Abstract
Gram-negative bacteria contain multiple secretion pathways that facilitate the translocation of proteins across the outer membrane. The two-partner secretion (TPS) system is composed of two essential components, a secreted exoprotein and a pore-forming β barrel protein that is thought to transport the exoprotein across the outer membrane. A putative TPS system was previously described in the annotation of the genome of Escherichia coli O157:H7 strain EDL933. We found that the two components of this system, which we designate OtpA and OtpB, are not predicted to belong to either of the two major subtypes of TPS systems (hemolysins and adhesins) based on their sequences. Nevertheless, we obtained direct evidence that OtpA and OtpB constitute a bona fide TPS system. We found that secretion of OtpA into the extracellular environment in E. coli O157:H7 requires OtpB and that when OtpA was produced in an E. coli K-12 strain, its secretion was strictly dependent on the production of OtpB. Furthermore, using OtpA/OtpB as a model system, we show that protein secretion via the TPS pathway is extremely rapid.
In the bacterial two-partner secretion (TPS) pathway, a single polypeptide (exoprotein) is translocated across the outer membrane (OM) by a dedicated β barrel pore that is generally encoded in the same operon. TPS exoproteins are large proteins that range in size from 100 kDa to more than 500 kDa (2, 4, 5, 10, 20, 25). They usually contain typical N-terminal signal peptides that target them for translocation across the inner membrane via the Sec pathway (20). The most characteristic feature of TPS exoproteins is a highly conserved N-terminal domain of approximately 250 residues known as the TPS domain (7, 17, 20). This domain is both necessary and sufficient for secretion and has been proposed to mediate recognition of the exoprotein by the transporter (12, 20, 21, 31, 33). The first ∼100 residues of the TPS domain are especially highly conserved and contain two nearly universal motifs, NPNL and NPNGI, that are separated by ∼35 residues (12, 18, 34). The exact function of these motifs is unclear, although in at least some cases they are required for secretion (20).
Many TPS exoproteins play a role in bacterial virulence. One group of TPS exoproteins, which includes Serratia marcescens ShlA, Proteus mirabilis HpmA, Photorhabdus luminescens PhlA, Haemophilus ducreyi HhdA, and Edwardsiella tarda EthA, have hemolytic/cytolytic activities and share extensive sequence homology (3, 4, 16, 28, 40, 45). A hemolysin-like protein from Pseudomonas putida (HlpA) has recently been shown to affect root colonization and iron uptake and may also be a member of this family (25). A second family of TPS proteins, which includes enterotoxigenic Escherichia coli EtpA, Bordetella pertussis FHA, and Haemophilus influenzae HMW1 and HMW2, function as adhesins (10, 37, 46). With the exception of FHA, these adhesins are closely related by sequence (36, 46). Outside of the TPS domain, however, the two major families show only limited sequence similarity. Two novel TPS exoproteins that do not appear to have either hemolytic or adhesive activities have recently been identified. The Burkholderia pseudomallei BpaA protein has no clear virulence function (5), and the E. coli E93 CdiA inhibits the growth of other E. coli strains in a contact-dependent fashion (2). Beyond the TPS domain, these proteins are not closely related to the two major exoprotein families (2, 5).
The TPS transporters are OM proteins that are predicted to have a typical β barrel structure (19, 22, 24, 39). In most characterized TPS systems, the β barrel appears to be sufficient for translocation of the coordinately produced exoprotein (20). The HMW1 and HMW2 systems are exceptions in that a separate glycosylase is required for secretion and stability (11, 38). In addition to mediating exoprotein secretion, some TPS transporters are also involved in activation or modification of their partner proteins. The TPS transporter ShlB, for example, is required for the activation of ShlA/HpmA hemolytic activity (15, 27, 47). Furthermore, recent studies with the EtpBA system suggest that the EtpB transporter has a second function as an adhesin (10).
Based on homology to TPS components in other organisms, a putative novel TPS system was identified in the enterohemorrhagic E. coli strain O157:H7 (14, 29). The genes that encode these putative TPS components reside on O island 47 (OI-47), which encodes a putative type 1 fimbria and is found almost exclusively in the verotoxin-producing serotypes most highly associated with hemolytic uremic syndrome (35). In the present study we used sequence analysis to examine the relationship of the putative E. coli O157:H7 TPS systems to previously characterized TPS systems. We also used cell fractionation experiments to examine the localization of OtpA (O157:H7 two-partner protein A) and OtpB (O157:H7 two-partner protein B) and to obtain direct evidence that the two proteins constitute a TPS system. Finally, we used OtpA/OtpB as a model system to perform the first kinetic analysis of secretion via the TPS pathway.
MATERIALS AND METHODS
Antisera, bacterial strains, and media.
Polyclonal rabbit antisera were generated against peptides derived from the N terminus of OtpA, the C terminus of OtpA, and the N terminus of OtpB (TINGMMEVAGDKADLIIANPNC, CDAVLGGFYGLALQANQYDN, and ADESTLRAHKLEKKDVYSNAQC, respectively) after coupling to keyhole limpet hemocyanin (Fig. 1). The bacteria used in this study were the E. coli K-12 strains MC4100 (F− araD139 Δ(argF-lac)U169 rpsL150 relA1 thi fib5301 deoC1 ptsF25 rbsR) (6), AD202 (MC4100 ompT::kan) (1), and HDB114 (MC4100 ara+ ompT::kan) (41) and the E. coli O157:H7 strain EDL933 (American Type Culture Collection). An EDL933 derivative lacking otpB (PC53) was created using an adaptation of the Lambda red recombinase system for enterohemorrhagic E. coli (8, 9). To disrupt otpB, a 1.5-kb fragment containing the kanamycin resistance gene (kan) from pKD4 (8) was amplified using the oligonucleotide pair PC21/PC22 (Fig. 1 and Table 1) and used to transform EDL933. In all experiments bacteria were grown at 37°C in either LB or a standard minimal medium (M9 containing 0.2% glycerol and 40 μg/ml of all of the l-amino acids except cysteine and methionine). Media were supplemented as needed with ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), or kanamycin (25 μg/ml).
