Abstract
Extracellular polysaccharides of many bacteria are synthesized by the Wzy polymerase-dependent mechanism, where long-chain polymers are assembled from undecaprenyl-phosphate-linked repeat units on the outer face of the cytoplasmic membrane. In gram-positive bacteria, Wzy-dependent capsules remain largely cell associated via membrane and peptidoglycan linkages. Like many Wzy-dependent capsules, the Streptococcus pneumoniae serotype 2 capsule is branched. In this study, we found that deletions of cps2K, cps2J, or cps2H, which encode a UDP-glucose dehydrogenase necessary for side chain synthesis, the putative Wzx transporter (flippase), and the putative Wzy polymerase, respectively, were obtained only in the presence of suppressor mutations. Most of the suppressor mutations were in cps2E, which encodes the initiating glycosyltransferase for capsule synthesis. The cps2K mutants containing the suppressor mutations produced low levels of high-molecular-weight polymer that was detected only in membrane fractions. cps2K-repaired mutants exhibited only modest increases in capsule production due to the effect of the secondary mutation, but capsule was detectable in both membrane and cell wall fractions. Lethality of the cps2K, cps2J, and cps2H mutations was likely due to sequestration of undecaprenyl-phosphate in the capsule pathway and either preclusion of its turnover for utilization in essential pathways or destabilization of the membrane due to an accumulation of lipid-linked intermediates. The results demonstrate that proper polymer assembly requires not only a functional transporter and polymerase but also complete repeat units. A central role for the initiating glycosyltransferase in controlling capsule synthesis is also suggested.
The capsular polysaccharides of Streptococcus pneumoniae are essential for virulence of this organism. In systemic infections, such as pneumonia and bacteremia, high levels of capsule are necessary to impede complement-mediated opsonophagocytosis (1, 31, 69), whereas in colonization, reduced amounts of capsule may be sufficient, as surface adhesins must be exposed (20, 39, 51, 58, 63). The 90 described S. pneumoniae serotypes vary in their sugar compositions, linkages, and branching patterns (8, 32, 57). Most S. pneumoniae capsules consist of repeating subunits that are synthesized by the Wzy-dependent mechanism, which is also used to synthesize capsules and exopolysaccharides in many other streptococci, lactococci, and staphylococci, as well as in gram-negative bacteria expressing group 1 capsules and lipopolysaccharide (LPS) O antigens (8, 18, 36, 48, 67, 71). In this mechanism, repeat unit synthesis is initiated by transfer of a sugar-phosphate to a lipid acceptor on the cytoplasmic face of the membrane, with subsequent addition of the remaining sugars to complete the subunit. In most S. pneumoniae serotypes, CpsE homologues catalyze the initiation step by transferring glucose-1-phosphate (Glc-1-P) to a polyprenol acceptor (15, 37, 47, 60), while unique glycosyltransferases catalyze each subsequent monosaccharide addition. The final subunit is translocated across the cytoplasmic membrane by a Wzx flippase, and the Wzy polymerase then links the repeat units into long-chain polymers, with growth occurring at the reducing end of the polysaccharide (50). In gram-negative bacteria, the capsule is ultimately transported and linked to the outer face of the outer membrane (67). In gram-positive bacteria, some or all of the polymer may be linked to the peptidoglycan (6, 17, 21, 54, 65), with the remainder being membrane associated (6). Modulation of capsule chain length and amount occurs, at least in part, through the action of a phosphoregulatory system that includes an autophosphorylating tyrosine kinase (6, 7, 44-46, 70). In S. pneumoniae, CpsC and CpsD represent the membrane-associated activation domain and cytoplasm-associated ATPase domain, respectively, of this kinase. CpsB is a phosphotyrosine phosphatase and kinase inhibitor that affects the level of CpsD phosphorylation (7, 41).
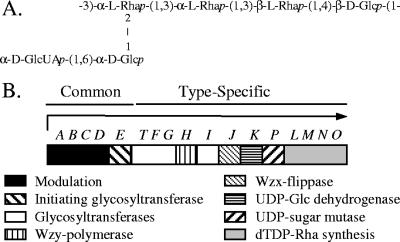
Although a general picture of capsule synthesis in gram-positive bacteria has emerged, much remains to be learned about specific aspects of this process. As a model system, we have used the S. pneumoniae serotype 2 capsule, in which the repeat unit contains a backbone of Glc-Rha-Rha-Rha and a side chain of Glc-GlcUA (Fig. 1A). As for all S. pneumoniae capsules assembled by the Wzy-dependent mechanism, the type 2 genetic locus exhibits a cassette-like arrangement, where genes unique to a specific serotype and essential for the biosynthesis of type-specific sugars, polymerases, and transporters are flanked by homologous sequences common to all serotypes (Fig. 1B) (3, 23, 30, 34). Putative roles for the type 2-specific genes have been assigned based on homology (34), but their functions have not been experimentally determined.
FIG. 1.
Type 2 capsule structure and genetic locus. (A) Structure of the repeat unit. Synthesis of the backbone initiates by addition of Glc-1-P to a polyprenol acceptor. (B) Genetic organization of the type 2 capsule locus (34). S. pneumoniae capsule loci are flanked by dexB upstream and aliA (also referred to as plpA) downstream. The arrow indicates the putative transcript containing cps2A to cps2O.
The type-specific gene cps2K is predicted to encode a UDP-glucose dehydrogenase (UDP-GlcDH) (34), which catalyzes the NAD+-dependent oxidation of UDP-Glc to UDP-glucuronic acid (UDP-GlcUA). Cps2K contains the same strictly conserved active site signature sequence of GGXCXXXD, as well as extensive homology to the signature NAD+- and UDP-sugar binding domains, found in other UDP-GlcDHs (14). UDP-GlcDHs play critical roles in the formation of many microbial capsules, including those of Streptococcus pyogenes (25, 66), Escherichia coli K5 (52), Cryptococcus neoformans (29), and many S. pneumoniae serotypes (57), as well as mammalian polymers such as hyaluronan, chondroitin sulfate, and heparan sulfate. In many of these polymers, GlcUA is part of the backbone structure, and for capsules such as type 3 in S. pneumoniae, mutations affecting the synthesis of UDP-GlcUA have severe effects on polysaccharide production and virulence (22, 31, 61). Less is known about the effects of eliminating GlcUA or other sugars from the side chains of microbial capsules. The C. neoformans capsule contains a side chain of GlcUA and xylose, both of which are derived from UDP-GlcUA. In mutants lacking UDP-Glc DH activity, capsule production appears to be completely eliminated (29). In Streptococcus agalactiae (group B streptococcus), mutants that fail to make the terminal sialic acid of the type III capsule side chain, due to mutation of either the CMP-sialic acid synthetase or the sialyltransferase, continue to produce an apparently normal capsule, albeit at greatly reduced levels (17, 65).
