Abstract
Bacillus subtilis undergoes a highly distinctive division during spore formation. It yields two unequal cells, the mother cell and the prespore, and septum formation is completed before the origin-distal 70% of the chromosome has entered the smaller prespore. The mother cell subsequently engulfs the prespore. Two different probes were used to study the behavior of the terminus (ter) region of the chromosome during spore formation. Only one ter region was observed at the time of sporulation division. A second ter region, indicative of chromosome separation, was not distinguishable until engulfment was nearing completion, when one was in the mother cell and the other in the prespore. Separation of the two ter regions depended on the DNA translocase SpoIIIE. It is concluded that SpoIIIE is required during spore formation for chromosome separation as well as for translocation; SpoIIIE is not required for separation during vegetative growth.
Formation of spores by Bacillus subtilis is a primitive differentiation process that is triggered by nutrient depletion (11, 29, 31). A critical early stage is an asymmetrically located cell division. The division during spore formation shares much of its machinery with vegetative division (12). However, it differs in several respects (17), of which three are important to the present study. First, it is asymmetrically located, resulting in two cell types, the larger mother cell and the smaller prespore (also called forespore), whose subsequent fates are very different. Second, these cells do not separate following division, but rather the mother cell engulfs the prespore; the sporulation division septum is much thinner than the vegetative septum, and its flexibility following autolysis is critical to engulfment. Third, septation is completed, in the sense of effective compartmentalization between the mother cell and the prespore, before a chromosome has been completely partitioned into the prespore. At the time of septum formation, only the origin-proximal 30% of the chromosome destined for the prespore is actually present in the prespore (47, 49). As a consequence, there is genetic asymmetry between the prespore and the mother cell when they are first formed. Indeed, the position of a gene on the chromosome (origin-distal or origin-proximal) can dramatically affect its expression and thus the efficiency of spore formation (10, 14, 21, 50).
The origin-distal 70% of the chromosome is translocated into the prespore by the SpoIIIE protein, which functions as a DNA translocase (3, 47). The SpoIIIE protein localizes to the middle of the sporulation division septum (38, 48). It forms an effective seal around the DNA during translocation, preventing the diffusion of proteins between the prespore and mother cell (16, 48). During engulfment, it migrates with the engulfing membrane to the cell pole, where it is required for completion of engulfment (38).
The time of translocation of the chromosome into the prespore has been estimated by two methods. The first used DAPI (4′,6′-diamidino-2-phenylindole) staining of nucleoids and suggested 10 to 15 min (32). The second used time of expression of a σF-directed gene fusion located at different positions and suggested about 20 min (21). Thus, both estimates were consistent with a fairly transient genetic asymmetry. The measurements suggested that separation of the two chromosomes might be completed before the start of engulfment, which was also estimated to initiate about 15 min after completion of septum formation (28). However, neither estimate of the translocation time directly addressed the possibility that the chromosome terminus (ter) region might behave differently: separation of the ter regions and movement of a ter region into the prespore might be delayed compared to movement of the rest of the chromosome. Given the importance that has been attached to transient genetic asymmetry (10) and to the events associated with completion of chromosome separation (8, 27), we have examined the behavior of the ter region in more detail.
Bacteria with circular genomes present the problem that during replication the two copies of the genome become intertwined and can be covalently joined as a result of homologous recombination (25). The separation of newly replicated genomes (chromosomes) has been most extensively studied with Escherichia coli. It requires the action of topoisomerase; if the two copies are covalently joined to form dimers, it also requires the action of the XerC/XerD recombinase acting at a specific site, dif, located near the chromosome terminus (13). Chromosome separation and partitioning are coordinated with the closure of the division septum by the C-terminal portion of the protein FtsK (4, 41). Coordination of chromosome segregation with the closure of the division septum is a striking feature of cell division in E. coli.
The process of division during vegetative growth of B. subtilis is very similar to that of E. coli (12). In particular, there is thought to be the same coordination between chromosome partitioning and septation. Similarities are seen in a requirement for topoisomerase (18) and in a recombinase homologous to XerC/XerD acting at a specific site, dif (36, 37). However, no proteins have been shown to have a role comparable to that of FtsK in coordinating chromosome separation with completion of septation during vegetative division. Two B. subtilis proteins have about 50% identity to the C-terminal portion of FtsK, namely, SpoIIIE and YtpT, but insertional inactivation of their structural genes singly or in combination has little if any effect on chromosome separation during vegetative growth (24, 36, 40). In contrast to the situation during vegetative growth, we report here that SpoIIIE is essential for chromosome separation during spore formation.
We monitored the movement of the chromosome ter region during sporulation using tandem repeats of the lac operator, lacO, inserted near the chromosome terminus and a plasmid encoding either a green fluorescent protein (GFP)-LacI or a LacI-cyan fluorescent protein (CFP) translational fusion. The binding of the fused protein to lacO marks the location of the terminus. Our results indicate that two termini are not separated until engulfment is nearing completion and that a ter region is not present in the prespore until that time. We obtained similar results using a replication termination protein (RTP)-yellow fluorescent protein (YFP) fusion (24), which binds near to the replication terminus. The separation of two ter regions was absolutely dependent on SpoIIIE.
MATERIALS AND METHODS
Media.
B. subtilis was grown in modified Ramaley and Burden (34) minimal medium (MRB) (7), in modified Schaeffer's sporulation medium (MSSM), or on Schaeffer's sporulation agar (30, 35). When required, the medium contained 5 μg or 20 μg chloramphenicol/ml, 1.5 μg erythromycin/ml, or 100 μg spectinomycin/ml. E. coli was grown on Luria-Bertani lysogeny broth agar, containing 100 μg ampicillin/ml when required.
Spore formation.
