |
| hutU (SMb21163) | Putative urocanate hydratase (urocanase) | +2.63 |
| hutH1 (SMb21165) | Putative histidine ammonia-lyase | +2.54 |
| hutI (SMb21166) | Putative imidazolonepropionase | +3.06 |
| hisV (SMc00670) | HisV/HutV histidine transport ABC transporter cytoplasmic ATP-binding cassette (10) | +2.80 |
| hisW (SMc00671) | HisW/HutW histidine transport ABC transporter inner membrane permease (10) | +3.04 |
| hisX (SMc00672) | HisX/HutX histidine transport ABC transporter periplasmic binding protein (10) | +3.49 |
| SMc00673 | Putative hydrolase | +3.06 |
| Chaperones and proteolytic processing | ||
| SMb21295 | Putative IpaA-like Hsp20 chaperone | +3.52 |
| SMc00360 | Putative periplasmic transglutaminase-like cysteine protease (DUF920) | −2.32 |
| SMc00539 | Putative M23 family membrane-bound metaloprotease (COG4942) | −2.18 |
| SMc00998 | Putative periplasmic transglutaminase-like cysteine protease (DUF920) | −2.72 |
| SMc02825 | Putative M17 family aminopeptidase | −2.44 |
| SMc04040 | Putative IpaA-like Hsp20 chaperone | +3.19 |
| Pilus assembly | ||
| cpaF1 (SMc02820) | Putative cytoplasmic pilus assembly protein | −2.12 |
| SMc04059 | Putative TadG-like pilus assembly protein | −2.23 |
| cpaE1 (SMc04109) | Putative pilus assembly protein | −2.29 |
| cpaC1 (SMc04111) | Putative outer membrane pilus assembly protein | −2.04 |
| Outer membrane proteins | ||
| Opacity-related subgroup of OmpA-like proteins | ||
| SMc00141 | Putative OMP associated with a DUF612 domain | +5.70 |
| SMc00489a,b | Putative Pseudomonas aeruginosa PagL lipid A 3-O-deacylase ortholog (69) | −2.31 |
| ropB1 (SMc00604)a | Putative Rhizobium leguminosarum RopB ortholog (14) | +2.36 |
| SMc00638 | Putative Mesorhizobium huakuii Opa22 ortholog | −2.39 |
| SMc00639 | Putative Mesorhizobium huakuii Opa22 ortholog | −2.05 |
| Miscellaneous OMP tspO (SMa1079) | Rhodobacter sphaeroides TspO homolog required for nutrient deprivation regulation of ndiAB (21) | +3.13 |
| SMc00986 | Putative OMP with two copies of the DUF1217 domain | −2.90 |
| Secreted extracellular proteins nex18 (SMa1077)a | Fasciclin-like protein (55) | +2.61 |
| SMb20838 | Putative NodO-like hemolysin Ca2+-binding metalloprotease | +3.74 |
| SMc03108 | Putative NodO-like hemolysin Ca2+-binding metalloprotease | +3.30 |
| SMc04236 | Putative glycine-rich outer membrane autotransporter protein | +3.95 |
Differentially expressed gene whose regulation was confirmed with a gene fusion.
Differentially expressed gene whose symbiotic phenotype was assayed; each mutant assayed was found to promote wild-type symbiosis with M. sativa and M. truncatula.
β-Glucuronidase activity assays.
The relative expression level of each uidA+ transcription fusion was determined by assaying β-glucuronidase activity as previously described (39) with the following modifications. The assay buffer was supplemented with 10 mM EDTA (pH 8.0) and 0.1% sarcosyl, and the enzyme assays were performed with 5 mM p-nitrophenyl β-d-glucuronide substrate (Sigma). At specific time points, the reaction was stopped and the sample was centrifuged for 5 min at 18,000 × g to remove cellular debris. p-Nitrophenol absorbance of the clarified supernatant was measured at 415 nm, and modified Miller units were determined with the following formula: 1,000(A415/tvp), where t is the reaction time in minutes, v is the volume of culture assayed in ml, and p is the concentration of total cell protein present in the reaction. We isolated total cell protein by the addition of 0.2 volume of 100% trichloroacetic acid (TCA), followed by centrifugation for 10 min at 18,000 × g. The resulting TCA pellet was washed with 100% acetone, dried, and resuspended in 50 mM Tris (pH 8). Protein concentrations were determined by a Bradford assay (Bio-Rad). For each strain assayed, the average and standard deviation were derived from four independent cultures.
Isolation and analysis of flagella.
S. meliloti cultures were vortexed vigorously three times for 15 s in order to shear flagellar filaments from the cell surface. The vortexed culture was centrifuged for 30 min at 3,500 × g, and the cleared supernatant was isolated. Flagellar filaments within the supernatant were further isolated by ultracentrifugation at 40,000 × g for 40 min. Protein in the flagellar pellet was solubilized in 50 mM Tris (pH 7.5) buffer containing 250 mM NaCl and 10% glycerol, with a volume of buffer normalized for total protein concentration of the originating culture. We isolated total protein from an aliquot of each vortexed culture by the addition of 0.2 volume of 100% TCA, followed by centrifugation at 18,000 × g at 4°C for 10 min. The resulting TCA pellet was washed with 100% acetone, dried, and resuspended in 50 mM Tris (pH 8). Protein concentrations were determined by a Bradford assay (Bio-Rad). The flagellar preparation was analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Flagellar protein was detected with Coomassie stain, and the resulting band was isolated and analyzed by matrix-assisted laser desorption ionization-time of flight at the MIT Biopolymers Core Facility to ensure it represented flagellar protein. A 1:25 dilution of the flagellar preparation was again subjected to 12% SDS-PAGE, and protein was detected using quantitative Sypro orange dye according to the manufacturer's instructions (Molecular Probes). The gel was scanned using a Typhoon 9400 model imager (Amersham Biosciences), and protein bands were quantified relative to an internal standard of bovine serum albumin (Sigma).
Bioinformatics analyses of differentially expressed genes.