FIG. 1.
otpAB region in E. coli O157:H7 strain EDL933 and mutant strain PC53. Organization of otpAB region within pathogenicity island OI-47. The small arrows indicate oligonucleotides used for PCR amplification. The gray segment of otpB represents the 675-bp fragment that was replaced with the kanamycin resistance gene in strain PC53. Asterisks indicate the approximate locations of the corresponding peptide segments used for antibody generation. Z1541 encodes a hypothetical protein, and Z1544 encodes a putative acyl-carrier protein synthase located immediately downstream from otpB.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′ to 3′)a | Description (reference) |
|---|---|---|
| AW102 | CAGAATCCAGAGGCC | Anneals to bp 137 to 152 of Z1544 |
| AW112 | GCGTTAGAAGCAAAC | Anneals to bp 3757 to 3771 of otpA |
| AW118 | CCACTGCTGTCTTTGGTTACATCTT | Anneals 25 bp downstream of otpA |
| AW123 | GGAATTCCCTACGCCGGAAGATCCCGTTCAGGT | Anneals to bp 1621 to 1646 of shlB |
| AW127 | GGAATTCCGTGCAGGAAAGGCGGCGTTATTTGCC | Anneals 30 to 55 bp downstream of shlA |
| AW128 | GGAATTCCCTCTGCGAAATTTCGTATTTCCCAC | Anneals 321 to 346 bp upstream of shlB |
| AW136 | GGAATTCCCCTGCAAAACTGTTGATTAAAAGGT | Anneals 17 bp downstream of shlB |
| PC5 | AAAATGAATAGCATCAATAAG | Anneals 17 to 37 bp upstream of otpA |
| PC7 | GCGGATCCATCAATAAGGAAAACGTTAG | Anneals 6 to 25 bp upstream of otpA |
| PC8 | GGGGATCCTGAGAAGATAATGCAATTACG | Anneals 10 bp upstream of otpB |
| PC9 | GGTCTAGAGGCAATTTCAACAATATCTGA | Anneals 29 to 48 bp downstream of otpB |
| PC21 | TTGAACTGGAAGATAACTTTCTGGGTGGTCAGTTGATTTCTGAAGGTGTAGGCTGGAGCTGCTTC | Homologous to bp 223 to 268 of otpA and contains priming site 1 (8) |
| PC22 | CATAATCCAGCGAGATGTACGAAAGCGGAATCACCTGCTGCGATTCATATGAATATCCTCCTTA | Homologous to bp 853 to 898 of otpB and contains priming site 2 (8) |
| PC38 | GAGGATCCTTACTTGCCGTTAGTATTGTCAA | Anneals to bp 1030 to 1050 of otpA |
Underlined sequences denote restriction sites.
Plasmid construction.
DNA fragments encoding otpAB, otpA, otpA(N), and otpB were amplified by PCR using EDL933 genomic DNA as a template and the oligonucleotide pairs PC7/PC9, PC5/AW118, PC7/PC38, and AW112/AW102, respectively (Fig. 1 and Table 1). shlA and shlB were amplified by PCR using S. marcescens genomic DNA (ATCC 27137D) as a template and the oligonucleotide pairs AW123/AW127 and AW128/AW136, respectively (Table 1). The six resulting amplicons were cloned into the TOPO XL PCR vector (Invitrogen) according to the manufacturer's instructions. The otpAB locus was then subcloned into the EcoRI site of pTrc99A (Pharmacia) to yield pPC19. otpA was subcloned into the BamHI and SalI sites of pTrc99A to yield pPC20, otpA(N) was subcloned into the BamHI site of pTrc99a to yield pPC69, and otpB was ligated into the KpnI and PstI sites of pBAD33 (13) to yield pPC11. shlA and shlB were ligated in succession into the EcoRI site of pTrc99A to yield pAJW215.
Sequence analysis.
The percent sequence identity was determined by use of BLASTP using the default setting without a filter. Sequence alignments and dendrograms were generated using the ClustalW method in the program Megalign (DNASTAR, Inc.). Signal peptide predictions were made using SignalP3.0 (http://www.cbs.dtu.dk/services/SignalP) and SIG-Pred (http://www.bioinformatics.leeds.ac.uk/prot_analysis/Signal.html).
Subcellular fractionations.
For experiments that used EDL933 or PC53 lacking a multicopy plasmid, 10 ml of LB was inoculated with 50 μl of a saturated overnight culture and incubated to an optical density at 550 nm (OD550) of 3.0. For experiments that used AD202 or EDL933 containing pPC19, cells were grown overnight in minimal medium, washed, and inoculated into 50 ml of fresh medium at an OD550 of 0.02. When cultures reached an OD550 of 0.2, otpAB expression was induced by the addition of 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG). After 5 min, cells were placed on ice. In all experiments, cells were harvested by centrifugation at 3,700 × g for 10 min at 4°C. Proteins secreted into the medium were precipitated with ice-cold 10% trichloroacetic acid (TCA), and cell pellets were resuspended in 1 ml 40% sucrose, 33 mM Tris (pH 8.0). Cells were treated with lysozyme (0.1 mg/ml) for 20 min on ice. After the addition of 1 mM MgSO4, spheroplasts were collected by centrifugation at 16,600 × g at 4°C, and the supernatant (periplasmic fraction) was precipitated with TCA. The spheroplasts were divided into cytoplasmic and membrane fractions as previously described (30). Protein concentrations were determined using the bicinchoninic acid assay (Pierce), and equal amounts of protein from each fraction were analyzed by Western blotting.
Kinetic analysis of OtpA and OtpB biogenesis and protein export.