In the present study, we examined the role of the side chain, and specifically the terminal GlcUA residue, in production of the S. pneumoniae type 2 capsule. Our results demonstrate that this residue is essential for proper assembly and processing of the capsule, and the inability to synthesize or process a complete repeat unit is detrimental to the cell, due at least in part to failure to transfer the polymer to the cell wall.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Strains and plasmids used are listed in Table 1. S. pneumoniae strains were grown at 37°C in THY (Todd-Hewitt broth supplemented with 0.5% yeast extract [Difco]), on BBL plates (Difco), or on blood agar plates (Blood Agar Base no. 2; Remel) containing 3% defibrinated sheep blood (Colorado Serum Company). Broth cultures were grown standing in a water bath or incubator; agar plate cultures were incubated in candle jars. E. coli DH5αF′, TOP10, and M15(pREP4) were grown in L broth (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl, and 1 g/liter glucose) or L agar (L broth containing 15 g/liter Bacto agar). Media were supplemented with the following antibiotics when appropriate: erythromycin (Em) (0.3 μg/ml for S. pneumoniae and 300 μg/ml for E. coli), ampicillin (100 μg/ml), or kanamycin (Km) (250 μg/ml for S. pneumoniae and 50 μg/ml for E. coli).
TABLE 1.
Strains and plasmids used in this study
| Strain(s) or plasmid(s) | Propertiesa | Reference or source |
|---|---|---|
| S. pneumoniae strains | ||
| AM1000 | Δ[cps2A to cps2H]; type 2 Cps− | 39 |
| BX505 | pBX110 × D39, Δcps2H, Cps−; cps2E5765delT (L244* premature stop) | This study |
| BX511 | pBX113 × D39, Δcps2K, Cpsr; cps2E5953G→T (G303V) | This study |
| BX512 | pBX113 × D39, Δcps2K, Cpsr; cps2E5920G→A (G292R) | This study |
| BX513 | pBX116 × BX511, cps3D repair of Δcps2K, Cpsr | This study |
| BX515 | pBX115 × BX511, cps2K repair, Cpsr | This study |
| BX516 | pJD377 × D39, Em marker insertion downstream of capsule locus, Cps+ | This study |
| BX518 | pBX115 × BX512, cps2K repair, Cpsr | This study |
| BX519 | BX516 × BX511, Emr, capsule replacement in BX511, Cps+ | This study |
| BX522 | BX516 × BX512, Emr; capsule replacement in BX511, Cps+ | |
| BX532, BX539 | pBX113 × D39, independent Δcps2K derivatives; Cpsr; cps2E6132C→A (P363T) | This study |
| BX533 | pBX113 × D39, Δcps2K, Cpsr; cps2E6319A→G (D425G) | This study |
| BX535 | pBX123 × D39, Em insertion upstream of capsule locus promoter, Cps+ | This study |
| BX540 | pBX115 × BX533, cps2K repair, Cpsr | This study |
| BX544 | pBX145 × BX518, cps2E repair in BX518, Cps+ | This study |
| BX545 | pBX145 × BX515, cps2E repair in BX515, Cps+ | This study |
| BX547, BX548, BX549 | pBX113 × D39, Δcps2K, Cpsr; independent derivatives with respective cps2E mutations 5544G→T (D167Y), 6350G→T (W435C), and 6015delA (L369* premature stop) | This study |
| BX550 | pBX113 × D39, Δcps2K, Cpsr; unknown suppressor mutation | This study |
| BX551 | pBX113 × D39, Δcps2K, Cpsr; A-to-G transition 4 bp downstream of −10 sequence of capsule promoter | This study |
| BX552 | pBX110 × D39, Δcps2H, Cps−; cps2E5539C→T (L199F) | This study |
| BX554, BX555 | pBX113 × D39, Δcps2K, Cpsr; independent derivatives with respective cps2E mutations 6198A→G (E385G) and 6201T→C (Y386H) | This study |
| BX556 | pBX113 × D39, Δcps2K, Cpsr; 1-kb vector insertion in cps2L region | This study |
| BX605-BX607, BX609-BX612 | pBX113 × D39, Δcps2K, Cpsr; independent derivatives with respective cps2E mutations 5632T→G (V196G), 5607insT (E191* premature stop), 6178C→G (T378R), 5984G→C (K312N), 6178C→G (T378R), 6349G→A (W407*stop), and 5539T→G (V165G) | This study |
| BX635 | pBX113 × D39, Δcps2K, Cpsr; cps2E6060T→C (F339L) | This study |
| BX667-BX669 | pBX190 × D39, Δcps2J, Cps−; independent derivatives with respective cps2E mutations 6276G→A (G411R), 5839T→G (I265S), and 6157G→C (G371A) | This study |
| D39 | Type 2 parent strain, Cps+ | 5 |
| KA1521 | Δcps2E, type 2, Cps− | 15 |
| WU2 | Type 3 parent strain, Cps+ | 11 |
| E. coli strains | ||
| BX163 | M15(pREP4, pBX163) | This study |
| BX165 | M15(pREP4, pBX165) | This study |
| DH5αF′ | F′ φ80lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Life Technologies, Inc. |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| M15(pREP4) | F− Nals Strs Rifs Thi− Lac− Ara+ Gal+ Mtl− RecA+ Uvr+ Lon+ | QIAGEN |
| Plasmids | ||
| pCR 2.1 TOPO | PCR cloning vector; Apr Kmr | Invitrogen |
| pJY4164 | S. pneumoniae suicide vector; Emr | 72 |
| pJD377 | pJY4164::type 3 plpA/tnpA region | 23 |
| pBX105 | pJY4164 plus PCR fragments from primer pairs Cps2-G0/Cps2-G2 and Cps2-I2/Cps2-I3, for cps2H deletion | This study |
| pBX108 | pJY4164 plus PCR fragments from primer pairs Cps2-J12303/Cps2-J2 and Cps2-P1/Cps2-P15468, for cps2K deletion | This study |
| pBX110 | Km resistance gene, aphA-3, between two fragments of pBX105 | This study |
| pBX113 | Km resistance gene, aphA-3, between two fragments of pBX108 | This study |
| pBX115, pBX121 | pJY4164 plus PCR fragment from primer pair Cps2-J1/Cps2-P2, for repair of cps2K deletion | This study |
| pBX116 | pJY4164 plus PCR fragment from primer pair Cps3D-F/Cps3D-R, for cps3D repair of cps2K deletion | This study |
| pBX123 | pJY4164 plus 900-bp fragment excised with EcoRI from pCV646 | This study |
| pBX145 | pJY4164 plus PCR fragment from primer pair Cps2-D3/Cps2-T1, for repair of cps2E | This study |
| pBX163 | pQE-40 containing the full-length cps2K lacking GTG start; from primer pair Cps2KORF-BglII/Cps2KORF-KpnI | This study |
| pBX165 | pQE-40 lacking dihydrofolate reductase region (BamHI-KpnI, deletion) | This study |
| pBX190 | pJY4164 plus PCR fragments from primer pairs Cps2-I4/Cps2-J8 and Cps2-J7/Cps2-K1, for deletion of cps2K; aphA-3 excised from pBX113 and inserted between two fragments | This study |
| pCV646 | pGEM cloning vector plus PCR fragment from c-ups1/c-ups2; Apr | This study |
| pQE40 | Expression vector, N-terminal His6 tag; Kmr | QIAGEN |
| pSF151 | Streptococcal shuttle vector containing the Km resistance gene, aphA-3 | 56 |
Cpsr, reduced capsule levels. cps2E superscripts indicate mutations and their locations based on GenBank accession no. AF026471. Amino acid changes are indicated in parentheses. del, deletion; ins, insertion; →, nucleotide change.
