Abstract
CbpA, an Escherichia coli DnaJ homolog, can function as a cochaperone for the DnaK/Hsp70 chaperone system, and its in vitro activity can be modulated by CbpM. We discovered that CbpM specifically inhibits the in vivo activity of CbpA, preventing it from functioning in cell growth and division. Furthermore, we have shown that CbpM interacts with CbpA in vivo during stationary phase, suggesting that the inhibition of activity is a result of the interaction. These results reveal that the activity of the E. coli DnaK system can be regulated in vivo by a specific inhibitor.
One of the major chaperone systems found in both prokaryotes and eukaryotes is the DnaK/Hsp70 system (3). It performs ATP-dependent protein remodeling and reactivation associated with diverse cellular functions. DnaK/Hsp70 acts in collaboration with two cochaperones, DnaJ/Hsp40 and GrpE. DnaJ participates in the delivery of substrates to DnaK, and GrpE stimulates nucleotide exchange.
In addition to DnaJ, Escherichia coli contains five other DnaJ homologs, including CbpA (curved DNA-binding protein A), which was first identified as a DNA-binding protein (15). CbpA binds DNA efficiently, with a preference for curved DNA, and has been localized to the nucleoids of stationary-phase cells (1, 8, 15). CbpA differs from DnaJ in that it is a type II DnaJ protein, lacking the zinc-binding domain characteristic of E. coli DnaJ and other type I DnaJ proteins. High-level expression of CbpA suppresses several phenotypes associated with dnaJ deletions, including temperature sensitivity and λ replication defects (15).
The cbpA gene is in an operon that has a σS-dependent promoter (17). Another gene, cbpM (CbpA modulator), lies downstream of cbpA within the same operon (4). cbpM encodes an 11-kDa protein that has structural homology to DafA of Thermus thermophilus (4). DafATth (DnaK assembly factor A) mediates the assembly of ring-like structures composed of a trimer each of DnaKTth, DnaJTth, and DafATth (13), and this complex is proposed to hold the DnaK chaperone system in an inactive state (5, 11). E. coli CbpM can bind to and inhibit CbpA in vitro, but it has not been shown to interact with DnaK (2, 4).
Here, we have examined the relationship between CbpA and CbpM within the cell. We discovered that CbpM specifically inhibits the in vivo activity of CbpA. Moreover, CbpM forms a stable complex with CbpA in vivo. This suggests that the interaction between CbpM and CbpA results in the inhibition of CbpA activity. These results demonstrate that the in vivo activity of the E. coli DnaK chaperone system can be modulated by CbpM.
CbpM specifically inhibits CbpA function in vivo.
To study the in vivo activities of CbpA and CbpM, we used the strains listed in Table 1. All strains used were derivatives of BW27784, a derivative of MG1655 (10), and transductions with P1vir were performed as described previously (12). Deletion/insertion mutations in cbpA, cbpM, and cbpAM were created as described previously (18), using chloramphenicol resistance cassettes that were amplified using the oligonucleotide primer pairs listed in Table 2. The ΔcbpA3 deletion removed bases 1 to 840 of cbpA, leaving the last 81 nucleotides. The ΔcbpM3 deletion removed bases 2 to 306 of cbpM. The ΔcbpAM3 deletion removed all DNA between the cbpA ATG start codon and the cbpM TGA stop codon. Mutations were confirmed by sequencing. Except as indicated for specific experiments, all strains were cultured at 30°C prior to experimental use, excluding ΔdnaJ ΔcbpAM3 mutants, which were cultured at 25°C.
TABLE 1.