Bacteria were inoculated into MRB or MSSM from isolated colonies of the appropriate strain. Cultures were incubated at 30°C (MRB) or 37°C (MSSM) in conical flasks such that the culture occupied no more than 12.5% of the flask volume. Flasks were shaken on an orbital shaker at 120 rpm. Growth was monitored by absorbance at 600 nm; the absorbance was converted to bacterial dry weight by means of a standard calibration curve. The end of exponential growth was defined as the start of spore formation.
Strains.
B. subtilis strains used in this study are listed in Table 1. B. subtilis 168 strain BR151 (trpC2 metB10 lys-3) was the parent of the SL strains. Strains DCL693, KPL475, MMB348, and MMB357 were kindly provided by Alan Grossman (Massachusetts Institute of Technology). Strains DCL693 and KPL475 contain thrC::Ppen-gfpmut2′-′lacIΔ11 (deletion of the LacI oligomerization domain; gene product referred to as GFP-LacI) mls; KPL475 has pAT14 integrated by single crossover at cgeD, and DCL693 has pAT15 integrated by single crossover at spoOJ (both plasmids harbor tandem repeats of lacO); cgeD is located near the terminus (181°) and spoOJ near the origin (359°) of the B. subtilis chromosome (44, 46). Strains MMB357 and MMB348 contain lacIΔ11′-′cfpW7 (gene product referred to as LacI-CFP) and rtp′-′yfpmut2 (RTP-YFP) translational fusions, respectively (24); lacIΔ11′-′cfpW7 is under the control of the Ppen promoter, and rtp′-′yfpmut2 is under the control of the rtp promoter. DNA from a derivative of MMB357 was used to introduce the lacIΔ11′-′cfpW7 fusion into a BR151 derivative containing cgeD::pAT14 (from KPL475) to yield SL13004. DNA from MMB348 was used to introduce the rtp′-′yfpmut2 fusion into BR151 to yield SL12976. DNA from a derivative of MMB357 was used to introduce the lacIΔ11′-′cfpW7 fusion into a BR151 derivative containing cgeD::pAT15 (from DCL693) to yield SL13209. Strain SL12931, dnaX′-′yfpmut2 (23) was a derivative of BR151 transformed with pKL183, obtained from Alan Grossman. The spoIIIE::spc cassette in strain SL13271 was derived from strain PL412 (33), kindly provided by Petra Anne Levin (Washington University, St. Louis, Mo.). Details of strain construction are available on request. The spoIIIG::neo construct was described previously (5). The spoIIR::neo construct was kindly provided by Vasant Chary.
TABLE 1.
B. subtilis strains used
| Strain | Relevant genotype | Source |
|---|---|---|
| BR151 | trpC2 metB10 lys-3 | Lab stock |
| KPL475 | cgeD (181°)::pAT14 (lacO cassette cat) thrC::(Ppen-gfpmut2′-′lacIΔ11 mls) | Alan Grossman |
| DCL693 | spoOJ (359°)::pAT15(lacO cassette cat) thrC::(Ppen-gfpmut2′-′lacIΔ11 mls) | Alan Grossman |
| MMB348 | rtp::pGK95 (rtp′-′yfpmut2 cat) | Alan Grossman |
| MMB357 | thrC::(Ppen-lacIΔ11′-′cfpW7 mls) | Alan Grossman |
| SL12931 | trpC2 metB10 lys-3 dnaX::pKL183 (dnaX′-′yfpmut2 spc) | This study |
| SL12976 | trpC2 metB10 lys-3 rtp′-′yfpmut2 cat | This study |
| SL13004 | trpC2 metB10 lys-3 cgeD (181°)::pAT14 (lacO cassette cat) thrC::(Ppen-lacIΔ11′-′cfpW7 mls) | This study |
| SL13209 | trpC2 metB10 lys-3 spoOJ (359°)::pAT15 (lacO cassette cat) thrC::(Ppen-lacIΔ11′-′cfpW7 mls) | This study |
| SL13271 | trpC2 metB10 lys-3 cgeD (181°)::pAT14 (lacO cassette cat) thrC::(Ppen-lacIΔ11′-′cfpW7 mls) spoIIIE::spc | This study |
| SL13272 | trpC2 metB10 lys-3 cgeD (181°)::pAT14 (lacO cassette cat) thrC::(Ppen-lacIΔ11′-′cfpW7 mls) spoIIR::neo | This study |
| SL13346 | trpC2 metB10 lys-3 cgeD (181°)::pAT14 (lacO cassette cat) thrC::(Ppen-lacIΔ11′-′cfpW7 mls) spoIIIG::neo | This study |
| SL13933 | trpC2 metB10 lys-3 dnaX::pKL183 (dnaX′-′yfpmut2) spoIIIE::cat | This study |
E. coli DH5α (Gibco-BRL) was used to maintain plasmids.
Fluorescence microscopy.
Cultures were grown in MRB at 30°C or MSSM at 37°C. A 200-μl volume of culture was mixed with 0.2 μl of 1 mg FM4-64 (Molecular Probes)/ml phosphate-buffered saline (Gibco-BRL). Samples were incubated at room temperature for 5 min, and 1 μl of unfixed sample was transferred to a microscope slide coated with 1.2% agarose. Images were captured using a DM IRE2 microscope with a TCS SL confocal system (Leica), using a 100× oil immersion objective and Leica imaging software. GFP emission was captured between 500 and 550 nm and FM4-64 emission between 600 and 730 nm; excitation for both fluorophores was at 488 nm. CFP emission was captured between 465 and 500 nm, with excitation at 458 nm. YFP emission was captured between 505 and 550 nm, with excitation at 488 nm. Fluorographs were generated from a single stack in the Z plane, with four-point line averaging. The image format was 512 by 512 pixels, and the scan speed was 400 image-lines/s. Cell length, the position of the prespore membrane, and the positions of fluorescent ter foci were measured using the ImageJ 1.36b program (1; http://rsb.info.nih.gov/ij/).