Expression and purification of Cps2K.
The open reading frame (ORF) of cps2K, minus the ribosome binding site and GTG start codon, was PCR amplified from S. pneumoniae D39 chromosomal DNA, using the primers Cps2KORF-BglII and Cps2KORF-KpnI, which incorporate a BglII site at the 5′ end of the PCR product and a KpnI site at the 3′ end, respectively. The fragment was cloned into the expression vector pQE-40, in which the dihydrofolate reductase region between the His6 tag and the mutlicloning site was excised using BamHI and KpnI. The resulting plasmid, pBX163, was electroporated into the E. coli expression strain M15(pREP4). Transformants were selected on L agar containing both Km and ampicillin.
For expression of recombinant Cps2K, a 100-ml culture of BX163 was grown from an overnight culture diluted 1:100 in L broth containing the appropriate antibiotics at 37°C with shaking to a cell density of ∼2 × 108 CFU/ml. Expression of cps2K was induced with isopropyl-thio-β-galactoside (IPTG) (0.8 mM final concentration) for 4 h at 37°C. Cultures were centrifuged at 20,000 × g for 10 min, and the pellet was stored overnight at −80°C until further purification. The pellet was resuspended in 4 ml of a phosphate buffer (50 mM sodium phosphate, 300 mM NaCl, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride) and treated with 1 mg/ml of lysozyme for 4 to 5 h at 4°C. The lysozyme-treated sample was sonicated using three 30-s bursts with a 2-min cooling time on ice in between each burst. Insoluble material was pelleted (20,000 × g for 10 min at 4°C) from this lysate, and the soluble His6-Cps2K was purified from the supernatant using Talon beads as per the manufacturer's protocol (BD Biosciences). Dithiothreitol (1 mM) was present throughout the entire purification procedure in order to stabilize the UDP-GlcDH (14, 53). The presence of the 44-kDa protein was confirmed by Coomassie blue staining of sodium dodecyl sulfate (SDS)-10% polyacrylamide gels in which the proteins from the lysates, supernatants, and His purification had been separated.
A UDP-GlcDH activity assay was done spectrophotometrically by following the accumulation of NADH at 340 nm, which results from the reduction of 2 mol of NAD+ for every mole of UDP-Glc oxidized (55). Briefly, 5 μl of the BX163 crude lysate or soluble fraction obtained as described above and containing 50 μg of total protein, or 50 μg of the His-purified protein from BX163, was added to 1 ml of a reaction mixture containing 100 mM Tris-HCl (pH 8.7), 10 mM MgCl2, 0.5 mM UDP-Glc, and 1 mM NAD+. The accumulation of NADH at room temperature was followed spectrophotometrically at 340 nm. Protein concentrations were determined using the Bio-Rad Bradford assay method. To determine the amount of NADH produced per minute, a standard curve was extrapolated from the absorbance of NADH standards (concentration range, 1 to 500 μM) at 340 nm. Purified bovine UDP-GlcDH (Sigma) was used as a positive control.
Plasmid and mutant constructions.
Primers used for the construction of plasmids and mutants are listed in Table 2. For an in-frame deletion of cps2K, the flanking regions were PCR amplified from D39 chromosomal DNA using primer pairs Cps2-J1/Cps2-J12303F and Cps2-P1/Cps2-P14568R. The two resulting PCR products were cloned separately into pCR 2.1-TOPO (Invitrogen) and transformed into TOP10 cells. Each cloned fragment was excised using EcoRI and KpnI and subcloned together into the S. pneumoniae suicide vector pJY4164, resulting in pBX108. The correct orientations of the inserts were confirmed by PCR and sequencing. The ORF of the Km resistance-encoding gene, aphA-3, was amplified from the pneumococcal shuttle vector pSF151 using the primer pair KM151-2/KM151-3, and the resulting PCR product was cloned into pCR 2.1-TOPO vector. pBX108 was partially digested with KpnI, and the aphA-3 fragment was excised from TOPO using KpnI and subsequently inserted between the two fragments in pBX108, resulting in pBX113. Correct orientation of all three inserted fragments was then confirmed by PCR and restriction digestions. pBX113 was transformed into competent D39, and Δcps2K mutants were selected by Km resistance and confirmed by PCR and sequencing. Constructions of in-frame deletions of cps2H and cps2J were performed as described for deletions of cps2K except that the primer pairs used were Cps2-G0/Cps2-G2 and Cps2-I2/Cps2-I3 for deletion of cps2H and Cps2-I4/Cps2-J8 and Cps2-J7/Cps2-K1 for deletion of cps2J.
TABLE 2.