Strains and plasmids utilized in this study
| Strain or plasmid | Description (parental strain in parentheses) | Source or reference |
|---|---|---|
| Strains | ||
| BW27784 | (BW25113) DE(araFGH) φ(ΔaraEp PCP18-araE) | 10 |
| DY330 | (W3110) ΔlacU169 gal490 λcI857 Δ(cro-bioA) | 18 |
| MC105 | (BW27784) ΔcbpA3::cat | This study |
| MC108 | (BW27784) ΔcbpM3::cat | This study |
| MC143 | (BW27784) ΔcbpAM3::cat | This study |
| MC144 | (MC143) ΔdnaJ::kana | This study |
| MC150 | (BW27784) ΔdnaJ::kana | This study |
| Plasmids | ||
| pBAD24 | 9 | |
| pcbpM+ | pBAD24 with cbpA insert | This study |
| pcbpA+ | pBAD24 with cbpM insert | This study |
The ΔdnaJ::kan allele was acquired from Tilly and Yarmolinsky (14).
TABLE 2.
Oligonucleotides used for plasmid and strain construction
| Function and name | Oligonucleotide sequence (5′-3′)a |
|---|---|
| Construction of plasmids | |
| cbpAfor | TAATGAATTCACCATGGAATTAAAGGATTATTACGCC |
| cbpArev | CATTAAGCTTTTATGCTTTCCCCCAATCTTT |
| cbpMfor | CCATCCATGGCTAATGTTACGGTGACTTTT |
| cbpMrev | AGTTAAGCTTTCACGGATGAGCTACAAACCG |
| Chromosomal mutations | |
| ΔcbpAfor | GGGGTTCCTTCAATTTGTGTTGATTTACGCGAGATAACGCTTGTGACGGAAGATCACTTCGC |
| ΔcbpArev | CGACTGGGCGTCTGCCAGTTGCTGCCACAGCGCGGCAGTGTTTTCTTACGCCCCGCCCTGCCACTCATCG |
| ΔcbpMfor | CCAGTCGTCTTTTGATCCACGTAAAGATTGGGGGAAAGCATAATGTGACGGAAGATCACTTCGC |
| ΔcbpMrev | GTAGACCGGTTAAGATGCGTCATCGCATCCGGCAAACACACATTACGCCCCGCCCTGCCACTCATCG |
Underlined bases indicate regions of homology with DNA in or near cbpA or cbpM.
Previously, it was shown that mutants containing deletions in both dnaJ and cbpA are unable to grow at 16°C or above 30°C, unlike strains with either of the genes intact (7, 16). It was further shown that CbpA expressed from a multicopy plasmid can suppress this growth defect in ΔdnaJ ΔcbpA strains (16). The observation that CbpA can functionally compensate for DnaJ allowed us to test whether CbpM, expressed from a multicopy plasmid, inhibits the function of CbpA in vivo. We constructed the pcbpA+ and pcbpM+ plasmids by amplifying cbpA and cbpM by PCR using the oligonucleotide primers indicated in Table 2. cbpA was digested with EcoRI and HindIII and cbpM with NcoI and HindIII. The inserts were ligated into pBAD24 (9), electroporated into DH5α, purified, sequenced, and electroporated into the BW27784 derivatives. We then expressed CbpM from the plasmid in various deletion strains and measured growth over a range of temperatures.