For those differentially expressed genes with no known function, we searched for homologous functional or conserved domains using HHPred (http://toolkit.tuebingen.mpg.de/hhpred), which uses both sequence identity and predictions of secondary structure in the query to search several protein databases. We used the PSORT algorithm (http://www.psort.org/) to predict protein localization. We identified the closest characterized PAS domain family member for those PAS domains present in CbrA, SMc02369, and SMc00888 by performing database searches with both HHPred and Phyre (http://www.sbg.bio.ic.ac.uk/phyre/), which is also a protein structure prediction program that aligns a query with related domains of a known tertiary structure and highlights functional residues within the alignment. These secondary structure-based searches were more successful at identifying PAS domain relatives than a protein BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) because PAS domain relatives share more structural identity than sequence identity (61). The sequence alignment of E. coli OmpA and S. meliloti OmpA family members was performed based on predictions of secondary structure using HHPred and Phyre and the known structure of the membrane-spanning domain of OmpA (57).
RESULTS
Microarray analysis identifies potential CbrA-regulated genes during free-living stationary phase growth.
CbrA is a putative two-component sensory histidine kinase that is required to establish an effective symbiosis between Sinorhizobium meliloti and the host legume Medicago sativa (32). Notably, we found that CbrA-dependent effects on gene expression are observed primarily during stationary phase growth rather than exponential growth. In order to achieve a comprehensive identification of CbrA-regulated genes, we performed a global analysis using Affymetrix GeneChips to compare the pattern of gene expression in the cbrA::Tn5 mutant to that of the wild type under stationary phase growth conditions. Overall, the microarray experiment identified 80 genes with significantly increased expression (see Table S1 in the supplemental material) and 82 genes with significantly decreased expression (see Table S2 in the supplemental material) in the cbrA::Tn5 mutant, compared to those in the wild type. Nearly half of these genes have no predicted function, and this is particularly true of the most strongly induced genes in the cbrA::Tn5 mutant (see Table S1 in the supplemental material). However, some of the genes identified did cluster into functional groups (Table 2).
We performed a preliminary validation of the microarray experiment by comparing the expression pattern of succinoglycan biosynthesis genes (Table 2) from this study to previous data (32). Consistent with our previous results, we observed a threefold increase in the expression of the succinoglycan modification genes exoH and exoK in the cbrA::Tn5 mutant. However, the overall pattern of exo gene expression observed with the microarray highlighted a slightly different subset of exo genes than we found in our previous studies using transcription fusions and reverse transcriptase PCR. This may be attributable to significant differences in culture conditions (batch growth in culture tubes versus flasks) or perhaps to the differential sensitivity of the methods used. For instance, previously, we found dramatic CbrA-dependent regulation of exoT; however, its expression was too low to be reliably detected by GeneChip analysis. We also found that exoB levels were unchanged, in contrast with our previous results, and that several additional exo genes were subject to CbrA regulation (including the genes exoA, exoL, exoN, exoW, and exoY).
Interestingly, our expression data suggest that CbrA may modulate the production or transport of other polysaccharides, in combination with succinoglycan, that promote infection thread invasion. The cbrA::Tn5 mutant exhibits a threefold increase in expression of ndvA (Table 2), which encodes an inner-membrane ABC-type transporter of the cyclic β-(1,2)-glucan critical for infection thread invasion (23, 52, 78). There may also be an increase in expression of sinI (Table 2), which encodes a quorum-sensing autoinducer synthase whose activity is required to establish symbiosis in the absence of succinoglycan via its role in regulating ExpR-dependent EPS II production (46).
CbrA affects the expression of multiple regulators during stationary phase.
Our microarray analysis indicated that CbrA affects the expression of eight regulatory proteins and may therefore be situated at the top of a complex regulatory network (Table 2). For instance, the cbrA::Tn5 mutant exhibits decreased expression of bioS (Table 2), which encodes a LysR-like transcription factor whose expression increases in response to biotin addition and during the transition from exponential growth to stationary phase growth (37, 79). While BioS autoregulation was found to contribute to biotin-dependent induction of bioS, the factor required for stationary phase regulation of bioS was not identified previously. We performed an allelic exchange and replaced bioS+ with a bioS′-uidA+ fusion that serves as a reporter for bioS promoter activity and disrupts bioS function, eliminating the effects of autoregulation. During stationary phase, we observed a fourfold decrease in bioS′-uidA+ expression in the cbrA::Tn5 mutant compared to that in the wild type (Fig. 1, Table 2). In contrast, during exponential growth, there was no significant difference in the bioS expression of the wild type and that of the cbrA::Tn5 mutant (Fig. 1). Thus, CbrA regulates bioS in a growth phase-dependent manner and is required for full stationary phase induction of bioS. As reported previously (79), we found that a bioS null mutant is symbiotically proficient on M. sativa and is able to compete effectively with a bioS+-carrying strain for nodule colonization; this observation is also true of the related diploid host Medicago truncatula (data not shown).
FIG. 1.
CbrA plays an important role in growth phase regulation of bioS. β-Glucuronidase activity expressed from a bioS′-uidA+ fusion was measured from either exponential phase or stationary phase wild-type and cbrA::Tn5 cultures. The uidA+ transcription fusion serves as a reporter for promoter activity and also disrupts gene function.
The cbrA::Tn5 mutant also exhibits decreased expression of a putative operon encoding two regulatory factors: one is an uncharacterized two-component histidine kinase with a PAS domain, SMc00888, and the other, SMc00887, is a putative enzyme involved in c-di-GMP [bis-(3′,5′)-cyclic dimeric GMP] metabolism. The secondary signaling molecule, c-di-GMP, has recently been found to regulate complex bacterial developmental processes in diverse bacterial species (12, 66). The intracellular concentration of c-di-GMP is modulated through the enzymatic activity of proteins containing a GGDEF domain, which functions as a diguanylate cyclase, or an EAL domain that functions as a phosphodiesterase. We were therefore intrigued to find that the expression of SMc00887, which encodes a protein containing an N-terminal GGDEF and a C-terminal EAL domain, is decreased fourfold in the cbrA::Tn5 mutant (Fig. 2, Table 2). However, SMc00887 contains an unorthodox GGDEF motif in which the canonical glycine residues are replaced with alanine and glutamate (ADDEF), suggesting that this domain is enzymatically inactive (42). The GGDEF domain of SMc00887 may instead function as a sensory motif, perhaps of GTP levels, that regulates the phosphodiesterase activity of the associated EAL domain. Thus, we predict that decreased expression of SMc00887 may lead to increased c-di-GMP levels in the cbrA::Tn5 mutant as a result of lower cellular phosphodiesterase activity, although this hypothesis remains to be tested directly.