Assessment of the stages of engulfment.
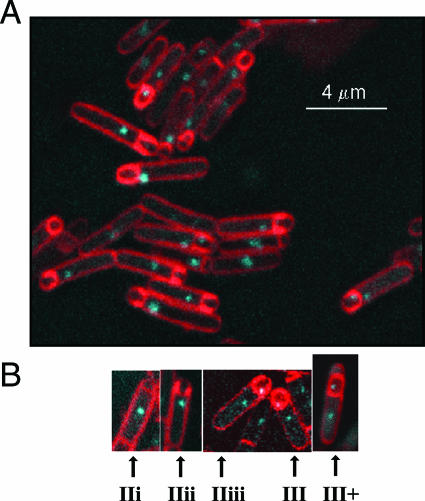
The names of morphological intermediates in the process of engulfment are essentially those suggested by Illing and Errington (19). The division septum is straight when first formed (stage IIi). The septum then loses its rigidity and bulges into the mother cell (stage IIii). After that, the points of attachment of the septal membrane to the peripheral cell membrane move towards the pole of the cell (stage IIiii). Engulfment is completed by membrane fusion at the cell pole, resulting in a detached prespore entirely within the mother cell (stage III). The stages were visualized using the membrane stain FM4-64 (38) (Fig. 1). Only organisms presenting complete longitudinal sections were scored. With the strains and conditions we used, FM4-64 efficiently stained completely engulfed prespores (unpublished observations), although it does not do so under other conditions (38). Organisms in which a presumptively completely engulfed prespore had clearly become detached from the cell pole of the mother cell had presumably reached stage III and might have progressed further in spore formation; they are designated III+. The membranes separating the prespore from the mother cell typically stained more strongly than the peripheral membrane for organisms at stages IIi to IIiii; however, by stage III+ the staining intensity was usually comparable to or weaker than that of the peripheral membrane, although it occasionally remained stronger.
FIG. 1.
Stages of engulfment. Strain SL13004 was induced to form spores in MRB at 30°C. Bacterial membranes were stained with FM4-64 (red). The positions of chromosome terminus (ter) regions were indicated by the binding to lacO, located at 181° on the chromosome, of LacI-CFP (cyan). (A) Mixed population of organisms at different stages of engulfment; (B) examples of organisms illustrating each of the stages (19). Stage IIi, organism with a newly formed, straight division septum. The septum then loses its rigidity and bulges into the mother cell (stage IIii). After that, the points of attachment of the septal membrane to the peripheral cell membrane move towards the pole of the cell (stage IIiii). Engulfment is completed by membrane fusion at the cell pole, resulting in a detached prespore entirely within the mother cell (stage III). In stage III+, a presumptively completely engulfed prespore has moved from the cell pole; the organism is presumed to have reached stage III and perhaps progressed further.
RESULTS
A single chromosome terminus region is detected when the sporulation septum is first formed.
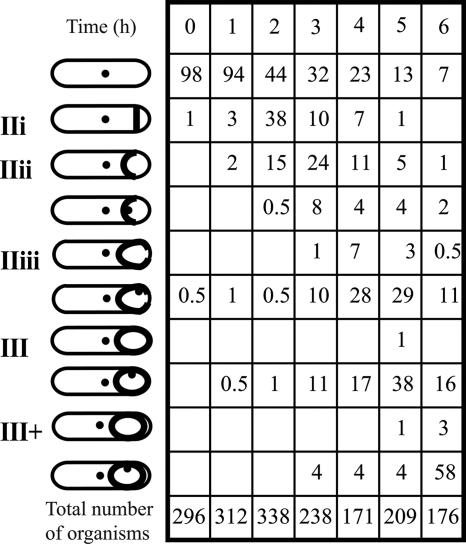
The positions of the origin and terminus regions (referred to as ori and ter, respectively) of the B. subtilis chromosome were identified by CFP fluorescence using strains containing the lac operator of E. coli inserted near the origin, at spo0J, or the terminus, at cgeD, and containing a plasmid encoding a LacI-CFP translational fusion (24). Bacteria were induced to form spores in MRB at 30°C. Of several systems tested, this one gave the clearest CFP signals. In this medium, growth stops and bacteria start to form spores when glucose becomes exhausted; no centrifugation, which might disrupt nucleoid architecture, is involved. The LacI-CFP/lacO system did not affect growth rate or spore formation. The doubling time during exponential growth in MRB at 30°C was about 120 min, which is thought to be longer than the time for chromosome replication, so that overlapping cycles of DNA replication (dichotomous replication) would not complicate analysis. Consistent with this interpretation, no exponentially growing bacteria that contained more than two ori regions were observed (unpublished observations). With strain SL13004, only 6% of organisms had undergone the sporulation division 1 h after the end of exponential growth. By hour 2, 38% of organisms were at stage IIi, and 17% had reached stage IIii or beyond (Fig. 2). One hour later, the proportion at stage IIi had declined to 10%, and 58% of organisms had reached stage IIii or beyond.
FIG. 2.
Time course of engulfment and of ter region separation. The spo+ strain SL13004 was incubated in MRB at 30°C. The strain contained a LacI-CFP fusion and copies of lacO inserted at cgeD, located at 181° on the chromosome. Schematic representations of the stages of engulfment are indicated, as are the location of ter regions. The ter regions detected in the prespore were generally adjacent to the membrane; their position was variable, and the most common position at each stage is represented. The time shown is the time after the end of exponential growth. Numbers in the columns are percentages of organisms at the indicated stages. The total number of organisms scored at each time is given at the bottom of each column.