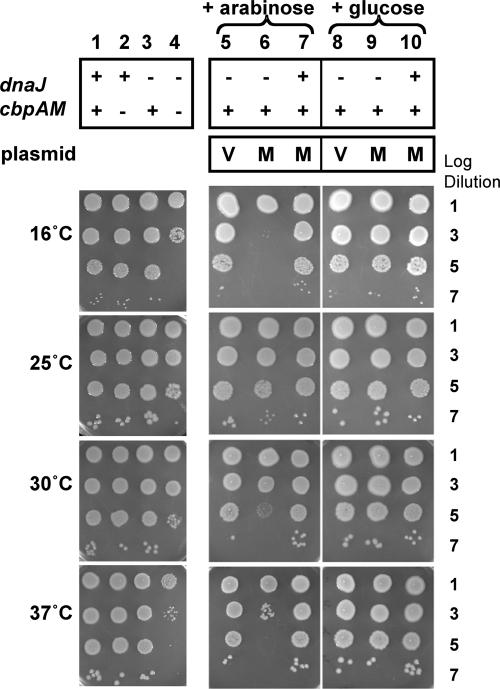
We first tested the deletion strains for their abilities to grow in the absence of pcbpM+. Strains with single deletions of dnaJ, double deletions of cbpAM, or triple deletions of dnaJ and cbpAM were grown at 25°C and plated at 16°C, 25°C, 30°C, and 37°C. As expected, the parental (Fig. 1, column 1) and ΔdnaJ (Fig. 1, column 3) strains grew well at all temperatures tested. Likewise, the ΔcbpAM3 (Fig. 1, column 2), ΔcbpA3 (data not shown), and ΔcbpM3 (data not shown) strains grew well under the conditions tested. In contrast, growth of the triple deletion strain that lacked dnaJ, cbpA, and cbpM was reduced about 10,000-fold at 16°C and 37°C (Fig. 1, column 4). At 25°C and 30°C, growth of the ΔdnaJ ΔcbpAM3 strain was reduced about 100-fold (Fig. 1, column 4). When pcbpM+ was introduced into a ΔdnaJ cbpAM+ strain and subsequently induced with arabinose at 16° and 37°C, there was a dramatic reduction of growth by about 10,000-fold (Fig. 1, column 6). At 25°C, there was no apparent effect on growth by induction of cbpM from the plasmid, while there was a small effect at 30°C (Fig. 1, column 6). The growth defects caused by high-level expression of CbpM in the ΔdnaJ cbpAM+ strain were very similar to those for the phenotypes of the ΔdnaJ ΔcbpAM3 mutant, suggesting that CbpM was inhibiting CbpA. ΔdnaJ cbpAM+ mutant cells carrying the pcbpM+ plasmid but not induced with arabinose exhibited decreased growth at 16°C and 37°C (about threefold lower than that of the vector-only control), indicating that low-level expression of CbpM was sufficient to elicit the growth defect (data not shown). In a control experiment in which pcbpM+ was present in the ΔdnaJ cbpAM+ strain but repressed by the addition of glucose to the medium, there was no inhibition of growth at 16°C or 37°C (Fig. 1, column 9). The vector alone did not inhibit the growth of ΔdnaJ cbpAM+ cells at any temperature in the presence of arabinose or glucose (Fig. 1, columns 5 and 8). Interestingly, induction of pcbpM+ by arabinose in the dnaJ+ cells did not inhibit growth at a high or low temperature, suggesting that CbpM does not inhibit DnaJ in vivo (Fig. 1, column 7). Overexpression of CbpM in dnaJ+ ΔcbpAM or ΔdnaJ ΔcbpAM strains had no significant effect on growth (data not shown), demonstrating that other DnaJ homologs were not inhibited by CbpM. Taken together, these data demonstrate for the first time that CbpM is able to specifically inhibit CbpA function in vivo.
FIG. 1.
Growth of ΔdnaJ and ΔcbpAM3 strains at different temperatures and the effects of CbpM overexpression. E. coli strains with the indicated dnaJ and cbpAM genotypes were grown for 24 h at 25°C and then serially diluted in LB. Ten microliters of the indicated dilutions was spotted on LB agar (plus 100 μg/ml ampicillin for strains containing plasmids) and incubated at the indicated temperatures for 24 h (30°C and 37°C), 48 h (25°C), or 96 h (16°C). Strains marked “V” contained the empty pBAD24 vector. Strains marked “M” contained the pcbpM+ construct. Arabinose (0.02%) or glucose (0.5%) was added to the culture medium for strains bearing plasmids as indicated.