Primers used in this study
| Primera | Sequenceb | Descriptionc |
|---|---|---|
| Cps2-D2 (+) | GGTTCTTATGGAGATTACGGGAA | cps2D4996-5018 |
| Cps2-D3 (+) | CTCACAGGCAAAATTGGATTTTG | cps2D4368-4390 |
| Cps2-E10 (+) | ATTTACTTCCTCACATTACATG | cps2E5343-5364 |
| Cps2-E11 (−) | AAACTACTTCGCTCCATCTCTC | cps2E6418-6396 |
| Cps2-T1 (−) | CTCATGACCATCTGGATTTAC | cps2T6449-6468 |
| Cps2-T2 (+) | TTATATCATTGGTTCAAAGGGG | cps2T6459-6481 |
| Cps2-G0 (+) | CAAGGACATGATGTGGTTTGTTA | cps2G8665-8688 |
| Cps2-G2 (−) | *CATTATAACTATCCATACTAATAA | cps2G9671-9647 |
| Cps2-I3 (−) | CACCTGAATTTGTCCCAATAAC | cps2I11906-11884 |
| Cps2-I2 (+) | *TAAAAATGGATGGGGAAATTCAA | cps2I10830-10854 |
| Cps2-I4 (+) | TTCGATAGTTGAGGATTCAGACTTT | cps2I11039-11065 |
| Cps2-J1 (+) | CTTGTAGTAAAATACTTGCTAAG | cps2J13034-13058 |
| Cps2-J12303F (+) | TTCTGAAGGGGTTCTTCGATTTGCA | cps2J12303-12327 |
| Cps2-J2 (−) | *CATTTTTCTCCTTTCAATACTCGT | cps2J13561-13527 |
| Cps2-J7 (+) | *TAAGAACCAATAAGTACGAGTAT | cps2K13522-13544 |
| Cps2-J8 (−) | *CAATTTTCTAGTTCCTTATATAGT | cps2J12116-12094 |
| Cps2-K1(−) | AACTACTCTTACTCCCTTAGCTTTTA | cps2K14627-14602 |
| Cps2KORF-BglII (+) | **AAAATAGCAGTAGCAGG | cps2K13564-13581 |
| Cps2KORF-KpnI (−) | *TTAATCTCTTTCAAAAATA | cps2K14798-14780 |
| Cps2-P1 (+) | *GAAAGAGATTAATTTAGTATATT | cps2P14787-14809 |
| Cps2-P15468R (−) | CTTCCTCTACTACACTAAGTATCC | cps2P15468-15445 |
| Cps2-P2 (−) | CATGCGTTATGACTGTCTTAGG | cps2P15283-15262 |
| Cps3D-F (+) | *GAGGACTGTAGTAAAAT | cps3D1012-1029 |
| Cps3D-R (−) | *CCCTTATTCTCTGCC | cps3D2215-2210 |
| CpsL-1 (+) | AGGTTATTTCATTATGAAAGG | cps2L15496-15517 |
| CpsL-2 (−) | CCGAAAAAATTATCTGTCATCTAG | cps2L16399-16375 |
| c-ups1 | GAGCCCATGTTTCTCAATAGG | cps2449-470 |
| c-ups2 | ATCTTAGTAGACTTCCCGCG | cps21373-1352 |
| KM151-2 (−) | *GTACTAAAACAATTCATCCA | aphA-32543-2523 |
| KM151-3 (+) | *GAGGAAGGAAATAATAA | aphA-31729-1746 |
| LDH-F (+) | GTCGGTGATGGTGCTGTAGGTTCATC | ldh164-189 |
| LDH-R (−) | GTCGATGTTAGCGTGTGACCAAACAG | ldh710-687 |
| Ugd-1 (+) | GGGCATTCTTCCATCTAAAAATGA | ugd148064-148088 |
| Ugd-2 (−) | GCATTTAAACTTCTCCTCTCAGC | ugd148627-148604 |
Forward and reverse primers are indicated by + and −, respectively.
* and **, KpnI (GGTACC) and BglII (AGATCT) sites present at the 5′ end.
Superscript numbers indicate the positions of the primer start and end in the homologous sequence of either type 2 capsule (cps2) (GenBank accession no. AF026471), type 3 capsule (cps3) (GenBank accession no. U15171), pDL276 aphA-3 (GenBank accession no. AF216803), ldh (TIGR4 sequence) (GenBank accession no. AE007422), or R6 ugd (GenBank accession no. NC003098).
For repair of the Δcps2K mutants, the cps2K ORF and 500 bp flanking each side were PCR amplified from D39 chromosomal DNA using primer pair Cps2-J1/Cps2-P2. The fragment was cloned into pCR 2.1-TOPO, subsequently excised using EcoRI, and ligated into pJY4164. The resulting construct, pBX115, was transformed into Δcps2K strains BX511, BX512, and BX533. The mixtures were plated on blood agar plates without selection. Strains BX515, BX518, and BX540, which contained repairs of the respective Δcps2K mutants, were obtained by screening for loss of Km resistance, and the repair of cps2K was confirmed by PCR and sequencing.
For repair of Δcps2K mutants with cps3D, the UDP-GlcDH gene from serotype 3 S. pneumoniae, the ORF of cps3D was PCR amplified from S. pneumoniae WU2 chromosomal DNA using primer pair Cps3D-F/Cps3D-R and cloned into pCR 2.1-TOPO. The forward primer Cps3D-F includes the Shine-Dalgarno sequence and start codon of cps3D. The cloned fragment was excised using KpnI and inserted between the two fragments in pBX108, resulting in pBX116. Correct orientation of all three fragments was confirmed by PCR. The resulting construct was transformed into BX511, and the reaction mixture was plated on blood agar plates without selection. Strain BX517, containing the allelic exchange of cps3D for aphA-3 in BX511, was obtained by screening for loss of Km resistance, which was then confirmed by PCR.
Repair of cps2E in the cps2K-repaired strains was conducted essentially as described above for the repair of Δcps2K mutants. cps2E, along with the 500 bp on either side, was PCR amplified from D39 chromosomal DNA and cloned into pCR 2.1-TOPO. The cloned fragment was excised using EcoRI and ligated into pJY4164, resulting in pBX145. pBX145 was transformed into BX515 and BX518, and the reaction mixture was plated on blood agar plates without selection. Repaired cps2E strains, BX545 and BX546, respectively, were identified by screening for large, glossy colonies, and repair of the mutations was confirmed by sequencing.
Capsule analyses.