FIG. 2.
CbrA regulates the expression of genes predicted to affect c-di-GMP signaling. β-Glucuronidase activity was measured from stationary phase wild-type and cbrA::Tn5 cultures. The uidA+ transcription fusion present in each strain is indicated below the graph. The SMc00888′-uidA+ fusion was integrated in order to leave the gene intact while the SMc00887′-uidA+ fusion disrupted the native gene.
The start codon of SMc00887 overlaps with the stop codon of the upstream gene SMc00888 (ATGA), suggesting that the two genes are translationally coupled and perhaps functionally related. Similar to SMc00887, the expression of the SMc00888 locus is decreased threefold in the cbrA::Tn5 mutant during stationary phase growth (Fig. 2 and Table 2). SMc00888 encodes an uncharacterized hybrid two-component histidine kinase associated with an N-terminal sensory PAS domain and a C-terminal response regulator domain. Based on a homology search of all previously characterized PAS domains, the SMc00888 PAS domain (amino acid residues 142 to 239) is most similar to that of the S. meliloti oxygen sensor FixL (18% identical) and the Escherichia coli redox-sensitive phosphodiesterase DOS (17% identical), both of which bind a heme ligand (33). Curiously, the CbrA PAS domain (residues 729 to 880) also shares similarity to the FixL (27% identity) and DOS (21% identity) PAS domains. Given the important role that c-di-GMP can play in various pathogenic bacterium-host interactions, we tested the possibility that SMc00887 and SMc00888 could also participate in the regulation of symbiotic development. However, a strain lacking both SMc00888 and SMc00887 was symbiotically competent on both hosts M. sativa and M. truncatula (M. J. Barnett and S. R. Long, data not shown). Therefore, if these regulators do play a role in symbiosis, it is not an essential one, at least under laboratory conditions.
The expression of an uncharacterized two-component histidine kinase with a sensory PAS/PAC domain, SMc02369, is also decreased in the cbrA::Tn5 mutant (Table 2). The SMc02369 PAS motif (residues 125 to 389) contains a repeat similar to that of the Drosophila melanogaster PERIOD circadian clock protein (residues 2 to 297), which is involved in mediating protein-protein interactions (88), with these PAS motifs sharing 12% identity. Interestingly, of those histidine kinases with a known function, SMc02369 most closely resembles that of PleC of Caulobacter crescentus (29% identical, 62% similar). In C. crescentus, pleC mutants have paralyzed flagella and defects in pilus assembly (74, 84). As discussed below, the cbrA::Tn5 mutant also exhibits decreased motility and lowered expression of pilus biosynthesis genes, which may be attributable to altered SMc02369 expression.
CbrA modulates flagellar biosynthesis and cellular motility.
Flagellar motility is a highly regulated bacterial behavior that is responsive to nutritional availability, population density, and a variety of environmental stimuli (72, 77). In S. meliloti, there is a pattern of decreased flagellar motility under growth conditions or in mutants that increases succinoglycan production (3), and this link was also observed with the cbrA::Tn5 mutant. The largest functional clustering of genes with differential expression in the mutant compared to that in the wild type is comprised of those encoding flagellar biosynthesis and chemotaxis functions (56 genes or 35% [Table 1 and see Table S2 in the supplemental material]). The expression of these genes is significantly decreased in the cbrA::Tn5 mutant, ranging from 2- to 10-fold.
The expression of two flagellar biosynthesis regulators, visN and rem (68, 75), appears to be decreased in the cbrA::Tn5 mutant (Table 2). In S. meliloti, flagellar genes are expressed in a temporal hierarchy: class I genes are LuxR-type regulators encoded by visN and visR (75). Expression of all class II (for instance the flagellar assembly gene flaM) and class III (for instance the flagellar filament genes flaA through flaD) genes is strictly visN and visR dependent (75). Our microarray results indicate that the expression of visN, but not visR (data not shown), is decreased twofold in the cbrA::Tn5 mutant. Expression of another regulatory gene in the flagellar cluster, rem, is decreased approximately threefold in the cbrA::Tn5 mutant. Rem is an orphaned OmpR-type response regulator whose expression is visNR dependent and whose function is also required for expression of class II and class III flagellar genes (68). Given that VisN and Rem positively regulate motility and chemotaxis, their down-regulation in the cbrA::Tn5 mutant is consistent with the phenotypic decrease in motility discussed below. Therefore, the motility defect of the cbrA::Tn5 mutant is likely derived from the cumulative effect of multiple regulatory factors and is probably indirect.
We tested our microarray results using uidA+ transcription fusions present at the native gene loci and confirmed that expression of the flagellin gene flaC and the chemotaxis genes mcpU and mcpX is significantly decreased in the cbrA::Tn5 mutant (Fig. 3A and Table 2). To assess the overall impact of CbrA-dependent regulation on flagellar biosynthesis, we isolated flagellar filaments from cultures grown to stationary phase. We analyzed purified flagellar filament protein by SDS-PAGE and quantified the relative amounts of flagellin isolated from wild-type and cbrA::Tn5 cultures. Although we found flagella associated with the cbrA::Tn5 mutant, the abundance was 7- to 10-fold lower than that from the wild type (Fig. 3B). We also assayed motility by measuring bacterial swarming efficiency on LB/MC medium containing 0.22% agar. The swarm diameter formed by the cbrA::Tn5 mutant (2.8 ± 0.1 cm) was significantly decreased relative to that formed by the wild type (4.6 ± 0.1 cm) after 3 days (Fig. 3C). Although flagellar motility is not strictly required for nodulation, mutants defective in flagellar chemotaxis are less competitive at colonizing sites of nodule initiation on the root surface (1). Thus, the cbrA::Tn5 mutant motility defect could account, at least in part, for the inability of the mutant to compete with the wild type for nodule colonization.
FIG. 3.
CbrA regulates flagellar motility and chemotaxis. (a) β-Glucuronidase activity was measured from stationary phase wild-type and cbrA::Tn5 cultures. The uidA+ transcription fusion present in each strain, indicated below the graph, was integrated into the genome in order to leave gene function intact. (b) Flagellar filaments isolated from wild-type and cbrA::Tn5 stationary phase cultures were analyzed by SDS-PAGE and Coomassie protein detection. (c) The swimming ability of wild-type and cbrA::Tn5 bacteria was assessed after 3 days' incubation on LB/MC medium supplemented with 0.22% agar.