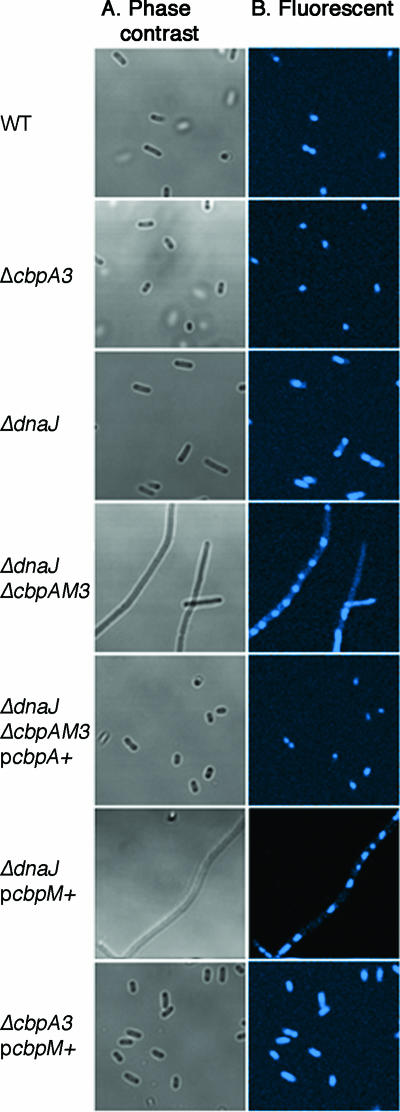
In addition to the narrow temperature range for growth, the ΔdnaJ ΔcbpA strain is defective in cell division, forming long filamentous cells (16). We wanted to determine whether high-level expression of CbpM could also cause a similar cell division defect in the ΔdnaJ cbpAM+ mutant. Cells were grown to late stationary phase at 25°C and examined by phase-contrast microscopy. We observed that the wild-type, ΔcbpA3, ΔdnaJ, ΔcbpM3, and ΔcbpAM3 strains all exhibited small, rod-shaped cells (Fig. 2A and data not shown). However, the ΔdnaJ ΔcbpAM triple mutant displayed a filamentous morphology (Fig. 2A) similar to that described for the ΔdnaJ ΔcbpA double mutant (16). Complementation of the ΔdnaJ ΔcbpAM3 strain with pcbpA+ reverted the cells to wild-type morphology, showing that CbpA activity in ΔdnaJ cells is necessary for proper cell division and that CbpM activity is dispensable (Fig. 2A). To determine whether the function of CbpA in cell division could be inhibited by CbpM, pcbpM+ was introduced into a ΔdnaJ strain and induced with arabinose. High-level expression of CbpM resulted in filamentous cells (Fig. 2A), suggesting that CbpM was able to counteract CbpA activity, yielding a ΔdnaJ ΔcbpA phenocopy. Expression of CbpM in a ΔcbpA dnaJ+ strain did not result in filamentous cells, indicating that CbpM was not able to inhibit DnaJ (Fig. 2A).
FIG. 2.
Cellular morphology of wild-type (WT), ΔcbpA3, ΔdnaJ, and ΔdnaJ ΔcbpAM3 strains and the effects of CbpA and CbpM overexpression. Strains were grown for 24 h at 25°C in LB and then examined by either phase-contrast (A) or fluorescent (B) microscopy. The relevant strain genotypes are indicated to the left of the figure, and strains bearing plasmids were grown in the presence of arabinose (0.02%) and ampicillin (100 μg/ml).
Filamentous cells can arise from defects in two separate processes in cell division: chromosome segregation and septum formation. In cells that do not segregate the chromosome properly, the filaments have irregularly distributed masses of DNA. In contrast, cells that have defects in septum formation have evenly spaced nucleoids throughout the length of the filament. ΔdnaJ ΔcbpA double mutants are known to be defective in septum formation (16). To determine the nature of the cell division defect seen in ΔdnaJ cells expressing high levels of CbpM, the DNA was stained with a fluorescent dye, Hoechs 33342 (Invitrogen), and nucleoid localization was examined by fluorescent microscopy using a Zeiss 510 NLO microscope as previously described (6). In the ΔdnaJ cbpAM+ cells expressing additional cbpM from a plasmid, discrete nucleoids were visible throughout the lengths of the filaments, indicating that the activity missing from the cells participates in septum formation, not chromosome segregation (Fig. 2B). Taken together, the restricted growth temperature and the altered cellular morphology demonstrate that CbpM is capable of inhibiting CbpA in vivo.
In vivo interaction of CbpA and CbpM.