Indirect capsule enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (31, 39) with slight modifications. In brief, duplicate cultures were grown in THY to a density of ∼3 × 108 CFU/ml, and 5 ml of each was centrifuged at 20,000 × g for 10 min. The culture supernatant was collected and filtered (0.45-μm-pore-size syringe filter; Corning). The pelleted cells were resuspended in phosphate-buffered saline (PBS) (342.5 mM NaCl, 6.75 mM KCl, 13.5 mM Na2HPO4, and 4.5 mM KH2PO4) and heat killed at 56°C for 20 min. All samples were normalized to the same optical density at 600 nm. Wells of polystyrene microtiter plates (Corning Inc.) were coated overnight at 4°C with twofold serial dilutions of the samples. Wells were washed three times in PBS containing 0.5% Tween (PBST) and blocked for 1 h with 200 μl of 1% bovine serum albumin (BSA) in PBS (BSA-PBS) at room temperature. A rabbit polyclonal anti-type 2 antiserum (Statens Serum Institute, Denmark) adsorbed against a nonencapsulated type 2 derivative, AM1000, was used for detection of capsule on the cell surface. For adsorption, 250 ml of AM1000 was grown in THY to a density of ∼3 × 108 CFU/ml and heat killed for 45 min at 56°C. The culture was centrifuged, washed once in PBS, centrifuged, and resuspended in 250 μl of anti-type 2 antiserum diluted 1/10 in PBS. Adsorption was conducted by rotating the solution overnight at 4°C. AM1000 was pelleted, and the supernatant containing the adsorbed anti-type 2 antiserum was filter sterilized (0.22-μm-pore-size syringe filter; Millipore) and stored at 4°C until use. The adsorbed anti-type 2 antiserum was diluted 1/5,000 in BSA-PBS, and 100 μl of this solution was added to each well, followed by incubation at room temperature for 1 h. The wells were washed three times with PBST and incubated with biotinylated goat anti-rabbit immunoglobulin conjugated to strepavidin-alkaline phosphatase for 1 h at room temperature. The wells were washed three times with PBST, followed by development with 1 mg/ml p-nitrophenolphosphate in glycine buffer (0.1 M glycine, 1 mM MgCl2, 0.1 M ZnCl2, pH 10.4). Absorbance was measured at 415 nm. Surface accessibility assays were performed in an identical manner except that the adsorbed type 2-specific antiserum was replaced with a nonadsorbed rabbit polyclonal antiserum raised against a type 19 strain (Statens Serum Institute, Denmark). This antiserum contains a high titer of antibodies to noncapsular surface antigens and provides an effective measure of blocking of the surface by the capsule (31). For competitive-inhibition ELISAs, cultures were grown as described above. Assays were performed as previously described (13). Briefly, wells of microtiter plates were coated as described above with heat-killed D39 at a density of 3 × 108 CFU/ml in PBS. Cell lysates used as inhibitors were prepared by growing 10-ml cultures of each strain to a density of 3 × 108 CFU/ml in THY. Samples were normalized to the same optical density and centrifuged at 20,000 × g for 10 min. Pellets were resuspended in 0.1 ml lysis buffer (0.1% sodium deoycholate, 0.01% SDS, 0.15 M sodium citrate) and incubated at 37°C for 10 min. To the lysed bacteria, 0.9 ml of SSC (0.15 M NaCl, 0.015 M sodium citrate) was added, and the samples were incubated at 65°C for 15 min. Twofold serial dilutions of the lysates or culture supernatants, together with polyclonal anti-type 2 antiserum diluted as described above, were added to the D39-coated microtiter plates. The remainder of the procedure was as described above for the indirect ELISAs.
Electron microscopy.
Bacteria were prepared for electron microscopy as described by Kolkman (38). Briefly, cultures were grown to a density of ∼3 × 108 CFU/ml in THY, and 5 ml of each culture was centrifuged at 20,000 × g for 10 min. The pellets were fixed in 500 μl of a 1% glutaraldehyde-4% formaldehyde solution for 30 min at 4°C. Fixed samples were further processed by the University of Alabama at Birmingham electron microscopy core facility for microscopy. In general, samples were postfixed in 1% osmium tetroxide, washed in phosphate buffer, dehydrated with ethanol, embedded with Polybed, dried, sectioned, and stained with uranyl acetate.
Protein analyses of Cps2D∼P, Cps2D, and Cps2E.
Western immunoblots of Cps2D and tyrosine-phosphorylated Cps2D [Cps2D∼P] were performed as previously described (6). In brief, cultures were grown to a density of ∼3 × 108 CFU/ml and centrifuged at 20,000 × g for 10 min at 4°C. The pellets were resuspended in water at a 50× concentration, and the samples were normalized to the same optical density at 600 nm. Twenty microliters of the cell suspensions was used for Cps2D blots, and 10 μl was used for Cps2D∼P blots. Samples were boiled in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, and proteins were separated by SDS-10% PAGE. Proteins were transferred to a nitrocellulose membrane. Cps2D was detected using a polyclonal rabbit Cps2D-specific antiserum as described previously (64). Cps2D∼P was detected using a monoclonal antibody to phosphotyrosine clone PT-66 conjugated to horseradish peroxidase (Sigma). ImageJ software (http://rsb.info.nih.gov/ij) was used for densitometry analyses.
Relative Cps2E protein levels were determined as described previously (15). In brief, 10 μg of total protein from isolated S. pneumoniae membranes was separated by SDS-10% PAGE and subsequently transferred to a nitrocellulose membrane. Cps2E was detected using a polyclonal rabbit antiserum directed against the C-terminal portion of Cps2E and diluted 1/5,000.
Capsule replacement experiments and linkage analyses to map suppressor mutations in Δcps2K mutants.
Em resistance markers were linked to the capsule locus by insertion of pJD377 (plpA insertion, downstream) and pBX123 (between dexB and the capsule promoter, upstream) into the D39 chromosome, resulting in strains BX516 and BX535, respectively. Chromosomal DNAs of BX516 and BX535 were isolated using a genomic column prep (QIAGEN) and used to transform the Δcps2K mutants BX511 and BX512. Em-resistant transformants were screened for the large-colony phenotype indicative of capsule production and also for loss of Km resistance.
To sequence cps2E, the gene was PCR amplified from chromosomal DNAs of the Δcps2K mutants using primer pairs Cps2-E10 and Cps2-E11. The fragment was gel extracted (gel extraction kit; QIAGEN) and sequenced at the sequencing core facility of the Helfin Center for Human Genetics at the University of Alabama at Birmingham.
Membrane isolations and Cps2E glycosyltransferase assays.
Cps2E glycosyltransferase activity in isolated membranes was tested as described previously (37), where Cps2E activity is defined as the ability to transfer [3H]Glc from UDP-[3H]Glc to an organically soluble product in a reaction conducted at 10°C. S. pneumoniae membranes were isolated as previously described (15, 16). Membranes containing 10 μg of total protein were incubated in a 100-μl reaction mixture of 5 mM Tris-acetate (pH 7.5), 10 mM MgCl2, and 1 μM UDP-[3H]Glc (1 Ci/mmol; Sigma) at 10°C for 10 min. The reaction was stopped by the addition of 1 ml chloroform-methanol (2:1), and the organic phase was extracted using 200 μl pure solvent upper phase (1.5 ml chloroform, 25 ml methanol, 23.5 ml H2O, and 0.183 g KCl). The amount of radioactivity incorporated into the organic phase was measured by liquid scintillation counting.
Analysis of capsule transcripts.