At stage IIi, a single ter focus was observed in the mother cell and none in the prespore (strain SL13004) (Fig. 2). This result is consistent with the finding that only the origin-proximal 30% of a chromosome is present in the prespore when it is first formed (47, 49). Further, it suggests that either chromosome replication has not yet been completed or replication has been completed and the ter regions have remained associated so that only a single ter focus is visualized by microscopy.
Organisms with two ter regions become predominant at stage IIiii, as engulfment nears completion.
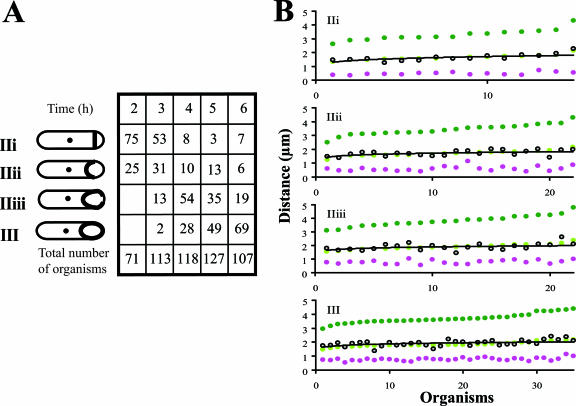
At stage IIii, about 80% of organisms still had a single ter focus, which was located in the mother cell; the remainder had two foci, one in the mother cell and one in the prespore (Fig. 2 and 3; the data in Fig. 2 and in Fig. 3 are from different experiments). By stage IIiii, about 80% of organisms had two ter foci (Fig. 2 and 3), one in the mother cell and the other in the prespore. The prespore ter focus often appeared to be associated with the engulfing membrane. The small size of the prespore precluded any firm conclusion about this association, but the prespore ori region was generally not associated with the engulfing membrane (data not shown). In IIii or IIiii organisms with two ter foci, those foci were almost always well separated (Fig. 3); further, in all organisms with two ter regions, one was in the mother cell and the other was in the prespore. We infer that once two ter regions were distinguishable, they separated rapidly, as has been reported previously for B. subtilis growing exponentially (45). Using a strain with the same labeling system to visualize the origin, two ori regions were detected in all organisms at the corresponding engulfment stages, one in the mother cell and one in the prespore (data not shown). Thus, it is unlikely that optical occlusion would prevent detection of a ter focus in the prespore at those stages. In contrast, in organisms that had progressed beyond stage III, the ori and ter foci in the prespore were often weaker than those in the mother cell; it is thought that the increased density associated with maturation of the prespore (29) reduced the fluorescent signal.
FIG. 3.
Positions of the terminus region during engulfment of a spo+ strain. (A) Positions of ter foci at different stages during engulfment of strain SL13004 incubated in MRB at 30°C. Data for a particular stage were pooled from samples at different times during spore formation (the data in Fig. 2 and 3 are from different experiments). Each organism is shown on the abscissa; organisms are ranked according to their length (ordinate). L, dark green; L/2, light green; M1, pink; M2, yellow; T1, gray if one ter region in the organism, black if two ter regions; best-fit line for the positions of T1 in each population is present as a black line; T2, blue. (B) Schematic representation of measurements of the position of different features in engulfing organisms. Measurements are from the prespore end of the sporulating organism (mother cell plus prespore) and are illustrated for an organism at stage III+. L, length of the sporulating organism; M1, position of the middle of the prespore membrane; M2 (III+ organisms only), position of the second prespore membrane; T1, position of the ter focus; T2, position of the second ter focus (when present; T2 was generally adjacent to the prespore membrane, as indicated).
Results from previous studies (9, 15, 28) have indicated that chromosome replication is completed before division and well before we observed two ter regions. In order to assess DNA replication, we used a DnaX-YFP translational fusion; the fusion efficiently replaces DnaX (23). DnaX (Tau) is the scaffolding protein of DNA polymerase and dissociates from the DNA when DNA is no longer being replicated (22, 23). Strain SL12931 was incubated in MRB at 30°C. During exponential growth, about 90% of bacteria displayed a single focus of YFP fluorescence located approximately at midcell. The proportion displaying such a focus dropped after the start of spore formation, and at 2 h only 25% of bacteria that were scored as stage 0 (no sporulation septum) had a focus. No focus was detected in any organism (0/76) scored as having formed the sporulation septum. We interpret this result to mean that DNA replication was completed before septum formation (stage IIi), although we cannot exclude the possibility that replication was stalled rather than completed.
The processes of engulfment and separation of the ter region were not altered when bacteria grown in a rich medium were induced to form spores.
The behavior of the ter and ori regions was also analyzed for bacteria forming spores in the rich medium MSSM. To enhance visualization of the ter and ori regions in this medium, a GFP-LacI fusion was used (44, 46). The GFP-LacI/lacO system did not affect growth rate or spore formation. In MSSM, the doubling time was about 30 min during exponential growth at 37°C, which requires dichotomous chromosome replication. Nevertheless, the behavior during spore formation of the ori region was similar to that for bacteria grown in MRB. In organisms of strain DCL693 that had reached stage IIi or later in MSSM, there was one ori focus in the mother cell and one in the prespore (data not shown). Presumably replication was no longer dichotomous at that stage.
The behavior of the ter region of strain KPL475 in MSSM was very similar to that of strain SL13004 in MRB. There was a single ter focus in the mother cell and none in the prespore of 35 organisms examined at stage IIi (Fig. 4A). By stage IIiii, the majority of organisms had two ter foci (10 of 14 organisms [Fig. 4A]), one in the mother cell and one in the prespore; all 40 organisms that had reached stage III or beyond had two ter foci. The position of the septum and the sizes of organisms at the different stages of engulfment in MSSM were comparable to those in the minimal medium MRB, with, if anything, fewer long organisms (compare Fig. 3B and 4A).