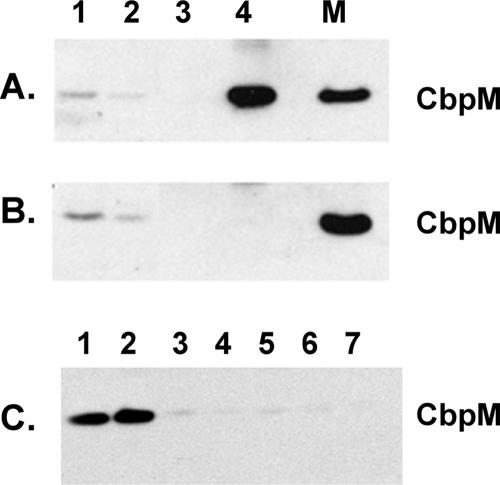
To determine whether CbpA and CbpM physically interact in vivo, we probed for a potential intermolecular interaction using coimmunoprecipitation. Wild-type and ΔcbpA3 strains were grown to early stationary phase (optical density at 595 nm of 3.0, 7 h of growth), and clarified cell lysates were incubated with polyclonal antibodies raised against CbpA. Antibody-protein complexes were precipitated with protein A-Sepharose (Amersham). The immunoprecipitated material was subjected to Western blot analysis using CbpM antiserum (Fig. 3A). The results revealed that significant amounts of CbpM were precipitated from the wild-type lysate with CbpA antiserum (Fig. 3A, lane 4) but not from the ΔcbpA3 lysate (Fig. 3B, lane 4). Lysates from late-stationary-phase wild-type cells (optical density at 595 nm of 5.0, 24 h of growth) were immunoprecipitated with CbpA, CbpM, or one of several other antisera, followed by Western blot analysis for detection of CbpM. CbpM again specifically coprecipitated with CbpA antiserum (Fig. 3C, lane 1) and also with CbpM antiserum as expected (Fig. 3C, lane 2). In control experiments, CbpM-containing complexes were not detected in significant amounts when preimmune serum or antiserum raised against other proteins (ClpB, ClpA, ClpP, and σS) was used for the immunoprecipitation (Fig. 3C, lanes 3 to 7). These results demonstrate that CbpA and CbpM form a complex in both early- and late-stationary-phase cells. They do not rule out the possibility that CbpA and CbpM are also associated with other proteins. The results suggest that it is the complex of CbpA and CbpM that results in the inhibition of CbpA in vivo.
FIG. 3.
Coimmunoprecipitation of CbpA and CbpM. (A) Coimmunoprecipitation was performed on early-stationary-phase wild-type cells by utilizing CbpA antibodies. Fractions from the procedure were analyzed for CbpM content by Western blotting with CbpM antibodies, using a WesternBreeze kit (Invitrogen). Lane 1, total cellular protein; lane 2, clarified lysate; lane 3, final wash of immunoprecipitate; lane 4; immunoprecipitate; lane M, purified CbpM. (B) Coimmunoprecipitation was performed on early-stationary-phase ΔcbpA3 cells by utilizing CbpA antibodies as described for Fig. 3A. (C) Coimmunoprecipitation was performed on late-stationary-phase wild-type cells, and the immunoprecipitates were analyzed for CbpM content by Western blotting. Immunoprecipitations were performed with antibodies raised against the following proteins: lane 1, CbpA; lane 2, CbpM; lane 3, σS; lane 4, ClpB; lane 5, ClpA; and lane 6, ClpP. Lane 7, preimmune serum.
In summary, this is the first report of a specific, in vivo modulator of the DnaK/Hsp70 system in E. coli. In the absence of DnaJ, CbpA is essential for proper cell division and growth at elevated and lowered temperatures. We have demonstrated that the activity of CbpA is specifically inhibited by CbpM in vivo, preventing CbpA from functioning in cell growth and division. We have also shown that CbpM forms a stable complex with CbpA in vivo, an interaction that may be the cause of the inhibition. Homologs of CbpM and CbpA exist in a number of bacteria, ranging from Shigella and Salmonella to Burkholderia and Synechococcus, suggesting that this mechanism of regulating the activity of DnaK is widely conserved.