Another cell surface structure whose biosynthesis may be regulated by CbrA is the type IV pilus, composed of PilA subunits. Although expression of pilA1 was unaltered, the expression of four genes required for pilus assembly was decreased in the cbrA::Tn5 mutant (Table 2). This cluster of pilus assembly genes closely resembles that of Caulobacter crescentus cpaA-cpaF, which is required for cell cycle-regulated biosynthesis of polar pili (73).
CbrA affects the expression of genes predicted to encode outer membrane and secreted extracellular proteins.
The cbrA::Tn5 mutant exhibits an envelope defect as reflected by its severe sensitivity to membrane-disrupting agents (32), so we looked closely at those differentially expressed genes predicted to encode extracytoplasmic envelope proteins. We were also interested in the regulation of genes encoding outer membrane and secreted extracellular proteins because these factors have the potential to be involved in direct communication with the host during symbiosis.
Based on the PSORT algorithm, we estimate that 28% of the genes differentially expressed between the cbrA::Tn5 mutant and the wild type encode cytoplasmic proteins (Fig. 4b), similar to the proportion of the genome predicted to encode cytoplasmic proteins (Fig. 4a). However, 27% of these differentially expressed cytoplasmic factors are involved in biosynthetic processes that ultimately affect cell surface physiology, such as the exo genes, and another 47% are involved in flagellar motility. We estimate that 6% of differentially expressed genes encode outer membrane proteins and another 6% encode secreted extracellular proteins. In contrast, only 1.2% of the genome is predicted to encode secreted outer membrane and extracellular proteins. Therefore, genes predicted to encode these secreted proteins are 10-fold enriched within our set of differentially expressed genes, indicating that an important function of CbrA is the regulation of bacterial outer-membrane and cell surface physiology.
FIG. 4.
Genes encoding secreted outer-membrane and extracellular proteins are highly enriched within the pool of CbrA-regulated genes. The subcellular localization of each protein was predicted using the PSORT algorithm. (a) The percentage of S. meliloti proteins predicted to be localized to each subcellular compartment or secreted extracellularly. (b) The percentage of genes differentially expressed in the cbrA::Tn5 mutant whose protein products are known or predicted to be localized to each subcellular compartment or secreted extracellularly. A few genes discussed in the text have been highlighted.
Given that little is known regarding the role of OMPs during either stationary phase adaptation or symbiosis, we were interested to find that the expression of several genes encoding known or putative OMPs was altered in the cbrA::Tn5 mutant, including ropB1 and tspO (Table 2). Like the product of ropB1, most of these OMPs belong to the opacity-related subgroup of OmpA-like proteins. Similar to OmpA, these OMPs are predicted to fold into an eight-stranded antiparallel β-barrel. However, sequence alignment based on secondary structure predictions suggests that none of these S. meliloti proteins contains the soluble OmpA periplasmic peptidoglycan-binding domain located at the C terminus, indicating that these OMPs likely impart an alternative function to the cell envelope. In particular, alignment of SMc00638 and SMc00639 with OmpA predicts significantly larger cell-surface-exposed loops in these S. meliloti OMPs (data not shown), suggesting the possibility of an extracellular function.
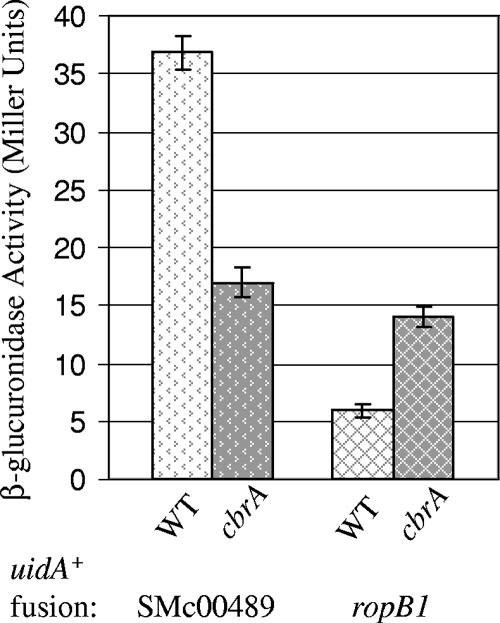
Expression of ropB1 is decreased dramatically in bacteroids compared that in free-living bacteria (4). Results from our microarray and uidA+ transcription fusion analyses indicate that ropB1 is also influenced by CbrA, such that the cbrA::Tn5 mutant exhibits a twofold increase in ropB1 expression compared to that in the wild type (Fig. 5 and Table 2). Although ropB1 is not required for symbiosis in S. meliloti (14), it is possible that unregulated or increased expression of this OMP could have a detrimental impact on symbiotic development. SMc00489 encodes another differentially expressed OmpA family member, and a SMc00489′-uidA+ fusion confirmed that expression is decreased twofold in the cbrA::Tn5 mutant relative to that in the wild type (Fig. 5 and Table 2). The protein encoded by SMc00489 has homology to PagL (69), which functions as a lipid A 3-O-deacylase and modifies the acylation pattern of LPS within the outer membrane (81). Since PagL function contributes to the virulence of Salmonella enterica serovar Typhimurium (40), we tested whether SMc00489 contributes to nodule development and symbiosis. The SMc00489′-uidA+ fusion disrupts gene function, so we tested the nodulation ability of this mutant with legume hosts M. sativa, M. truncatula, and Melilotus officinalis. We observed no obvious symbiotic defect: the SMc00489′-uidA+ mutant elicited the development of pink nitrogen-fixing nodules that support wild-type levels of plant growth, and the mutant was able to compete effectively with SMc00489+ bacteria for nodule occupancy (data not shown).
FIG. 5.
CbrA regulates the expression of genes encoding outer membrane proteins. β-Glucuronidase activity was measured from stationary phase wild-type and cbrA::Tn5 cultures. The uidA+ transcription fusion present in each strain is indicated below the graph. The SMc00489 fusion disrupts gene function while the ropB1 fusion leaves the gene intact.