Overnight cultures were washed and diluted in fresh minimal medium at an OD550 of 0.02. When cultures reached an OD550 of 0.2, otpA/otpB expression was induced by the addition of 100 μM IPTG. After 30 min, pulse-chase labeling was performed as previously described (44). Cells were poured over ice and harvested by centrifugation (2,900 × g, 5 min, 4°C), and secreted proteins were precipitated with TCA. Cell pellets were resuspended in 1 ml M9 salts containing 0.2% glycerol and divided in half. One half was immediately precipitated with TCA, and the other half was treated with proteinase K (PK) as previously described (44) prior to precipitation with TCA. All samples were then used for immunoprecipitations as previously described (26). The level of radioactivity in individual protein bands was quantitated using a Fuji BAS-2500 phosphorimager. The percentage of OtpA that was secreted was estimated using the formula [(OtpAcell−PK − OtpAcell+PK + 0.5 OtpAsecreted)/(OtpAcell−PK + 0.5 OtpAsecreted)] × 100. Similarly, the percentage of OtpA(N) that was present as a preprotein was estimated using the formula {pre-OtpA(N)cell−PK/[pre-OtpA(N)cell−PK + OtpA(N)cell−PK + 0.5 OtpA(N)secreted] } × 100.
Uncoupled expression of otpA and otpB.
Overnight cultures of HDB114 transformed with pPC20 and pPC11 were inoculated into 50 ml of minimal medium at an OD550 of 0.02. When the cultures reached an OD550 of 0.2 an uninduced 1-ml sample was removed. Portions of each culture were then supplemented with 100 μM IPTG to induce otpA expression or 100 μM IPTG plus 0.1% arabinose to induce otpA and otpB expression. Samples (1 ml) were removed 10, 20, and 30 min after induction. Uninduced and induced samples were centrifuged for 5 min (2,900 × g, 4°C), and both the supernatants and cell pellets were precipitated with TCA.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis.
Protein samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 8 to 16% polyacrylamide minigels (Invitrogen). For Western blots, antigen-antibody complexes were detected using the SuperSignal West Pico Chemiluminscent kit (Pierce).
RESULTS
Sequence analysis of E. coli O157:H7 OtpA and OtpB.
Open reading frame Z1542 of E. coli O157:H7 strain EDL33 has been predicted to encode an ∼130 kDa TPS exoprotein (14, 35). We have thus designated Z1542 as otpA and its gene product as OtpA. SIG-Pred and SignalP3.0 predict that the first 31 residues of OtpA constitute a canonical signal peptide. The N-terminal ∼250 residues of the mature form of OtpA are highly homologous to the TPS domains associated with all known TPS exoproteins. We found an especially highly conserved segment (amino acids 44 to 159) within this domain that is more than 45% identical to the corresponding segment in at least 50 characterized and putative TPS exoproteins. This segment contains the two NPNL and NPNGI motifs that are characteristic of all TPS exoproteins (residues 95 to 98 and 135 to 139) (Fig. 2A) (20). Phylogenetic analysis shows that this highly conserved segment of OtpA is most closely related to the TPS domains of the hemolysins and FHA even though it is the outlier among this cluster (Fig. 2B). Unlike the hemolysins, however, OtpA lacks the sequence CXXC two residues downstream from the NPNGI motif (Fig. 2A). Although OtpA is annotated in several sequence databases as a putative hemolysin or adhesin, most of the sequence similarity is found between residues 44 and 159. In fact, phylogenetic analysis indicates that full-length OtpA is only distantly related to TPS hemolysins and adhesins (Fig. 2C). In contrast, within their respective families the TPS hemolysins and HMW1-like adhesins show a high degree of sequence identity across their entire protein sequence (Fig. 2C). Our sequence analysis strongly suggests that OtpA is a TPS exoprotein even though it is not clearly predicted to belong to either of the well-characterized exoprotein families.
FIG. 2.
Alignment of TPS exoproteins. (A) ClustalW alignment of conserved residues at the N termini of TPS exoproteins. Residues shadowed in black match the consensus sequence. Brackets denote conserved NPNL and NPNGI motifs. The gray bar indicates the conserved CXXC motif found in TPS hemolysins. (B) Phylogenetic tree showing the relationship between the selected TPS exoproteins that make up the segment corresponding to OtpA residues 44 to 159. The scale bar represents nucleotide substitutions (in hundreds). The dotted line indicates a negative branch length. (C) Phylogenetic tree showing the relationship of the complete sequence of the following selected TPS exoproteins (accession numbers of TPS exoproteins are in parentheses): OtpA (AAG55657); BpaA, Burkholderia pseudomallei TPS exoprotein (AAO19442); CdiA, E. coli EC93 contact-dependent inhibitor (AAZ57198); EihA, Edwardsiella ictaluri (AAQ16190); EthA: E. tarda hemolysin (BAA21097); EtpA, enterotoxigenic E. coli adhesin (AAX13509); F11, E. coli F11 (ZP_00726065); FHA, B. pertussis filamentous hemagglutinin (P12255); HecA, Erwinia chrysanthemi adhesin (AAN38708); HhdA, H. ducreyi hemolysin (AAC43538); H. inf 1, H. influenzae HMW1 (AAS77299); H. inf 2, H. influenzae 86-028NP (YP_249393); HMW1A and HMW2A, H. influenzae adhesins (AAA20527 and AAA20524); HpmA, P. mirabilis hemolysin (P16466); PhlA, P. luminescens hemolysin (CAD18998); RB50, Bordetella bronchiseptica RB50 (NP_888481); ShlA, S. marcescens hemolysin (P15320); Tohama I, Bordetella pertussis Tohama I (CAE42943); UTI89, E. coli UTI189 (YP_543880); XhlA, Xenorhabdus nematophila (AAV33651); YhlA, Yersinia ruckeri hemolysin (ABG49104).