FIG. 4.
Changes of medium and of probe do not affect assessment of position of the terminus region during engulfment of spo+ strains. (A) Position as indicated by GFP-LacI binding to lacO at cgeD. Strain KPL475 was induced to form spores in MSSM at 37°C. Measurements are as in Fig. 2. (B) Position as indicated by an RTP-CFP fusion binding to Ter sites. Strain SL12976 was induced to form spores in MRB at 30°C. Measurements are as in Fig. 3.
The separation during engulfment of the terminally located RTP-binding region was very similar to that of the cgeD-lacO region.
The position of the terminus region was also assayed with an RTP-YFP translational fusion (24). This fused protein provides a way to visualize the terminus region that is distinct from the method described above, which utilized the binding of LacI to tandem repeats of the lacO region inserted near the terminus. The RTP binds at up to nine sites very near the terminus of replication, called Ter sites, so as to prevent DNA replication complexes from overshooting the terminus region (8). Two RTP dimers potentially bind to each site (8). The RTP-YFP fusion protein has been shown to substitute for RTP, making visualization of the terminus region possible (24). Presumably because fewer molecules are involved with RTP-YFP, the fluorescence signals were weaker; nevertheless, more than 75% of organisms examined displayed at least one RTP-YFP focus. The RTP-YFP fusion did not affect growth rate or spore formation.
A single RTP-YFP focus was observed at stage IIi of spore formation (16 of 16 organisms examined [Fig. 4B]), located in the mother cell. The bulk of the organisms (12 of 13) at stage IIii had a similar, single focus. However, by stage IIiii, there were usually two foci (18 of 27 organisms at stage IIiii; 28 of 31 organisms that had reached stage III or beyond) (Fig. 4B), one in the mother cell and the other in the prespore. Thus, although fewer cells were examined, the results obtained for the location of the terminus region using RTP-YFP are very similar to those obtained with the lacO/LacI-CFP and lacO/GFP-LacI systems.
Mutation in spoIIIE blocks the appearance of a second ter region during spore formation.
The spoIIIE locus encodes a DNA translocase, which is required to transfer the origin-distal 70% of the chromosome into the prespore. Its homolog in E. coli, FtsK, is involved in chromosome separation during vegetative growth. Inactivation of SpoIIIE has little effect on vegetative growth of B. subtilis, and so presumably SpoIIIE does not have a comparable role during vegetative growth. However, we wished to test if SpoIIIE did have a role in chromosome separation during spore formation.
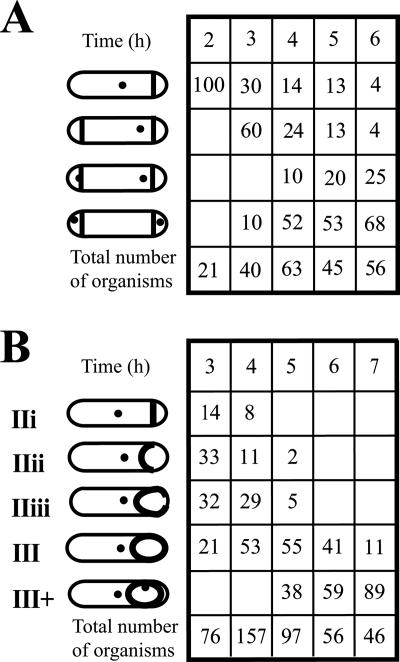
Sporulation was initiated in MRB for strain SL13271, in which much of spoIIIE had been replaced with an spc cassette. The strain contains lacO inserted near the chromosome terminus, at cgeD. Consistent with the function of SpoIIIE as a DNA translocase, no focus of LacI-CFP fluorescence was detected in the prespore in any samples, indicating that the ter region had not entered the prespore. Importantly, even in organisms that had reached stage IIiii or III, there was just a single ter focus (Fig. 5A). The same result was obtained using LacI-GFP as a tag and using RTP-YFP as an indicator of the terminus region (data not shown).
FIG. 5.
Effect of insertional inactivation of spoIIIE on the behavior of the terminus region during engulfment. The spoIIIE::spc strain SL13271 was incubated in MRB at 30°C. Strain SL13271 contained a LacI-CFP fusion and copies of lacO inserted at cgeD, located at 181° on the chromosome. (A) Time course of engulfment. Schematic representations of the stages of engulfment are presented (based on Fig. 1), and the locations of ter regions are indicated. No organisms reached stage III+, and no organisms had two ter regions. The time shown is the time after the end of exponential growth. Numbers in the columns are percentages of organisms at the indicated stages. The total number of organisms scored at each time is given at the bottom of each column. Only organisms with a sporulation septum were scored. (B) Positions of the ter region during engulfment of strain SL13271. Data for a particular stage were pooled from samples at different times. Measurements are as in Fig. 3.
All characterized mutations in spoIIIE that block sporulation also block DNA translocation. However, some point mutations are less pleiotropic than the null mutation described above (39, 48). Consequently, we also tested the effect of such a point mutation, spoIIIE36. The effect of spoIIIE36 on ter localization was essentially the same as that of the spoIIIE::spc mutation. Namely, there was no ter focus in the prespore, and there was just a single ter focus in the mother cell (data not shown). Thus, both classes of spoIIIE mutation blocked ter separation. We infer that when DNA translocation was blocked by a spoIIIE mutation, then separation of chromosome ter regions was also blocked. Disruption of spoIIIE did not affect DNA replication assayed with SL13933 as for the spo+ strain SL12931: about 90% of bacteria displayed a DnaX-YFP focus during exponential growth, whereas no focus was detected in any organism (0/56) that had formed the sporulation septum.