RNA was isolated from 50-ml S. pneumoniae cultures using a previously described hot-acid-phenol procedure (27). Serial twofold dilutions of RNA samples were used in slot blot analyses to determine the relative amounts of transcripts. Detection of transcripts and densitometry were performed as previously described (6). PCR probes were digoxigenin labeled (Roche), and the amount of labeling was visualized using Pierce SuperSignal chemiluminescent substrate. ImageJ software was used for densitometry analyses. The intensity of each band was normalized to lactate dehydrogenase (ldh) transcripts, and these ratios were compared for the parent and mutant strains.
Capsule immunoblots.
Fractionation of S. pneumoniae into cell wall and protoplast fractions was performed as previously described with minor modifications (73). This method results in minimal cross contamination of fractions (6). In brief, S. pneumonie cultures were grown to a density of ∼3 × 108 CFU/ml, and cells were sedimented at 20,000 × g for 10 min at 4°C. Pellets were suspended in protoplast buffer (20% sucrose, 50 mM MgSO4, 50 mM Tris [pH 7.4]) at 1/100 the original culture volume. Forty units of mutanolysin (Sigma) was added to each milliliter, and the sample was incubated overnight at room temperature (the S. pneumoniae autolysin LytA is also active under these conditions). After incubation, the formation of protoplasts was confirmed by light microscopy. Protoplasts were sedimented at 10,000 × g for 10 min. The supernatant containing the cell wall fraction was filtered (0.22-μm-pore-size syringe filter; Millipore), and the sedimented protoplasts were resuspended in protoplast buffer in a volume equal to the cell wall extract. For samples concentrated 2- or 10-fold, pellets were suspended in 1/200 or 1/500 of the original culture volume.
The fractions were further processed and analyzed for capsule and teichoic acids in immunoblots as previously described (6). In brief, 20 μl of sample containing either cell walls or protoplasts was combined with 10 μl of buffer B1 (50 mM EDTA, 0.5% Tween 20, 0.5% Triton X-100, 50 mM Tris [pH 8] [QIAGEN]) and 2 μl of Qiaprotease (20 μg/μl; QIAGEN) and incubated at 37°C for 30 min. Ten microliters of SDS-PAGE loading dye was added to each sample, followed by heating at 100°C for 8 min. The samples were separated by SDS-10% PAGE and then transferred to nitrocellulose membranes. Capsular polysaccharides were detected using a rabbit polyclonal antiserum against the type 2 capsule (Statens Serum Institute, Denmark) that had been adsorbed against the nonencapsulated AM1000 (as described above) and diluted 1/1,000. The presence of teichoic acid (C-polysaccharide) in the cell wall fractions was detected using a rabbit polyclonal antiserum diluted 1/5,000 (Statens Serum Institute, Denmark).
Isolation and characterization of capsule produced by Δcps2K mutants.
Cultures (250 ml) of D39 and a Δcps2K mutant, BX511, were grown to mid-exponential phase in chemically defined media (59) containing 0.0005% choline chloride, 0.25% sodium bicarbonate, and 0.073% cysteine-HCl. The polysaccharide isolation procedure was based on previously described methods (28, 68) with modifications, as described below. Cultures were centrifuged at 20,000 × g for 10 min, and the pellet was resuspended in 5 ml of water. Water-saturated phenol was added to a final concentration of 1%, and the suspension was incubated overnight at room temperature. Microscopy was used to confirm lysis of the bacteria. Cellular debris was pelleted by centrifugation at 20,000 × g for 30 min at 4°C. The supernatant was collected, and ethanol and sodium acetate were added to final concentrations of 60% and 7.2%, respectively, to precipitate the polysaccharide. The solution was centrifuged at 20,000 × g for 30 min at 4°C. The resulting pellet was dissolved in 5 ml of water, and the pH was adjusted to 7.5 with 1 M NaOH. Forty units of DNase (Promega) and 40 μg of RNase (QIAGEN) were added, and the suspension was incubated at 37°C for 4 h. Fifty units of proteinase K (QIAGEN) was then added, and the sample was incubated at 37°C overnight. Low-molecular-weight contaminants were removed by dialysis at 4°C overnight using 6,000- to 8,000-molecular-weight-cutoff dialysis tubing. Additional debris was removed by centrifugation (20,000 × g for 10 min at 4°C), and the supernatant containing the partially purified polysaccharide was collected and stored at 4°C.
The phenol-sulfuric acid method was used to determine total hexose present in the polysaccharide sample (4). A methylpentose assay was used to determine the amount of rhamnose present in the extracted polysaccharide samples (24). Carbazole and m-hydroxydiphenyl assays for measurement of total hexuronic acids (9, 26) were used to assess the GlcUA content in extracted polymer and whole cells. For whole cells, 10 ml of S. pneumoniae cultures was grown to a density of ∼3 × 108 CFU/ml and centrifuged at 20,000 × g for 10 min at 4°C. Pellets were resuspended in 500 μl of water. Serial dilutions of lysates were analyzed for total uronic acid as described previously (9, 26).
RESULTS
Cps2K exhibits UDP-GlcDH activity.
To determine whether cps2K encoded an authentic UDP-GlcDH, the gene was cloned from the S. pneumoniae capsule type 2 strain D39 into the expression vector pQE-40 and expressed in E. coli, as described in Materials and Methods. To facilitate purification of the recombinant protein, it was expressed with an N-terminal His6 tag. UDP-GlcDH activity was assayed spectrophotometrically by following the reduction of NAD+ to NADH during the oxidation of UDP-Glc to UDP-GlcUA, as described in Materials and Methods. The observed activities for bovine Ugd and Cps2K were 0.36 and 0.21 μmol NADH/min/μg purified protein, respectively (activity for the vector control E. coli strain was 0.0032 μmol NADH/min/μg total protein). As described below, repair of an S. pneumoniae D39 cps2K deletion mutant with cps3D, the S. pneumoniae type 3 UDP-GlcDH (2, 23), complemented the defect, further confirming the function of Cps2K.
cps2K deletion mutants exhibit severe reductions in capsule synthesis and fail to transfer polymer to the cell wall.