Open reading frame Z1543 is predicted to encode a ∼58 kDa TPS transporter/activator (14, 35). We have thus designated Z1543 as otpB and its gene product as OtpB. SIG-Pred and SignalP3.0 predict that the first 20 residues of OtpB constitute a typical signal peptide. Phylogenetic analysis indicated that OtpB is most closely related to the adhesin transporter FhaC (Fig. 3). OtpB and FhaC loosely cluster with the TPS hemolysin transporters, whereas the HMW1-like adhesin transporters form a completely separate cluster (Fig. 3B). Curiously, the adhesin transporter EtpB is more closely related to the hemolysin transporters than the HMW1-like adhesin transporters, and the hemolysin transporter HhdB does not cluster tightly with other hemolysin transporters (Fig. 3B). These observations suggest that the phylogenetic placement of a transporter does not necessarily reflect the function of its exoprotein partner. It is worth noting, however, that a pair of cysteine residues spaced 28 residues apart is found at the N terminus of OtpB (Fig. 3A). These cysteines are invariant in the hemolysin transporter/activator subclass of TPS transporters but are absent in the adhesin transporters (20).
FIG. 3.
Alignment of TPS transporters. (A) ClustalW alignment of TPS transporters. Residues shadowed in black match the consensus sequence. Asterisks indicate invariant cysteine residues in the hemolysin TPS transporters. (B) Phylogenetic tree showing the relationship between selected TPS transporters. The scale bar represents nucleotide substitutions (in hundreds). Dotted lines indicate negative branch lengths. Accession numbers of the following TPS transporters are in parentheses: ShlB, ShlA transporter (S. marcescens) (P15321); HpmB, HpmA transporter (P. mirabilis) (P16465); HhdB, HhdA transporter (H. ducreyi) (AAC43537); PhlB, PhlA transporter (P. luminescens) (CAD18997); FhaC, FHA transporter (B. pertussis) (P35077); HMW1B and HMW2B, HMW1 and HMW2 transporters (H. influenzae) (AAX88734 and AAX88268); EtpB, EtpA transporter (enterotoxigenic E. coli) (AAX13508).
Localization of OtpA and OtpB.
Based on our sequence analysis, we expected that OtpA and OtpB would localize to the secreted fraction and OM, respectively. To test this prediction, cellular and secreted fractions of EDL933 cultures were initially analyzed by Western blotting using antisera generated against OtpA N-terminal and C-terminal peptides. Both antisera detected a faint ∼130-kDa protein that was found exclusively in the secreted fraction (Fig. 4A, lane 4). This protein was absent from the secreted fraction of the E. coli K-12 strain MC4100, which does not encode OtpA (Fig. 4A, lanes 1 and 2). A strong band of ∼100 kDa was detected with the N-terminal antiserum, but analysis by mass spectrometry showed that this band corresponds to the passenger domain of the autotransporter EspP, a relatively abundant secreted protein (41) that presumably contains a cross-reactive epitope or binds the antibodies nonspecifically (data not shown). These results strongly suggest that OtpA is produced at a very low level under our experimental conditions and secreted into the medium as a full-length protein. Surprisingly, the predicted OtpB protein could not be detected with an anti-OtpB antipeptide antiserum (data not shown). Our inability to detect OtpB was not due to the nonreactivity of the antiserum (see below) but presumably resulted from the weak expression of the otpAB locus. We were unable to increase the expression of otpAB by altering the pH, oxygen tension, or composition of the culture medium or by incubating EDL933 with either HEp-2 or Caco-2 cells (data not shown).
FIG. 4.
Localization of OtpA and OtpB. (A) MC4100 and EDL933 cultures were divided into cellular (cell) and secreted (S) fractions, and each fraction was then analyzed by Western blotting using antisera raised against OtpA N-terminal (N-term.) and C-terminal (C-term.) peptides. Each lane contained 10 μg of protein. (B) AD202 and EDL933 transformed with pPC19 (Ptrc-otpAB) were incubated with IPTG and divided into secreted and subcellular fractions. Fractions were then analyzed by Western blotting using antisera raised against OtpA (N-terminal) and OtpB peptides. Each lane contained 4 μg of protein. C, cytoplasm; I, inner membrane; P, periplasm; O, OM; S, secreted; m, molecular weight markers.
To more easily determine the location of OtpA and OtpB, both EDL933 and an E. coli K-12 strain (AD202) were transformed with a multicopy plasmid (pPC19) that contains the otpAB locus under the control of an IPTG-inducible promoter. Cells were harvested after a 5-min incubation in the presence of 100 μM IPTG and fractionated, and the individual fractions were subjected to Western blot analysis. A ∼130-kDa protein that was localized predominantly to the secreted fraction of both strains was observed on blots that were probed with the N-terminal anti-OtpA antiserum (Fig. 4B, lanes 5 and 11). Presumably as a result of OtpA overproduction, a small amount of the protein was trapped in the cytoplasm in EDL933 (Fig. 4B, lane 7). Furthermore, a protein of the predicted molecular weight of OtpB (∼58 kDa) was detected almost exclusively in the OM fraction in both strains using the anti-OtpB antiserum (Fig. 4B, lanes 4 and 10). These results provide direct evidence that OtpA is a secreted protein and that OtpB is an OM protein.
OtpB is required for the secretion of OtpA.
The sequence and location of OtpA and OtpB suggest that they constitute a TPS system. To test this hypothesis, an EDL933 derivative (PC53) in which otpB is disrupted by kan was created using the Lambda red system. Proper integration of kan into otpB was verified by amplifying the otpB locus by PCR and analyzing the PCR product by restriction digest (data not shown). When PC53 cultures were analyzed by Western blotting using either the N- or C-terminal anti-OtpA antiserum, the ∼130-kDa band observed in the secreted fraction of the parent otpB+ strain was no longer detected (Fig. 5A). Since the disruption of otpB should not affect the expression of the upstream otpA gene, the complete absence of OtpA is presumably due to the instability of the protein that is trapped in the periplasm. These results provided the first indication that OtpB is required for the secretion of OtpA.