We identified several genes encoding known or putative secreted extracellular proteins as differentially expressed in the cbrA::Tn5 mutant (Table 2), including the well-characterized succinoglycan modification gene exoK (89) and nex18 (55). The ExoK endoglycanase promotes bacterial invasion of host tissues during infection thread growth by converting high-molecular-weight forms of succinoglycan to the symbiotically active low-molecular-weight forms (58, 89). The nex18 locus encodes a secreted extracellular protein with sequence similarity (37%) to the Mycobacterium tuberculosis protein MPB70, which has a structural similarity to the C-terminal FAS1 domain of the cell adhesion protein fasciclin I and was postulated to mediate physical interaction between invading bacterial cells and the extracellular matrix of the host (16). In S. meliloti, nex18 is likely expressed during nodule invasion because its promoter is sufficient to drive in planta bacA expression for normal symbiosis (55). A nex18 null mutant was subsequently found to play a role in promoting nodule development: nearly half of the plants assayed were stunted for growth and displayed reduced nitrogen fixation, compared to those of the wild-type symbiosis (55). We utilized the nex18′-bacA+-′uidA+ fusion to further examine CbrA-dependent regulation of nex18 and found, despite the low basal β-glucuronidase activity derived from this fusion, that the cbrA::Tn5 mutant consistently expressed twofold more β-glucuronidase activity (2.0 ± 0.4 Miller units) than the wild type (0.9 ± 0.15 Miller units). Thus, CbrA affects the expression of extracellular proteins that contribute to successful host invasion and nodule development. The identification of differentially expressed genes in the cbrA::Tn5 mutant brought to light a few uncharacterized extracellular proteins that may also influence bacterial invasion. We identified SMc03108 and SMb20838 as differentially expressed genes with increased expression in the cbrA::Tn5 mutant (Table 2). These two genes are predicted to encode secreted extracellular proteins of the RTX (repeat in toxin) family that contribute to the pathogenesis of a wide variety of virulent bacteria (20, 41). The Rhizobium leguminosarum nodulation protein NodO, another member of the RTX family, was previously shown to play a positive role in promoting invasion of R. leguminosarum during infection thread growth (31, 85). It will therefore be of interest to test whether SMc03108 and SMb20838 play a similar role during S. meliloti invasion.
DISCUSSION
CbrA is a regulatory factor whose function is critically important for establishing an effective symbiosis between S. meliloti and M. sativa (32). The cbrA gene encodes a putative two-component histidine kinase containing a sensory PAS domain. In our original study, we observed that a Tn5 disruption within cbrA leads to altered stationary phase expression of several exo genes involved in succinoglycan production (32). This result suggested that CbrA could play an important role in regulating stationary phase physiology in S. meliloti, which lacks any gene encoding the RpoS sigma factor critical for the regulation of stationary phase physiology in enteric bacteria (28, 38). In order to gain a more comprehensive understanding of CbrA-dependent regulation, we used microarray analysis to identify additional genes differentially expressed between the cbrA::Tn5 mutant and the wild type during stationary phase growth.
The results presented here further indicate that CbrA affects gene expression primarily during stationary phase growth conditions. For instance, we show that stationary phase induction of bioS expression is primarily dependent on CbrA. Although little is known about the targets of BioS regulation, it was observed that bioS function contributes significantly to the resumption of growth during the exit from the stationary phase (79). CbrA-mediated induction of bioS expression may therefore promote adaptive physiology in cells growing under stationary phase conditions. Global gene expression studies have suggested that S. meliloti growth is relatively rapid while the bacteria are contained within the infection thread but that cellular growth slows considerably once bacteria are located within the host cell cytoplasm (15). Consistent with this hypothesis is the observation that the stringent response regulator RelA is required for symbiosis (86). It has also been suggested that stationary phase physiology contributes to successful adaptation to the intracellular host environment in the closely related Brucella spp. (29, 65, 67), which also contain a CbrA ortholog and lack a candidate rpoS gene. One outstanding issue for future study is the precise nature of the growth phase signal that modulates CbrA activity, as this stimulus may also contribute to the developmental progression of the S. meliloti symbiont during nodule invasion and differentiation.
In addition to bioS, there are seven regulatory factors whose expression is responsive to CbrA activity. These include the SMc00887 enzyme, predicted to affect levels of the secondary signaling molecule c-di-GMP, the two-component histidine kinase genes SMc00888 and SMc02369, and cspA5 and sinI (Table 2). Disruptions in several of the regulatory genes identified in this microarray screen, including bioS (79), sinI (46, 47), SMc00887, and SMc00888, have been tested for symbiosis and found to result in no developmental defect. This is presumably also true of the flagellar regulators visN and rem, since flagella are not strictly required for symbiosis (1). The cbrA::Tn5 mutant phenotype may therefore result from the misexpression of genes that are direct targets of the CbrA two-component pathway. Alternatively, the cbrA::Tn5 symbiosis defect may derive from the combined effect of this mutation on several downstream regulatory pathways that control redundant, or overlapping, functions. Detailed studies with each of these regulators will be required to uncover the organizational structure of this network and to determine whether individual genes differentially expressed in the cbrA::Tn5 mutant are a direct or indirect target of the CbrA two-component pathway. This regulatory circuit is further complicated in that it contains three orphan histidine kinases, CbrA, SMc00888, and SMc02369. Curiously, each of these histidine kinases contains a sensory PAS domain that may monitor cellular redox status, suggesting that redox changes could play an important role in determining the physiological output of this particular regulatory circuit.
During early infection, bacteria are exposed to plant-derived reactive oxygen species but later find themselves in a microaerobic environment that is required for nitrogen fixation. Thus, the environment of the infection thread is quite different from that of the host cell cytoplasm, and we were intrigued by the possibility that CbrA may function as a developmental redox sensor able to distinguish between these two niches. Taken together, our results are consistent with the model in which cbrA::Tn5 bacteria are locked into a physiological state that is specialized for early infection thread invasion but are subsequently unable to properly differentiate within the host cell cytoplasm. For instance, the mutant has increased expression of the genes exoH, exoK, and ndvA, which play key roles in modifying or transporting the succinoglycan and cyclic β-(1,2)-glucan required for infection thread invasion. There may also be an increase in expression of sinI, which regulates ExpR-dependent EPS II production (46). It will therefore be interesting to test whether the genes encoding Nex18 and the NodO-like RTX factors SMc03108 and SMb20838, which also exhibit increased expressed in the cbrA::Tn5 mutant, specifically contribute to infection thread growth.