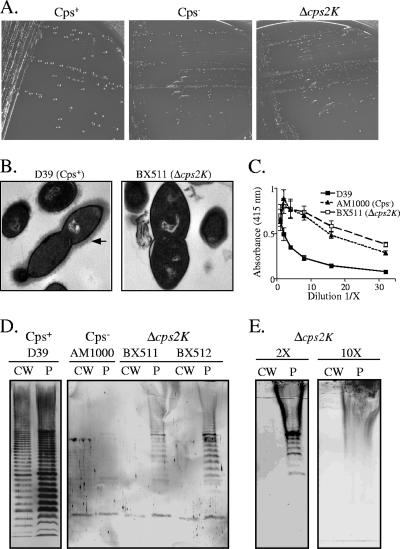
In-frame deletion mutants of S. pneumoniae D39 were generated by allelic replacement of cps2K with an aphA-3-containing fragment encoding resistance to Km, as described in Materials and Methods. Multiple independent cps2K mutants were derived in separate transformation reactions with D39. In contrast to the large, glossy colonies of the encapsulated D39 parent, all of the resulting Km-resistant transformants exhibited a small, rough colony morphology (Fig. 2A). When individual colonies were plated to determine CFU/colony, the numbers were the same for the parent and mutant strains (∼4 × 107 CFU/colony). However, microscopic observation revealed fewer bacteria per chain for the mutants. Thus, an overall lower number of bacteria were present in each colony, suggestive of a possible growth defect (discussed further below). Using a polyclonal antiserum to the type 2 polysaccharide in indirect and competitive-inhibition ELISAs, no capsule was detectable using intact cells, cell lysates, or culture supernatants from two independent cps2K mutants (data not shown). Further, no surface-localized capsule was detectable by electron microscopy (Fig. 2B). Consistent with a severe reduction in capsule synthesis, whole cells of the cps2K mutants exhibited the same high reactivity as a nonencapsulated mutant (AM1000, Δcps2A to Δcps2H) in ELISAs with a polyclonal antiserum containing a high titer of antibodies to noncapsular surface antigens (Fig. 2C). In this surface accessibility assay, binding of the antibodies is blocked in proportion to the amount of cell-associated capsule (31).
FIG. 2.
Phenotypes of Δcps2K mutants. (A) S. pneumoniae serotype 2 parent strain D39, the nonencapsulated D39 derivative AM1000, and the Δcps2K mutant BX511 streaked for isolation on blood agar. (B) Electron micrographs of D39 and the Δcps2K mutant, BX511. The arrow indicates the capsule. (C) Indirect ELISA for surface accessibility using whole cells and a polyclonal antiserum to surface antigens. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of two experiments. (D) Capsule immunoblots of cell wall (CW) and protoplast (P) fractions reacted with polyclonal antiserum against type 2 capsule. (E) Capsule immunoblots for BX512. Prior to fractionation to yield cell walls and protoplasts, the bacterial samples were concentrated 2- and 10-fold more than in panel D. The loadings in this panel therefore represent 2-fold (2×) and 10-fold (10×) more sample than in panel D. The smear in the protoplast lanes results from the heavily overloaded capsule-containing protoplast sample. The lack of capsule in the similarly overloaded cell walls is evident.
To further assess capsule production in the cps2K mutants, isolated cell fractions were examined in immunoblot analyses with the type 2-specific antiserum. These analyses revealed the presence of low levels of high-molecular-weight polymer on the membrane-containing protoplast fractions, but no polymer was detected on cell wall fractions (Fig. 2D), even when the latter were concentrated 2- or 10-fold (Fig. 2E). Teichoic acid was present in similar amounts in the cell wall fractions of the parent and mutant strains (data not shown), confirming that fractionation of the mutants had released the peptidoglycan from the cell and that synthesis of teichoic acid was not affected by the mutations.
The reduction in capsule was further demonstrated by assaying total hexose and methylpentose (for rhamnose) in polymer extracted from whole cells. In the cps2K mutants, the levels of both sugars were approximately 5% of the parental levels (Table 3). The presence of capsular polysaccharide in cell wall fractions was examined by using the methylpentose assay to assay for rhamnose. Here, the cps2K mutant BX511 was not different from the nonencapsulated strain (Table 3). Using a carbazole or m-hydroxydiphenyl assay to measure total uronic acid, GlcUA was undetectable in whole cells or extracted polymer from the Δcps2K mutants (data not shown).
TABLE 3.
Hexose and methylpentose levels in cps2K mutants
| Strain | Level (μg/108 CFU)a
|
||
|---|---|---|---|
| Hexose, isolated polymer | Methylpentose
|
||
| Isolated polymer | Cell wall | ||
| D39 (Cps+ parent) | 6.7 ± 0.9 (6.7) | 0.67 (0.67) | 0.736 (0.727) |
| BX511 (Δcps2K) | 0.35 ± 0.01 (0.35) | 0.037 (0.034) | 0.01 (0) |
| AM1000 (Cps−) | 0.004 ± 0.001 (0) | 0.003 (0) | 0.01 (0) |
| BX515 (cps2K repair of BX511) | NDb | ND | 0.0156 (0.0056) |
Polymers isolated from 250-ml cultures were assayed for both hexose and methylpentose, and cell walls isolated from a 250-ml culture were assayed for methylpentose. The hexose values represent means ± standard errors for two independent cultures. Values in parentheses are after the subtraction of the Cps− value for AM1000.
ND, not determined.
To confirm that the small amounts of capsule produced by the Δcps2K mutants were not due to undetectable levels of GlcUA arising from the activity of a non-Cps2K UDP-GlcDH, we deleted ugd in both the parent D39 and the Δcps2K mutant BX511. This gene is identified in the genome sequence of strain R6, a derivative of D39 (33). It is located outside the capsule locus and is predicted to encode a UDP-GlcDH with 40% identity and 61% similarity to Cps2K. The phenotypes of the ugd deletion mutants of D39 and BX511 were identical to those of their respective parents (data not shown), indicating that this gene does not contribute to capsule synthesis in these strains.
cps2K deletion mutants contain suppressor mutations.
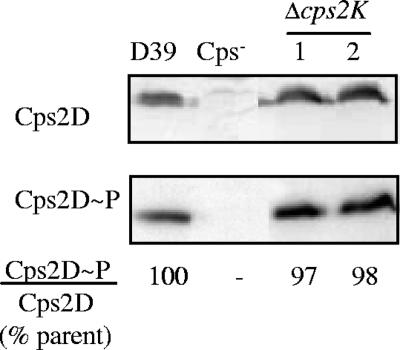
The alterations in capsule synthesis following deletion of cps2K were more severe than what had been anticipated at the outset of this study. To confirm that these effects were not due to any polar or feedback effects on transcription of the capsule locus, RNA slot blot analyses were performed. The probes used were specific for cps2C and cps2M, which lie upstream and downstream, respectively, of cps2K (Fig. 1B). For both independent cps2K mutants, transcription was unchanged from that of the parent D39 (data not shown). In addition, the levels of two capsule proteins, Cps2D and Cps2E, as well as the level of Cps2D tyrosine phosphorylation, were unchanged in the mutants (shown for Cps2D and Cps2D∼P in Fig. 3). The presence of Rha in the mutant polymer (described above) indicated that proteins encoded by the downstream genes cps2LMNO and necessary for synthesis of TDP-Rha (a precursor for subunit assembly) were present. The results of experiments described in the next section further indicated that the cps2K deletions did not affect translation of the downstream region.