FIG. 5.
OtpA secretion requires the synthesis of OtpB. OtpA and OtpB were detected by Western blotting with antipeptide antisera. (A) EDL933 and PC53 cultures were divided into cellular (cell) and secreted (S) fractions, and each fraction was then analyzed by Western blotting using antisera raised against OtpA N-terminal (N term.) and C-terminal (C term.) peptides. Each lane contained 10 μg of protein. (B) HDB114 transformed with pPC20 (Ptrc-otpA) and pPC11 (PBAD-otpB) were incubated with the indicated inducer(s) and harvested at various times postinduction. Cellular and secreted fractions were then analyzed by Western blotting using antisera raised against OtpA (N-terminal) and OtpB peptides. (C) The experiment from panel B was repeated except that cells were transformed with pPC69 [PBAD-otpA(N)] and pPC11 (PBAD-otpB). m, molecular weight markers.
To obtain additional evidence that OtpA and OtpB function as a TPS system, the two proteins were produced independently. HDB114 (AD202 ara+) was transformed with pPC20, which encodes otpA under the control of an IPTG-inducible promoter, and pPC11, which encodes otpB under the control of an arabinose-inducible promoter. Cultures were incubated with one or both inducers for various amounts of time, and cells were harvested by centrifugation. The cell pellets and culture supernatants were then probed by Western blotting with anti-OtpA and anti-OtpB antisera. When cultures were supplemented with IPTG only, OtpA was restricted to the cellular fraction even after a 30-min induction (Fig. 5B, top). However, when cultures were supplemented with both IPTG and arabinose, OtpA was found in the extracellular environment within 10 min, and the fraction of the protein that was secreted increased over time (Fig. 5B, middle). As expected, OtpB was found only in the cellular fraction (Fig. 5B, bottom). The prominent ∼62-kDa band that was detected by the anti-OtpB antiserum is a cross-reactive cellular protein (data not shown). The lag in OtpA secretion correlated with a delay in the synthesis of OtpB after the addition of arabinose. These results not only demonstrate that OtpB is required for the secretion of OtpA but also show that coordinate transcription of otpA and otpB is not required for OtpA secretion.
Based on the homology of the N terminus of OtpA to the TPS domains of other exoproteins, we predicted that this segment would be sufficient to facilitate secretion. To test this hypothesis, we transformed HDB114 with pPC11 and pPC69, a plasmid that encodes the first 350 residues of OtpA [OtpA(N)] under the control of an IPTG-inducible promoter. As expected, an ∼33-kDa fragment corresponding to OtpA(N) appeared in the culture medium when arabinose was added to induce synthesis of OtpB but was restricted to the cellular fraction in the absence of arabinose (Fig. 5C). These results demonstrate that the N terminus of OtpA functions as a bona fide TPS domain and corroborate the conclusion that OtpA and OtpB constitute a TPS system.
Kinetic analysis of OtpA secretion.
To obtain additional evidence that OtpB facilitates OtpA secretion as well as to gain insight into the mechanism of protein secretion via the TPS pathway, we next used pulse-chase experiments to monitor OtpA biogenesis in the presence and absence of OtpB. AD202 transformed with pPC19 or pPC20 was subjected to pulse-chase labeling after the addition of 100 μM IPTG. Cells were separated from the culture medium by centrifugation and divided in half, and one portion was treated with PK. OtpA and OtpB were then immunoprecipitated from each sample with antipeptide antisera generated against the N terminus of each protein. In cells that produced both OtpA and OtpB, roughly half of the OtpA reached the cell surface during the 30-s pulse-labeling period (Fig. 6A, top, lanes 1 to 3). A small fraction (∼10 to 20%) of the protein was present in the culture medium. In addition, about 40% of the OtpA was cell-associated but reduced in size by PK to an ∼100-kDa N-terminal fragment (Fig. 6A, top, lane 2). Much smaller amounts of this fragment appeared to be present throughout the time course in both protease-treated and untreated samples. Because this fragment was virtually absent when OtpA was produced without OtpB (Fig. 6B), it likely represents a population of secreted OtpA molecules whose N terminus is resistant to digestion by either PK or cellular proteases that contaminate the medium. Within 2 min, most of the OtpA was translocated across the OM (Fig. 6A, top, lanes 4 to 6). More than half of the OtpA was released into the culture medium, and much of the cell-associated protein was completely degraded by PK. For reasons that are unclear, a small fraction of the OtpA was resistant to protease digestion even after a 10-min chase and presumably remained trapped in an intracellular compartment (Fig. 6A, top, lanes 10 to 12). As expected, all of the OtpB was cell-associated (Fig. 6A, bottom). Furthermore, OtpB was completely resistant to protease digestion either because it folds tightly or does not have any accessible extracellular loops. In contrast, in the absence of OtpB, all of the OtpA remained cell-associated and was insensitive to protease digestion (Fig. 6B). Taken together, these results not only show that OtpB is essential for OtpA secretion but also demonstrate that OtpA is transported into the extracellular space very soon after its synthesis.
FIG. 6.
Kinetic analysis of OtpA secretion. AD202 transformed with pPC19 (Ptrc-otpAB) (A), pPC20 (Ptrc-otpA) (B), or pPC11 (PBAD-otpB) and pPC69 [PBAD-otpA(N)] (C) were subjected to pulse-chase labeling after the addition of IPTG. Radiolabeled samples were divided into cellular (cell) and secreted (S) fractions. Half of the cellular fraction was treated with PK. Immunoprecipitations were then performed using antisera raised against OtpA (N-terminal) and OtpB peptides. Because the entire secreted fraction was used in each immunoprecipitation, the secreted fractions were derived from twice as much culture volume as the cellular fractions. The length of the chase is indicated. m, molecular weight markers.