Comparatively little is known about the requirements for bacterial adaptation and differentiation after cells are taken up into the host cell cytoplasm. However, cbrA::Tn5 mutant bacteria appear to be unable to induce proper nitrogenase gene expression within the nodule, based on the level of β-glucuronidase activity expressed from a nifH′-uidA+ fusion (K. E. Gibson and G. C. Walker, unpublished data), indicating that bacteroid differentiation is somehow perturbed. We find that CbrA affects the expression of several genes predicted to encode secreted outer membrane proteins. In fact, these genes are enriched within the set of differentially expressed genes identified in our microarray analysis. We verified CbrA-dependent regulation of both ropB1 and SMc00489, whose products closely resemble E. coli OmpA, with the exception that they lack the periplasmic peptidoglycan-binding domain. Although neither ropB1 nor SMc00489 is required for symbiosis, it is possible their proteins play a role in free-living stationary phase physiology. It will be of interest to determine whether other CbrA-regulated OmpA homologs play a role in either stationary phase physiology or nodule development. For instance, the OMPs encoded by SMc00638 and SMc00639 share a high degree of similarity (65% and 61%, respectively) to the Opa22 protein of Mesorhizobium huakuii, which is absolutely required for nodule formation (17).
Interestingly, the cbrA::Tn5 mutant shares several phenotypic characteristics with the exoS96::Tn5 mutant, which is altered in the expression and activity of the ExoS two-component histidine kinase (19): both are succinoglycan overproducers with motility defects (32, 87). In fact, a variety of growth conditions lead to increased succinoglycan production and a concomitant decrease in flagellar motility, suggesting that the regulation of these phenotypes is closely linked (3). Perhaps the tight regulatory link between succinoglycan production and flagellar motility is required to coordinate a complex developmental program such as biofilm formation in the soil or rhizobial infection thread invasion of its host. Regardless, the cbrA::Tn5 mutant has a severe sensitivity to membrane-disrupting agents that is not observed with the exoS96::Tn5 mutant (K. E. Gibson and G. C. Walker, unpublished observations). Similarly, the cbrA::Tn5 mutant has a dramatic nodule development defect while the exoS::Tn5 mutant elicits formation of pink, nitrogen-fixing nodules (22, 32). Therefore, the apparent envelope defect of the cbrA::Tn5 mutant may contribute significantly to the nodule development defect of the mutant. We will continue to dissect the roles CbrA-regulated OMPs play in both free-living stationary phase physiology and symbiotic development, individually and in combination, as a means of gaining a better understanding of the function of the cell envelope during various stages of symbiotic development.
Supplementary Material
Acknowledgments
We thank Valerie Oke (University of Pittsburg) for providing us with the nex18-′bacA+-uidA+ fusion used in our studies. We are indebted to Steve Bell (Massachusetts Institute of Technology) for allowing us the use of a Beckman Optima TL ultracentrifuge and Typhoon 9400 imager, which were critical for our flagella analysis. The authors thank Hajime Kobayashi, Kathryn Jones, and Melanie Barker Berkman for insightful discussions about the microarray results; and Robert Fisher and Hajime Kobayashi for critical reading of the manuscript.
This work was supported by National Institutes of Health grant GM31010 (to G.C.W.), a National Science Foundation postdoctoral fellowship (to K.E.G.), and the Hoover Circle Fund (to S.R.L.).
G.C.W. is an American Cancer Society research professor and a Howard Hughes Medical Institute professor.
Footnotes
Published ahead of print on 19 January 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ames, P., and K. Bergman. 1981. Competitive advantage provided by bacterial motility in formation of nodules by Rhizobium meliloti. J. Bacteriol. 148:728-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1991. Current protocols in molecular biology, vol. 1. John Wiley & Sons Inc., Hoboken, NJ.
- 3.Barnett, M. J., and R. F. Fisher. 2006. Global gene expression in the rhizobial-legume symbiosis. Symbiosis 42:1-24. [Google Scholar]
- 4.Barnett, M. J., C. J. Toman, R. F. Fisher, and S. R. Long. 2004. A dual-genome Symbiosis Chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc. Natl. Acad. Sci. USA 101:16636-16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batut, J., S. G. Andersson, and D. O'Callaghan. 2004. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2:933-945. [DOI] [PubMed] [Google Scholar]
- 6.Baudouin, E., L. Pieuchot, G. Engler, N. Pauly, and A. Puppo. 2006. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interact. 19:970-975. [DOI] [PubMed] [Google Scholar]
- 7.Becker, A., A. Kleickmann, W. Arnold, and A. Puhler. 1993. Analysis of the Rhizobium meliloti exoH/exoK/exoL fragment: ExoK shows homology to excreted endo-beta-1,3-1,4-glucanases and ExoH resembles membrane proteins. Mol. Gen. Genet. 238:145-154. [DOI] [PubMed] [Google Scholar]
- 8.Becker, A., A. Kleickmann, M. Keller, W. Arnold, and A. Puhler. 1993. Identification and analysis of the Rhizobium meliloti exoAMONP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol. Gen. Genet. 241:367-379. [DOI] [PubMed] [Google Scholar]
- 9.Blumwald, E., M. G. Fortin, P. A. Rea, D. P. Verma, and R. J. Poole. 1985. Presence of host-plasma membrane type H-ATPase in the membrane envelope enclosing the bacteroids in soybean root nodules. Plant Physiol. 78:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boncompagni, E., L. Dupont, T. Mignot, M. Osteras, A. Lambert, M. C. Poggi, and D. Le Rudulier. 2000. Characterization of a Sinorhizobium meliloti ATP-binding cassette histidine transporter also involved in betaine and proline uptake. J. Bacteriol. 182:3717-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7:191-226. [DOI] [PubMed] [Google Scholar]
- 12.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 311:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell, G. R., B. L. Reuhs, and G. C. Walker. 2002. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl. Acad. Sci. USA 99:3938-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell, G. R., L. A. Sharypova, H. Scheidle, K. M. Jones, K. Niehaus, A. Becker, and G. C. Walker. 2003. Striking complexity of lipopolysaccharide defects in a collection of Sinorhizobium meliloti mutants. J. Bacteriol. 185:3853-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capela, D., C. Filipe, C. Bobik, J. Batut, and C. Bruand. 2006. Sinorhizobium meliloti differentiation during symbiosis with alfalfa: a transcriptomic dissection. Mol. Plant-Microbe Interact. 19:363-372. [DOI] [PubMed] [Google Scholar]