The ter focus in the spoIIIE mutant SL13271 was located at or very close to midorganism (mother cell plus prespore) for all organisms that had formed the spore septum, including those that advanced towards the completion of engulfment (best-fit line, stages IIii, IIiii, and III [Fig. 5B]). The same result was obtained with RTP-YFP as the indicator of terminus position (data not shown). This position contrasts with its position in spo+ strains. In spo+ organisms at stages IIii and IIiii with a single ter region, the ter region was located on the prespore side of midorganism (Fig. 3B and 4A and B). In spo+ organisms at stage IIi, however, its position was more nearly at midorganism (Fig. 3B and 4A and B), suggesting that the ter region was near midorganism when the septum was first formed and then moved towards the prespore.
Mutations in spoIIR and spoIIIG did not block appearance of the ter region in the prespore.
The results described in the section above may have been the specific consequence of mutating spoIIIE or a more general consequence of blocking sporulation during engulfment. In order to distinguish between these possibilities, the effects of spoIIR and spoIIIG mutations were examined; these mutations do not affect expression of spoIIIE (11, 31). In contrast to the effect of a spoIIIE mutation, neither the spoIIR nor the spoIIIG mutation blocked the appearance of a second ter focus in the prespore (strains SL13272 and SL13346, respectively) (Fig. 6). The spoIIR mutation blocks sporulation at stage IIi and results in an abortively disporic phenotype in which sporulation septa are formed near both poles (22, 29). Typically the septum at one end is formed after the septum at the other end (20, 29). The results (Fig. 6A) are consistent with one prespore receiving a ter region after the other. Mutation in spoIIIG causes sporulation to be blocked after completion of engulfment (19, 43). In the spoIIIG mutant, SL13346, the ter focus was clearly seen in the prespore only when engulfment appeared to be complete, as indicated by detachment of both ends of prespore from the mother cell (Fig. 6B). In spo+ strains, a second ter focus was commonly seen at stage IIiii (strain SL13004) (Fig. 3A), but this was not the case for the spoIIIG mutant.
FIG. 6.
Effect of insertional inactivation of spoIIR and of spoIIIG on behavior of the terminus region during engulfment. The spoIIR::neo strain SL13272 and the spoIIIG::neo strain SL13346 were induced to form spores in MRB at 30°C. They contained a LacI-CFP fusion and copies of lacO inserted at cgeD, located at 181° on the chromosome. Schematic representations of the stages of engulfment are presented, and the locations of ter regions are indicated. The ter foci detected in the prespore were generally adjacent to the membrane; their position was variable, and the most common position is represented. The positions of ter foci in the mother cell were also variable, and the most common position is indicated. (A) Time course of engulfment for SL13272. All organisms with a single septum had a single ter focus, which was located in the mother cell. (B) Time course for SL13346. Only organisms at stage III+ had two ter foci; all III+ organisms had two ter foci. In both panels, the time shown is the time after the end of exponential growth. Numbers in the columns are percentages of organisms at the indicated stages. The total number of organisms scored at each time is given at the bottom of each column. Only organisms with a sporulation septum were scored.
DISCUSSION
Separation of replicated chromosomes is an integral part of the bacterial cell cycle. It has generally been found to be coordinated with closure of the division septum, so that each of the resulting daughter cells has a complete genome (26). However, division during bacterial spore formation is different: division precedes complete chromosome partitioning, and the prespore receives the ori-distal 70% of its chromosome after the division is completed (47). We explored here the separation of chromosome termini during spore formation. We found that there is just one ter region observable in sporulating organisms for some time after formation of the sporulation division septum; a second ter region appeared only as engulfment of the prespore by the mother cell was nearing completion. Results from previous studies indicate that chromosome replication is completed before division during spore formation (9, 15, 28). The observed loss of DnaX-YFP foci, which mark DNA replication complexes (22, 23), supports the conclusion that replication is completed before division and hence well before we observed separation of two ter regions. We presume that the duplicated ter regions remain associated during this interval and so appear as a single focus. However, we cannot formally exclude the possibility that the two ter regions separate soon after replication but one is somehow occluded until engulfment is nearing completion. Continued association of duplicated ter regions has been reported for E. coli during vegetative growth, where they were estimated to remain associated for about 25 min after duplication (25, 42).
The SpoIIIE protein functions as a DNA translocase (3, 47) and is also critical to the completion of engulfment (38). We suggest here a third role for this 787-residue protein, in chromosome separation during spore formation. Only one ter focus was observed in spoIIIE mutants under sporulation conditions, indicating that ter separation requires SpoIIIE action (Fig. 5A). One possible explanation is that separation of two ter regions is a secondary, passive consequence of SpoIIIE-mediated translocation of one chromosome into the prespore. However, for three reasons we think it more likely that SpoIIIE has an active role in ter separation that is distinct from its role as a translocase. First, when two ter foci are detected in a sporulating organism, they are almost always well separated, and one is in the mother cell while the other is in the prespore. This observation suggests that the chromosomes are separated by an active mechanism, preceded by the associated ter regions of the two chromosomes being pulled as one towards SpoIIIE, which is located in the engulfing membrane (38; our unpublished observations). Concomitant with completion of translocation of one chromosome into the prespore, SpoIIIE mediates separation of the two ter regions, which then move apart rapidly. Second, in spo+ strains at stage IIii and stage IIiii with a single ter focus, that focus is generally on the prespore side of midorganism (Fig. 3B); if ter separation were merely a passive consequence of translocation, then presumably two ter foci should be distinguishable under those circumstances, with one being at midorganism and the other being pulled towards the prespore. Third, a homolog of SpoIIIE, FtsK, plays just such an active role in E. coli during vegetative growth (4, 13, 41). FtsK functions to resolve replicated chromosome in concert with the action of topoisomerase and of the XerC/D recombinase. It seems plausible that SpoIIIE functions similarly to FtsK in resolving replicated chromosome during spore formation, although it is also possible that SpoIIIE functions to disrupt some other sort of interaction that has kept termini associated during spore formation. Whatever the explanation, SpoIIIE is not required for separation of ter regions during vegetative growth of B. subtilis, pointing to a difference in the mechanism of chromosome separation between vegetative growth and spore formation. The nature of that difference is unknown.