Pulse-chase experiments in which otpA(N) and otpB were coexpressed in HDB114 showed that the N-terminal fragment of OtpA was secreted at least as rapidly as the full-length protein. Remarkably, the rate-limiting step in the secretion of OtpA(N) appeared to be the translocation of the protein across the inner membrane. About half of the pulse-labeled OtpA(N) was isolated as a preprotein and presumably still resided in the cytoplasm (Fig. 6C, lanes 1 to 3). All of the mature form of the protein that was generated during the pulse-labeling period, however, was either present in the culture medium or was sensitive to PK digestion and therefore was already in the extracellular space. Within 2 min, all of the residual pre-OtpA(N) was converted to mature OtpA(N) and was translocated across the OM (Fig. 6C, lanes 4 to 6).
OtpA does not display adhesive or hemolytic activity.
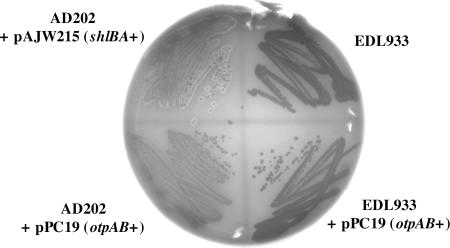
A variety of TPS exoproteins have been shown to lyse red blood cells when they are secreted either from their native hosts or from E. coli K-12 strains (3, 4, 16, 28, 40). In each case the synthesis of the TPS exoprotein and its cognate transporter is sufficient to produce hemolytic activity in E. coli K-12; no other factors from the native organism are required. To test whether OtpA has hemolytic activity, we streaked EDL933, EDL933 containing pPC19, or AD202 containing pPC19 on tryptic soy agar containing 5% sheep's blood and 100 μm IPTG. As a positive control, AD202 containing pAJW215, a plasmid that encodes the S. marcescens shlBA locus under the control of the trc promoter, was also plated in parallel. Consistent with previous results (27), a zone of hemolysis was observed around AD202 that secreted ShlA (Fig. 7). In contrast, no hemolytic activity was observed when OtpA was secreted from either EDL933 or AD202. Hemolytic activity was also not observed in cells that expressed both otpAB and Z1544, a gene that likely resides in the otpAB operon and that encodes a putative acyl-carrier protein synthase (Fig. 1 and data not shown). These results corroborate our sequence analysis and suggest that OtpA either is not a hemolysin or encodes a cryptic hemolytic activity that cannot be observed under standard assay conditions.
FIG. 7.
Assay for OtpA hemolytic activity. AD202 or EDL933 containing the indicated plasmid was streaked on tryptic soy agar plates supplemented with 5% sheep blood and 100 μM IPTG and incubated at 37°C.
The possibility that OtpA functions as an adhesin was also tested by monitoring bacterial adherence to HEp-2 cells and Caco-2 colorectal epithelial cells using a standard adherence assay and microscopic visualization of Giemsa-stained monolayers after bacterial infection. Synthesis of OtpA and OtpB did not increase the adherence of AD202 to either cell line (data not shown). Thus, OtpA did not function as an adhesin under our experimental conditions.
DISCUSSION
The results described in this study provide direct evidence that the previously uncharacterized E. coli O157:H7 otpAB locus encodes a TPS system. We found that disruption of the otpB gene in the native host abolished secretion of OtpA into the extracellular medium. Moreover, experiments in which recombinant otpA and otpB were expressed independently showed conclusively that OtpA secretion is strictly dependent on OtpB in both E. coli O157:H7 and E. coli K-12. Our results do not completely rule out the possibility that OtpB is merely a cofactor that promotes the secretion of OtpA by another unidentified transporter. The sequence similarity of OtpA and OtpB to components of known TPS systems and the finding that no E. coli O157:H7 proteins other than OtpB are required for OtpA secretion in E. coli K-12, however, strongly suggest that OtpB does indeed function as the OtpA translocase.
In the present study we also report the first detailed kinetic analysis of TPS secretion. Although OtpA secretion very likely requires both the prior integration of OtpB into the OM and the targeting of OtpA to the translocase, we found that a large fraction of OtpA emerges in the extracellular milieu within seconds of its synthesis in the cytoplasm. Because the assembly of OMPs into oligomeric complexes is often a slow process (32), the data raise the possibility that OtpB is monomeric like ShlB (22). The observation that OtpA secretion continues for ∼2 to 5 min after synthesis suggests that OtpA can exist as a secretion-competent intermediate in the cytoplasm or periplasmic space. Because a small fraction of the OtpA was not secreted even after a 10-min chase, however, it appears that the protein eventually adopts a secretion-incompetent conformation in the heterologous host. Indeed, a partial defect in exoprotein secretion has been observed in other studies in which TPS systems were reconstituted in E. coli K-12 (12, 33). Unexpectedly, our kinetic analysis also revealed the existence of an ∼100-kDa N-terminal fragment of the secreted form of OtpA that is resistant to PK digestion. It should be intriguing to determine whether this fragment represents a discrete folded domain of OtpA.
Although our results indicate that OtpA/OtpB constitute a bona fide TPS system, we also obtained evidence that OtpA has unique functional properties. Consistent with our sequence analysis, which showed that OtpA is only weakly related to the hemolysin family of exoproteins and lacks the signature CXXC motif, OtpA did not lyse red blood cells in a standard hemolysis assay. Indeed, OtpA has especially limited homology to the C-terminal portion of the hemolysins, which is required for hemolytic activity (27, 33). The results imply that if OtpA is a hemolysin, it must either function under very different conditions than other TPS hemolysins characterized to date or require an unidentified cofactor or modification. As previously suggested (14), the cluster of fatty acid modification genes directly downstream of otpAB might activate OtpA. Indeed, at least one E. coli hemolysin (HlyA) requires acylation for cytotoxic activity (48, 49), but it is not a TPS exoprotein, and its cognate acyltransferase is unrelated to proteins encoded in OI-47, in which otpAB resides.