- 16.Carr, M. D., M. J. Bloemink, E. Dentten, A. O. Whelan, S. V. Gordon, G. Kelly, T. A. Frenkiel, R. G. Hewinson, and R. A. Williamson. 2003. Solution structure of the Mycobacterium tuberculosis complex protein MPB70: from tuberculosis pathogenesis to inherited human corneal disease. J. Biol. Chem. 278:43736-43743. [DOI] [PubMed] [Google Scholar]
- 17.Cheng, G. J., Y. G. Li, and J. C. Zhou. 2006. Cloning and identification of opa22, a new gene involved in nodule formation by Mesorhizobium huakuii. FEMS Microbiol. Lett. 257:152-157. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coote, J. G. 1992. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol. Rev. 8:137-161. [DOI] [PubMed] [Google Scholar]
- 21.Davey, M. E., and F. J. de Bruijn. 2000. A homologue of the tryptophan-rich sensory protein TspO and FixL regulate a novel nutrient deprivation-induced Sinorhizobium meliloti locus. Appl. Environ. Microbiol. 66:5353-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty, D., J. A. Leigh, J. Glazebrook, and G. C. Walker. 1988. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J. Bacteriol. 170:4249-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dylan, T., P. Nagpal, D. R. Helinski, and G. S. Ditta. 1990. Symbiotic pseudorevertants of Rhizobium meliloti ndv mutants. J. Bacteriol. 172:1409-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson, G. P., A. Datta, R. W. Carlson, and G. C. Walker. 2005. Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol. Microbiol. 56:68-80. [DOI] [PubMed] [Google Scholar]
- 25.Finan, T. M., E. Hartweig, K. LeMieux, K. Bergman, G. C. Walker, and E. R. Signer. 1984. General transduction in Rhizobium meliloti. J. Bacteriol. 159:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraysse, N., F. Couderc, and V. Poinsot. 2003. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 270:1365-1380. [DOI] [PubMed] [Google Scholar]
- 27.Gage, D. J. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68:280-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 29.Gee, J. M., M. W. Valderas, M. E. Kovach, V. K. Grippe, G. T. Robertson, W. L. Ng, J. M. Richardson, M. E. Winkler, and R. M. Roop II. 2005. The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect. Immun. 73:2873-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geurts, R., E. Fedorova, and T. Bisseling. 2005. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr. Opin. Plant Biol. 8:346-352. [DOI] [PubMed] [Google Scholar]
- 31.Geurts, R., R. Heidstra, A. E. Hadri, J. A. Downie, H. Franssen, A. Van Kammen, and T. Bisseling. 1997. Sym2 of pea is involved in a nodulation factor-perception mechanism that controls the infection process in the epidermis. Plant Physiol. 115:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson, K. E., G. R. Campbell, J. Lloret, and G. C. Walker. 2006. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J. Bacteriol. 188:4508-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilles-Gonzalez, M. A., and G. Gonzalez. 2005. Heme-based sensors: defining characteristics, recent developments, and regulatory hypotheses. J. Inorg. Biochem. 99:1-22. [DOI] [PubMed] [Google Scholar]
- 34.Glazebrook, J., A. Ichige, and G. C. Walker. 1993. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 7:1485-1497. [DOI] [PubMed] [Google Scholar]
- 35.Glazebrook, J., J. W. Reed, T. L. Reuber, and G. C. Walker. 1990. Genetic analyses of Rhizobium meliloti exopolysaccharides. Int. J. Biol. Macromol. 12:67-70. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez, J. E., G. M. York, and G. C. Walker. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141-146. [DOI] [PubMed] [Google Scholar]
- 37.Heinz, E. B., D. A. Phillips, and W. R. Streit. 1999. BioS, a biotin-induced, stationary-phase, and possible LysR-type regulator in Sinorhizobium meliloti. Mol. Plant-Microbe Interact. 12:803-812. [DOI] [PubMed] [Google Scholar]
- 38.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferson, R. A., S. M. Burgess, and D. Hirsch. 1986. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc. Natl. Acad. Sci. USA 83:8447-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J. Biol. Chem. 279:20044-20048. [DOI] [PubMed] [Google Scholar]
- 41.Kerr, J. R. 1999. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol. Pathol. 52:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirillina, O., J. D. Fetherston, A. G. Bobrov, J. Abney, and R. D. Perry. 2004. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol. Microbiol. 54:75-88. [DOI] [PubMed] [Google Scholar]
- 43.Lang, E. A., and M. V. Marques. 2004. Identification and transcriptional control of Caulobacter crescentus genes encoding proteins containing a cold shock domain. J. Bacteriol. 186:5603-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long, S. R. 2001. Genes and signals in the rhizobium-legume symbiosis. Plant Physiol. 125:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marketon, M. M., S. A. Glenn, A. Eberhard, and J. E. Gonzalez. 2003. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 185:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marketon, M. M., M. R. Gronquist, A. Eberhard, and J. E. González. 2002. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J. Bacteriol. 184:5686-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier, V. M., P. Muschler, and B. Scharf. 2007. Functional analysis of nine putative chemoreceptor proteins in Sinorhizobium meliloti. J. Bacteriol. 189:1816-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metcalf, W. W., and B. L. Wanner. 1993. Construction of new beta-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene 129:17-25. [DOI] [PubMed] [Google Scholar]
- 50.Miyake, K. 2004. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 51.Muller, P., M. Keller, W. M. Weng, J. Quandt, W. Arnold, and A. Puhler. 1993. Genetic analysis of the Rhizobium meliloti exoYFQ operon: ExoY is homologous to sugar transferases and ExoQ represents a transmembrane protein. Mol. Plant-Microbe Interact. 6:55-65. [DOI] [PubMed] [Google Scholar]
- 52.Nagpal, P., S. P. Khanuja, and S. W. Stanfield. 1992. Suppression of the ndv mutant phenotype of Rhizobium meliloti by cloned exo genes. Mol. Microbiol. 6:479-488. [DOI] [PubMed] [Google Scholar]