The ter region of the spoIIIE mutants was located at or very close to midorganism (mother cell plus prespore) in organisms at all stages of engulfment (Fig. 5B). In spo+ organisms at stage IIi, the ter region was often also near midorganism. We infer that the position of the ter region at septation is determined independently of the presence of the spore septum (presumably midway between two ori regions, which are located at the opposite poles of the sporulating organism [46]). However, in spo+ organisms at stages IIii and IIiii with a single ter region, that region was located on the prespore side of midorganism, though still generally remaining at some distance from the engulfing membrane (Fig. 3B). At those stages of engulfment, SpoIIIE is observable as a single focus in the engulfing membrane (38, 48; our unpublished observations). We deduce that the DNA translocase activity of SpoIIIE contributes to the movement of the ter region towards the prespore even when the ter region is not close to SpoIIIE. Presumably translocation of the midchromosome region, which is directly mediated by SpoIIIE, results in movement of the distal part of the chromosome, including the ter region.
Intriguingly, the second ter region was observed only in organisms that had clearly completed engulfment (stage III+) in a spoIIIG mutant (Fig. 6B). Why the mutant should differ from the spo+ parent in showing no IIiii organisms with two ter regions is not clear, as spoIIIG encodes σG, which is thought to become active as a transcription factor only after completion of engulfment (6, 43). One possibility is that σG has some role that it plays before it becomes active as a transcription factor. In this context, there are reports of other σ factors having roles distinct from their roles as transcription factors (2, 5). An interpretation of the result is that in the spoIIIG mutant background, separation of the ter regions occurs upon completion of engulfment.
Separation of two ter regions coincided with the appearance of a ter region in the prespore. In spo+ strains, this separation generally occurred as engulfment of the prespore was nearing completion, at stage IIiii. Partridge and Errington (28) estimated that engulfment started about 15 min after septation and was completed about 40 min after septation during sporulation. Extrapolating to their system, the prespore lacked a ter region for, at most, 40 min. This places an upper limit on the time of the genetic asymmetry between mother cell and prespore with respect to the ter region. Transient genetic asymmetry has attracted considerable interest as a mechanism for establishing compartmentalized gene expression, with particular attention to the activation of σF in the prespore following septation (10, 14). It may be that transient genetic asymmetry, in the sense of the delay of entry of the ter region into the prespore until engulfment is nearing completion, is important for the activation of σG, the later-expressed prespore-specific sigma factor.
Acknowledgments
This work was supported by Public Health Service grant GM43577 from the National Institutes of Health.
We thank Bettina Buttaro and Vasant K. Chary for many helpful discussions. We are very grateful to Alan Grossman and Melanie Berkmen for advice about visualizing the ter region.
Footnotes
Published ahead of print on 23 February 2007.
REFERENCES
- 1.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 11:36-42. [Google Scholar]
- 2.Aldridge, P. D., J. E. Karlinsey, C. Aldridge, C. Birchall, D. Thompson, J. Yagasaki, and K. T. Hughes. 2006. The flagellar-specific transcription factor, σ28, is the type III secretion chaperone for the flagellar-specific anti-σ28 factor FlgM. Genes Dev. 15:2315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bath, J., L. J. Wu, J. Errington, and J. C. Wang. 2000. Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science 290:995-997. [DOI] [PubMed] [Google Scholar]
- 4.Bigot, S., J. Corre, J. M. Louarn, F. Cornet, and F. X. Barre. 2004. FtsK activities in Xer recombination, DNA mobilization and cell division involve overlapping and separate domains of the protein. Mol. Microbiol. 54:876-886. [DOI] [PubMed] [Google Scholar]
- 5.Chary, V. K., M. Meloni, D. W. Hilbert, and P. J. Piggot. 2005. Control of the expression and compartmentalization of σG activity during sporulation of Bacillus subtilis by regulators of σF and σE. J. Bacteriol. 187:6832-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chary, V. K., P. Xenopoulos, and P. J. Piggot. 2006. Blocking chromosome translocation during sporulation of Bacillus subtilis can result in prespore-specific activation of σG that is independent of σE and of engulfment. J. Bacteriol. 188:7267-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lencastre, H., and P. J. Piggot. 1979. Identification of different sites of expression for spo loci by transformation of Bacillus subtilis. J. Gen. Microbiol. 114:377-389. [DOI] [PubMed] [Google Scholar]
- 8.Duggin, I. G., and R. G. Wake. 2002. Termination of chromosome replication, p. 87-95. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 9.Dunn, G., P. Jeffs, N. H. Mann, D. M. Torgersen, and M. Young. 1978. The relationship between DNA replication and the induction of sporulation in Bacillus subtilis. J. Gen. Microbiol. 108:189-195. [Google Scholar]
- 10.Dworkin, J. 2003. Transient genetic asymmetry and cell fate in a bacterium. Trends Genet. 19:107-112. [DOI] [PubMed] [Google Scholar]
- 11.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errington, J., R. A. Daniel, and D. J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espeli, O., and K. J. Marians. 2004. Untangling intracellular DNA topology. Mol. Microbiol. 52:925-931. [DOI] [PubMed] [Google Scholar]