OtpA also did not display adhesive activity in standard cell culture-based assays. This result was not surprising given the weak homology of OtpA to known adhesins and the absence of a glycosylase gene immediately downstream from otpAB. Expression of a linked glycosylase gene has been shown to be required for the adhesive activity of HMW1 proteins and EtpA (10, 11, 38). Interestingly, OI-47 encodes a putative fimbrial cluster similar to both the Salmonella long polar fimbriae and the E. coli type 1 fimbriae (35). Both types of fimbriae have been implicated in adhesion to mammalian tissues (9, 42, 43). It is conceivable that OtpA functions as a fimbrial adhesin (despite lacking homology to the previously characterized E. coli fimbrial adhesins FimH, FedF, and FaeG) or only exhibits adhesive activity when it is synthesized in conjunction with the fimbrial proteins. Although we found that the otpAB locus is weakly expressed under laboratory conditions, previous work has shown that the expression of the fimbrial gene cluster in OI-47 is also constitutively low regardless of variations in culture medium, pH, temperature, oxygen tension, or growth phase (23). Perhaps the expression of otpAB and the adjacent fimbrial operon is coordinately upregulated in response to specific events during infection, such as contact with specific cell types. In any case, the fact that OI-47 is found almost exclusively in E. coli O157 serotypes associated with hemolytic uremic syndrome raises the possibility that the otpAB plays a role in virulence (35).
Given that we could not observe a hemolytic activity associated with OtpA, the identification of highly conserved cysteine residues that are a hallmark of hemolysin transporters in OtpB is somewhat puzzling. This observation suggests that sequence features of TPS transporters do not necessarily specify the function of their cognate exoproteins. It is conceivable that common sequence motifs in transporters simply reflect common biochemical or structural properties of exoproteins that have not yet been recognized. The mechanism of secretion via the TPS pathway is still very poorly understood, and the functional significance of sequence elements found in distinct subsets of TPS transporters may become more apparent as new insights emerge and additional members of the TPS superfamily are characterized.
Acknowledgments
We thank Susan Buchanan for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published ahead of print on 23 February 2007.
REFERENCES
- 1.Akiyama, Y., and K. Ito. 1990. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the ompT protease in vitro. Biochem. Biophys. Res. Commun. 167:711-715. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, S. K., R. Pamma, A. D. Hernday, J. E. Bickham, B. A. Braaten, and D. A. Low. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245-1248. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V., B. Neuss, Y. Ruan, E. Schiebel, H. Schoffler, and G. Jander. 1987. Identification of the Serratia marcescens hemolysin determinant by cloning into Escherichia coli. J. Bacteriol. 169:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillard, J., E. Duchaud, N. Boemare, F. Kunst, and A. Givaudan. 2002. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. J. Bacteriol. 184:3871-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, N. F., C. A. Logue, J. A. Boddey, R. Scott, R. G. Hirst, and I. R. Beacham. 2004. Identification of a novel two-partner secretion system from Burkholderia pseudomallei. Mol. Genet. Genomics 272:204-215. [DOI] [PubMed] [Google Scholar]
- 6.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 7.Clantin, B., H. Hodak, E. Willery, C. Locht, F. Jacob-Dubuisson, and V. Villeret. 2004. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc. Natl. Acad. Sci. USA 101:6194-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doughty, S., J. Sloan, V. Bennett-Wood, M. Robertson, R. M. Robins-Browne, and E. L. Hartland. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 70:6761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleckenstein, J. M., K. Roy, J. F. Fischer, and M. Burkitt. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect. Immun. 74:2245-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grass, S., A. Z. Buscher, W. E. Swords, M. A. Apicella, S. J. Barenkamp, N. Ozchlewski, and J. W. St. Geme III. 2003. The Haemophilus influenzae HMW1 adhesin is glycosylated in a process that requires HMW1C and phosphoglucomutase, an enzyme involved in lipooligosaccharide biosynthesis. Mol. Microbiol. 48:737-751. [DOI] [PubMed] [Google Scholar]
- 12.Grass, S., and J. W. St. Geme III. 2000. Maturation and secretion of the non-typable Haemophilus influenzae HMW1 adhesin: roles of the N-terminal and C-terminal domains. Mol. Microbiol. 36:55-67. [DOI] [PubMed] [Google Scholar]
- 13.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 15.Hertle, R., S. Brutsche, W. Groeger, S. Hobbie, W. Koch, U. Konninger, and V. Braun. 1997. Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol. Microbiol. 26:853-865. [DOI] [PubMed] [Google Scholar]
- 16.Hirono, I., N. Tange, and T. Aoki. 1997. Iron-regulated haemolysin gene from Edwardsiella tarda. Mol. Microbiol. 24:851-856. [DOI] [PubMed] [Google Scholar]
- 17.Hodak, H., B. Clantin, E. Willery, V. Villeret, C. Locht, and F. Jacob-Dubuisson. 2006. Secretion signal of the filamentous haemagglutinin, a model two-partner secretion substrate. Mol. Microbiol. 61:368-382. [DOI] [PubMed] [Google Scholar]
- 18.Jacob-Dubuisson, F., C. Buisine, E. Willery, G. Renauld-Mongenie, and C. Locht. 1997. Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J. Bacteriol. 179:775-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob-Dubuisson, F., C. El-Hamel, N. Saint, S. Guedin, E. Willery, G. Molle, and C. Locht. 1999. Channel formation by FhaC, the outer membrane protein involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J. Biol. Chem. 274:37731-37735. [DOI] [PubMed] [Google Scholar]