- 53.Niemann, H. H., W. D. Schubert, and D. W. Heinz. 2004. Adhesins and invasins of pathogenic bacteria: a structural view. Microbes Infect. 6:101-112. [DOI] [PubMed] [Google Scholar]
- 54.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oke, V., and S. R. Long. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 32:837-849. [DOI] [PubMed] [Google Scholar]
- 56.Oke, V., and S. R. Long. 1999. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 2:641-646. [DOI] [PubMed] [Google Scholar]
- 57.Pautsch, A., and G. E. Schulz. 2000. High-resolution structure of the OmpA membrane domain. J. Mol. Biol. 298:273-282. [DOI] [PubMed] [Google Scholar]
- 58.Pellock, B. J., H. P. Cheng, and G. C. Walker. 2000. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 182:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pellock, B. J., M. Teplitski, R. P. Boinay, W. D. Bauer, and G. C. Walker. 2002. A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J. Bacteriol. 184:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pizarro-Cerda, J., and P. Cossart. 2006. Bacterial adhesion and entry into host cells. Cell 124:715-727. [DOI] [PubMed] [Google Scholar]
- 61.Ponting, C. P., and L. Aravind. 1997. PAS: a multifunctional domain family comes to light. Curr. Biol. 7:R674-R677. [DOI] [PubMed] [Google Scholar]
- 62.Prell, J., and P. Poole. 2006. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14:161-168. [DOI] [PubMed] [Google Scholar]
- 63.Ramu, S. K., H. M. Peng, and D. R. Cook. 2002. Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol. Plant-Microbe Interact. 15:522-528. [DOI] [PubMed] [Google Scholar]
- 64.Reuber, T. L., and G. C. Walker. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269-280. [DOI] [PubMed] [Google Scholar]
- 65.Robertson, G. T., and R. M. Roop, Jr. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690-700. [DOI] [PubMed] [Google Scholar]
- 66.Romling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signaling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 67.Roop, R. M., II, J. M. Gee, G. T. Robertson, J. M. Richardson, W. L. Ng, and M. E. Winkler. 2003. Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 57:57-76. [DOI] [PubMed] [Google Scholar]
- 68.Rotter, C., S. Muhlbacher, D. Salamon, R. Schmitt, and B. Sharf. 2006. Rem, a new transcriptional activator of motility and chemotaxis in Sinorhizobium meliloti. J. Bacteriol. 188:6932-6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutten, L., J. Geurtsen, W. Lambert, J. J. Smolenaers, A. M. Bonvin, A. de Haan, P. van der Ley, M. R. Egmond, P. Gros, and J. Tommassen. 2006. Crystal structure and catalytic mechanism of the LPS 3-O-deacylase PagL from Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 103:7071-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos, R., D. Herouart, S. Sigaud, D. Touati, and A. Puppo. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interact. 14:86-89. [DOI] [PubMed] [Google Scholar]
- 71.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 72.Scharf, B., and R. Schmitt. 2002. Sensory transduction to the flagellar motor of Sinorhizobium meliloti. J. Mol. Microbiol. Biotechnol. 4:183-186. [PubMed] [Google Scholar]
- 73.Skerker, J. M., and L. Shapiro. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 19:3223-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sommer, J. M., and A. Newton. 1989. Turning off flagellum rotation requires the pleiotropic gene pleD: pleA, pleC, and pleD define two morphogenic pathways in Caulobacter crescentus. J. Bacteriol. 171:392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sourjik, V., P. Muschler, B. Scharf, and R. Schmitt. 2000. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J. Bacteriol. 182:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sourjik, V., W. Sterr, J. Platzer, I. Bos, M. Haslbeck, and R. Schmitt. 1998. Mapping of 41 chemotaxis, flagellar and motility genes to a single region of the Sinorhizobium meliloti chromosome. Gene 223:283-290. [DOI] [PubMed] [Google Scholar]
- 77.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27:505-523. [DOI] [PubMed] [Google Scholar]
- 78.Stanfield, S. W., L. Ielpi, D. O'Brochta, D. R. Helinski, and G. S. Ditta. 1988. The ndvA gene product of Rhizobium meliloti is required for β-(1→2)glucan production and has homology to the ATP-binding export protein HlyB. J. Bacteriol. 170:3523-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Streit, W. R., and D. A. Phillips. 1997. A biotin-regulated locus, bioS, in a possible survival operon of Rhizobium meliloti. Mol. Plant-Microbe Interact. 10:933-937. [DOI] [PubMed] [Google Scholar]
- 80.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trent, M. S., W. Pabich, C. R. Raetz, and S. I. Miller. 2001. A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083-9092. [DOI] [PubMed] [Google Scholar]
- 82.Ugalde, R. A. 1999. Intracellular lifestyle of Brucella spp: common genes with other animal pathogens, plant pathogens, and endosymbionts. Microbes Infect. 1:1211-1219. [DOI] [PubMed] [Google Scholar]
- 83.Vance, C. P. 2001. Symbiotic nitrogen fixation and phosphorus acquisition: plant nutrition in a world of declining renewable resources. Plant Physiol. 127:390-397. [PMC free article] [PubMed] [Google Scholar]
- 84.Viollier, P. H., N. Sternheim, and L. Shapiro. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J. 21:4420-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker, S. A., and J. A. Downie. 2000. Entry of Rhizobium leguminosarum bv. viciae into root hairs requires minimal Nod factor specificity, but subsequent infection thread growth requires nodO or nodE. Mol. Plant-Microbe Interact. 13:754-762. [DOI] [PubMed] [Google Scholar]
- 86.Wells, D. H., and S. R. Long. 2002. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 43:1115-1127. [DOI] [PubMed] [Google Scholar]
- 87.Yao, S. Y., L. Luo, K. J. Har, A. Becker, S. Ruberg, G. Q. Yu, J. B. Zhu, and H. P. Cheng. 2004. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 186:6042-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yildiz, O., M. Doi, I. Yujnovsky, L. Cardone, A. Berndt, S. Hennig, S. Schulze, C. Urbanke, P. Sassone-Corsi, and E. Wolf. 2005. Crystal structure and interactions of the PAS repeat region of the Drosophila clock protein PERIOD. Mol. Cell 17:69-82. [DOI] [PubMed] [Google Scholar]
- 89.York, G. M., and G. C. Walker. 1997. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol. Microbiol. 25:117-134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.