Acknowledgments
We thank Joel Hoskins, Nadim Majdalani, Carin Vanderpool, Susan Garfield, and Preeti Srivastava for their technical assistance. Additionally, we thank Joel Hoskins for his helpful discussions and comments during manuscript preparation.
This research was supported by the Intramural Research Program of the NIH National Cancer Institute Center for Cancer Research.
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Ali Azam, T., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird, J. G., S. Sharma, S. C. Roshwalb, J. R. Hoskins, and S. Wickner. 2006. Functional analysis of CbpA, a DnaJ homolog and nucleoid-associated DNA-binding protein. J. Biol. Chem. 281:34349-34356. [DOI] [PubMed] [Google Scholar]
- 3.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 4.Chae, C., S. Sharma, J. R. Hoskins, and S. Wickner. 2004. CbpA, a DnaJ homolog, is a DnaK co-chaperone, and its activity is modulated by CbpM. J. Biol. Chem. 279:33147-33153. [DOI] [PubMed] [Google Scholar]
- 5.Dumitru, G. L., Y. Groemping, D. Klostermeier, T. Restle, E. Deuerling, and J. Reinstein. 2004. DafA cycles between the DnaK chaperone system and translational machinery. J. Mol. Biol. 339:1179-1189. [DOI] [PubMed] [Google Scholar]
- 6.Fekete, R. A., and D. K. Chattoraj. 2005. A cis-acting sequence involved in chromosome segregation in Escherichia coli. Mol. Microbiol. 55:175-183. [DOI] [PubMed] [Google Scholar]
- 7.Genevaux, P., F. Schwager, C. Georgopoulos, and W. L. Kelley. 2001. The djlA gene acts synergistically with dnaJ in promoting Escherichia coli growth. J. Bacteriol. 183:5747-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gur, E., C. Katz, and E. Z. Ron. 2005. All three J-domain proteins of the Escherichia coli DnaK chaperone machinery are DNA binding proteins. FEBS Lett. 579:1935-1939. [DOI] [PubMed] [Google Scholar]
- 9.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khlebnikov, A., K. A. Datsenko, T. Skaug, B. L. Wanner, and J. D. Keasling. 2001. Homogeneous expression of the P(BAD) promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241-3247. [DOI] [PubMed] [Google Scholar]
- 11.Klostermeier, D., R. Seidel, and J. Reinstein. 1999. The functional cycle and regulation of the Thermus thermophilus DnaK chaperone system. J. Mol. Biol. 287:511-525. [DOI] [PubMed] [Google Scholar]
- 12.Miller, J. H. 1972. Experiments in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 13.Motohashi, K., M. Yohda, I. Endo, and M. Yoshida. 1996. A novel factor required for the assembly of the DnaK and DnaJ chaperones of Thermus thermophilus. J. Biol. Chem. 271:17343-17348. [DOI] [PubMed] [Google Scholar]
- 14.Tilly, K., and M. Yarmolinsky. 1989. Participation of Escherichia coli heat shock proteins DnaJ, DnaK, and GrpE in P1 plasmid replication. J. Bacteriol. 171:6025-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueguchi, C., M. Kakeda, H. Yamada, and T. Mizuno. 1994. An analogue of the DnaJ molecular chaperone in Escherichia coli. Proc. Natl. Acad. Sci. USA 91:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueguchi, C., T. Shiozawa, M. Kakeda, H. Yamada, and T. Mizuno. 1995. A study of the double mutation of dnaJ and cbpA, whose gene products function as molecular chaperones in Escherichia coli. J. Bacteriol. 177:3894-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashino, T., M. Kakeda, C. Ueguchi, and T. Mizuno. 1994. An analogue of the DnaJ molecular chaperone whose expression is controlled by sigma s during the stationary phase and phosphate starvation in Escherichia coli. Mol. Microbiol. 13:475-483. [DOI] [PubMed] [Google Scholar]
- 18.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]