- 20.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 21.Kajava, A. V., N. Cheng, R. Cleaver, M. Kessel, M. N. Simon, E. Willery, F. Jacob-Dubuisson, C. Locht, and A. C. Steven. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol. Microbiol. 42:279-292. [DOI] [PubMed] [Google Scholar]
- 22.Konninger, U. W., S. Hobbie, R. Benz, and V. Braun. 1999. The haemolysin-secreting ShlB protein of the outer membrane of Serratia marcescens: determination of surface-exposed residues and formation of ion-permeable pores by ShlB mutants in artificial lipid bilayer membranes. Mol. Microbiol. 32:1212-1225. [DOI] [PubMed] [Google Scholar]
- 23.Low, A. S., N. Holden, T. Rosser, A. J. Roe, C. Constantinidou, J. L. Hobman, D. G. Smith, J. C. Low, and D. L. Gally. 2006. Analysis of fimbrial gene clusters and their expression in enterohaemorrhagic Escherichia coli O157:H7. Env. Microbiol. 8:1033-1047. [DOI] [PubMed] [Google Scholar]
- 24.Meli, A. C., H. Hodak, B. Clantin, C. Locht, G. Molle, F. Jacob-Dubuisson, and N. Saint. 2006. Channel properties of TpsB transporter FhaC point to two functional domains with a C-terminal protein-conducting pore. J. Biol. Chem. 281:158-166. [DOI] [PubMed] [Google Scholar]
- 25.Molina, M. A., J. L. Ramos, and M. Espinosa-Urgel. 2006. A two-partner secretion system is involved in seed and root colonization and iron uptake by Pseudomonas putida KT2440. Env. Microbiol. 8:639-647. [DOI] [PubMed] [Google Scholar]
- 26.Newitt, J. A., and H. D. Bernstein. 1998. A mutation in the Escherichia coli secY gene that produces distinct effects on inner membrane protein insertion and protein export. J. Biol. Chem. 273:12451-12456. [DOI] [PubMed] [Google Scholar]
- 27.Ondraczek, R., S. Hobbie, and V. Braun. 1992. In vitro activation of the Serratia marcescens hemolysin through modification and complementation. J. Bacteriol. 174:5086-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the hemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 29.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 30.Qi, H. Y., J. B. Hyndman, and H. D. Bernstein. 2002. DnaK promotes the selective export of outer membrane protein precursors in SecA-deficient Escherichia coli. J. Biol. Chem. 277:51077-51083. [DOI] [PubMed] [Google Scholar]
- 31.Renauld-Mongenie, G., J. Cornette, N. Mielcarek, F. D. Menozzi, and C. Locht. 1996. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J. Bacteriol. 178:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouviere, P. E., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 33.Schiebel, E., H. Schwarz, and V. Braun. 1989. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J. Biol. Chem. 264:16311-16320. [PubMed] [Google Scholar]
- 34.Schonherr, R., R. Tsolis, T. Focareta, and V. Braun. 1993. Amino acid replacements in the Serratia marcescens haemolysin ShIA define sites involved in activation and secretion. Mol. Microbiol. 9:1229-1237. [DOI] [PubMed] [Google Scholar]
- 35.Shen, S., M. Mascarenhas, R. Morgan, K. Rahn, and M. A. Karmali. 2005. Identification of four fimbria-encoding genomic islands that are highly specific for verocytotoxin-producing Escherichia coli serotype O157 strains. J. Clin. Microbiol. 43:3840-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St. Geme, J. W., III. 1994. The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect. Immun. 62:3881-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St. Geme, J. W., III, S. Falkow, and S. J. Barenkamp. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. USA 90:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St. Geme, J. W., III, and S. Grass. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol. Microbiol. 27:617-630. [DOI] [PubMed] [Google Scholar]
- 39.Surana, N. K., S. Grass, G. G. Hardy, H. Li, D. G. Thanassi, and J. W. Geme III. 2004. Evidence for conservation of architecture and physical properties of Omp85-like proteins throughout evolution. Proc. Natl. Acad. Sci. USA 101:14497-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swihart, K. G., and R. A. Welch. 1990. Cytotoxic activity of the Proteus hemolysin HpmA. Infect. Immun. 58:1861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabady, R. L., J. H. Peterson, K. M. Skillman, and H. D. Bernstein. 2005. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc. Natl. Acad. Sci. USA 102:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres, A. G., J. A. Giron, N. T. Perna, V. Burland, F. R. Blattner, F. Avelino-Flores, and J. B. Kaper. 2002. Identification and characterization of lpfABCC′DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:5416-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres, A. G., K. J. Kanack, C. B. Tutt, V. Popov, and J. B. Kaper. 2004. Characterization of the second long polar (LP) fimbriae of Escherichia coli O157:H7 and distribution of LP fimbriae in other pathogenic E. coli strains. FEMS. Microbiol. Lett. 238:333-344. [DOI] [PubMed] [Google Scholar]
- 44.Ulbrandt, N. D., J. A. Newitt, and H. D. Bernstein. 1997. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88:187-196. [DOI] [PubMed] [Google Scholar]
- 45.Uphoff, T. S., and R. A. Welch. 1990. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172:1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urisu, A., J. L. Cowell, and C. R. Manclark. 1986. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect. Immun. 52:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker, G., R. Hertle, and V. Braun. 2004. Activation of Serratia marcescens hemolysin through a conformational change. Infect. Immun. 72:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Worsham, L. M., K. G. Langston, and M. L. Ernst-Fonberg. 2005. Thermodynamics of a protein acylation: activation of Escherichia coli hemolysin toxin. Biochemistry 44:1329-1337. [DOI] [PubMed] [Google Scholar]
- 49.Worsham, L. M., M. S. Trent, L. Earls, C. Jolly, and M. L. Ernst-Fonberg. 2001. Insights into the catalytic mechanism of HlyC, the internal protein acyltransferase that activates Escherichia coli hemolysin toxin. Biochemistry 40:13607-13616. [DOI] [PubMed] [Google Scholar]