- 14.Frandsen, N., I. Barak, C. Karmazyn-Campelli, and P. Stragier. 1999. Transient gene asymmetry during sporulation and establishment of cell specificity in Bacillus subtilis. Genes Dev. 13:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser, P. M., and J. Errington. 1995. Characterization of cell cycle events during the onset of sporulation in Bacillus subtilis. J. Bacteriol. 177:3923-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilbert, D. W., V. K. Chary, and P. J. Piggot. 2004. Contrasting effects of σE on compartmentalization of σF activity during sporulation of Bacillus subtilis. J. Bacteriol. 186:1983-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, W. M., J. L. Libbey, P. van der Hoeven, and S. X. Yu. 1998. Bipolar localization of Bacillus subtilis topoisomerase IV, an enzyme required for chromosome segregation. Proc. Natl. Acad. Sci. USA 95:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of σE and σF in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karow, M. L., P. Glaser, and P. J. Piggot. 1995. Identification of a gene, spoIIR, that links the activation of σE to the transcriptional activity of σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 92:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khvorova, A., V. K. Chary, D. W. Hilbert, and P. J. Piggot. 2000. The chromosomal location of the Bacillus subtilis sporulation gene spoIIR is important for its function. J. Bacteriol. 182:4425-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemon, K. P., and A. D. Grossman. 1998. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 282:1516-1519. [DOI] [PubMed] [Google Scholar]
- 23.Lemon, K. P., and A. D. Grossman. 2000. Movement of replicating DNA through a stationary replisome. Mol. Cell 6:1321-1330. [DOI] [PubMed] [Google Scholar]
- 24.Lemon, K. P., I. Kurtser, and A. D. Grossman. 2001. Effects of replication termination mutants on chromosome partitioning in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesterlin, C., F. X. Barre, and F. Cornet. 2004. Genetic recombination and the cell cycle: what we have learned from chromosome dimers. Mol. Microbiol. 54:1151-1160. [DOI] [PubMed] [Google Scholar]
- 26.Margolin, W. 2001. Spatial regulation of cytokinesis in bacteria. Curr. Opin. Microbiol. 4:647-652. [DOI] [PubMed] [Google Scholar]
- 27.Neylon, C., A. V. Kralicek, T. M. Hill, and N. E. Dixon. 2005. Replication termination in Escherichia coli: structure and antihelicase activity of the Tus-Ter complex. Microbiol. Mol. Biol. Rev. 69:501-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partridge, S. R., and J. Errington. 1993. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 8:945-955. [DOI] [PubMed] [Google Scholar]
- 29.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piggot, P. J., and C. A. M. Curtis. 1987. Analysis of the regulation of gene expression during Bacillus subtilis sporulation by manipulation of the copy number of spo-lacZ fusions. J. Bacteriol. 169:1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 32.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. Perez, Y. L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogliano, K., A. E. Hofmeister, and R. Losick. 1997. Disappearance of the σE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 179:3331-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramaley, R. F., and L. Burden. 1970. Replacement sporulation of Bacillus subtilis 168 in a chemically defined medium. J. Bacteriol. 101:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sciochetti, S. A., P. J. Piggot, and G. W. Blakely. 2001. Identification and characterization of the dif site from Bacillus subtilis. J. Bacteriol. 183:1058-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sciochetti, S. A., P. J. Piggot, D. J. Sherratt, and G. W. Blakely. 1999. The ripX locus of Bacillus subtilis encodes a site-specific recombinase involved in proper chromosome partitioning. J. Bacteriol. 181:6053-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp, M. D., and K. Pogliano. 1999. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc. Natl. Acad. Sci. USA 96:14553-14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharp, M. D., and K. Pogliano. 2003. The membrane domain of SpoIIIE is required for membrane fusion during Bacillus subtilis sporulation. J. Bacteriol. 185:2005-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharpe, M. E., and J. Errington. 1995. Postseptational chromosome partitioning in bacteria. Proc. Natl. Acad. Sci. USA 92:8630-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherratt, D. J., B. Søballe, F.-X. Barre, S. Filipe, I. Lau, T. Massey, and J. Yates. 2003. Recombination and chromosome segregation. Philos. Trans. R. Soc. Lond. B 359:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steiner, W. W., and P. L. Kuempel. 1998. Cell division is required for separation of dimer chromosomes at the dif locus of Escherichia coli. Mol. Microbiol. 27:257-268. [DOI] [PubMed] [Google Scholar]
- 43.Stragier, P. 1989. Temporal and spatial control of gene expression during sporulation: from facts to speculations, p. 243-254. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC.
- 44.Teleman, A. A., P. L. Graumann, D. C.-H. Lin, A. D. Grossman, and R. Losick. 1998. Chromosome arrangement within a bacterium. Curr. Biol. 8:1102-1109. [DOI] [PubMed] [Google Scholar]
- 45.Webb, C. D., P. L. Graumann, J. A. Kahana, A. A. Teleman, P. A. Silver, and R. Losick. 1998. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol. 28:883-892. [DOI] [PubMed] [Google Scholar]
- 46.Webb, C. D., A. Teleman, S. Gordon, A. Straight, A. Belmont, D. C. Lin, A. D. Grossman, A. Wright, and R. Losick. 1997. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell 88:667-674. [DOI] [PubMed] [Google Scholar]
- 47.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 48.Wu, L. J., and J. Errington. 1997. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 16:2161-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu, L. J., and J. Errington. 1998. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol. Microbiol. 27:777-786. [DOI] [PubMed] [Google Scholar]
- 50.Zupancic, M. L., H. Tran, and A. E. Hofmeister. 2001. Chromosomal organization governs the timing of cell type-specific gene expression required for spore formation in Bacillus subtilis. Mol. Microbiol. 39:1471-1481. [DOI] [PubMed] [Google Scholar]