FIG. 3.
Cps2D and Cps2D∼P in Δcps2K mutants. Cps2D and Cps2D∼P were detected in Western immunoblots. Cps2D∼P/Cps2D ratios were normalized to those of the parent D39 to obtain percent values. The Cps− strain was AM1000, in which cps2A to cps2H are deleted. The independent Δcps2K mutants were BX511 (lane 1) and BX512 (lane 2).
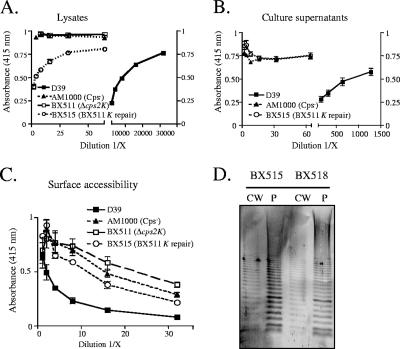
We next undertook repair of the cps2K deletions to confirm that the observed phenotype was due to only the mutation we constructed. Clones containing the entire cps2K gene and the 500-bp flanking regions were used to transform the cps2K mutants. Transformants in which the allelic exchange of cps2K and aphA-3 had occurred were identified by screening for loss of Km resistance and then confirming the presence of cps2K by PCR. Unexpectedly, repair of the cps2K deletion only partially restored capsule production. The colonies of the repaired mutants, though larger than those of the cps2K deletion mutants, were still extremely small. Using cell lysates in competitive-inhibition ELISAs, only 0.1% of the antibody-reactive capsular material produced by the parent was detectable with the cps2K-repaired strains (Fig. 4A), and no capsule was detectable in culture supernatants of these strains (Fig. 4B). Consistent with this low level of capsule, reactivity of the repaired mutants in the surface accessibility assay remained high, although it was less than that of both the nonencapsulated strain AM1000 and the cps2K deletion mutant (Fig. 4C). In immunoblot analyses, the full range of high- to low-molecular-weight polymer was observed in both the protoplast and cell wall fractions (Fig. 4D). Analysis by the methylpentose assay demonstrated the presence of rhamnose in the cell wall fraction of the repaired strain (Table 3, BX515). The UDP-GlcDH gene of S. pneumoniae serotype 3, cps3D, was also used to repair the cps2K deletion. This repair resulted in the same phenotype as that obtained using cps2K (data not shown). These results suggested that the cps2K deletion mutants contained suppressor mutations that affected proper capsule synthesis.
FIG. 4.
Capsule production by cps2K-repaired mutants. (A and B) Relative capsule amounts were determined by competitive inhibition ELISA for cell lysates (A) and culture supernatants (B). Results are shown for the Δcps2K mutant BX511 and its derivative BX515 obtained by repair of cps2K only. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of two experiments. Identical results were obtained for the independent Δcps2K mutant BX512 and its respective derivative BX518. (C) Indirect ELISA for surface accessibility using a polyclonal antiserum to surface antigens. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of two experiments. BX515 was significantly different from AM1000 and BX511 (P = 0.002 and 0.003, respectively, by a paired t test to compare dilutions 1/8 to 1/32). BX511 and AM1000 were not different. (D) Capsule immunoblots reacted with type 2-specific polyclonal antiserum. BX515, cps2K repair of BX511; BX518, cps2K repair of BX512. CW, cell wall fraction; P, protoplast fraction.
Suppressor mutations map to cps2E.
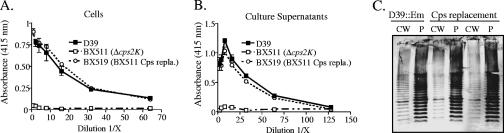
To determine whether the suppressor mutations were linked to the capsule locus, linkage analyses were performed using derivatives of the parent D39 as donors. These strains contained an Em resistance marker either upstream or downstream of the capsule locus, and chromosomal DNA from each was used to transform the cps2K deletion mutants. Our expectation for these experiments was that transformation of the entire capsule locus would repair the cps2K deletion and any other mutation(s) that might be contained in this region, resulting in a parental capsule phenotype if no other mutations were present elsewhere in the chromosome. To screen for the parental phenotype, Em-resistant transformants were examined for the presence of large, glossy colonies, which are indicative of capsule production. Approximately 2% of the Em-resistant transformants obtained with the upstream insertion and approximately 1% of those obtained with the downstream insertion exhibited large colonies. All of the large-colony Em-resistant transformants examined were Km sensitive, denoting repair of cps2K. ELISAs and immunoblot analyses of the Km-sensitive isolates demonstrated that full capsule production, including release of capsule into the culture supernatant and transfer to the cell wall, had been restored (Fig. 5). These results indicated that the suppressor mutations were located in or near the capsule locus.
FIG. 5.
Capsule production by cps2K mutants repaired by capsule locus replacement. (A and B) Relative capsule amounts were determined using intact cells (A) and culture supernatants (B) in indirect ELISAs. Results are shown for the Δcps2K mutant BX511 and its derivative BX519, obtained by replacement of the entire capsule locus. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of three experiments. Identical results were obtained for the independent Δcps2K mutant BX512 and its respective derivative BX522. Capsule levels for the D39 derivative BX516, containing an Em marker downstream of the capsule locus and used to replace the entire capsule locus, were identical to those for D39 (data not shown). (C) Capsule immunoblots reacted with type 2-specific polyclonal antiserum. CW, cell wall fraction; P, protoplast fraction. D39::Em, BX516 donor for capsule replacements; Cps replacement for left CW/P fractions, BX519 (BX511 repair); Cps replacement for right CW/P fractions, BX522 (BX512 repair).
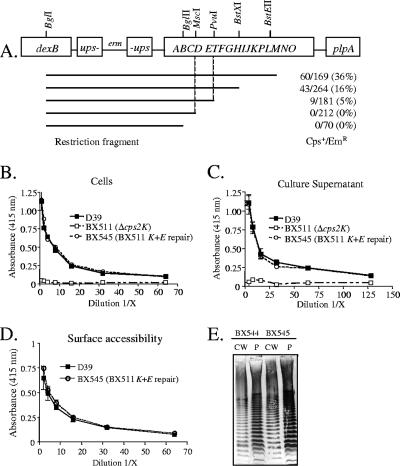
To map the suppressor mutations, we used as recipients the cps2K mutants in which the deletion had been repaired using cps2K and the 500-bp flanking regions. These strains should contain only the suppressor mutations, and their repair should result in parental capsule synthesis. Restriction enzyme-digested chromosomal DNAs from the D39 derivatives containing the Em resistance markers flanking the capsule locus were used to transform the recipients. Em-resistant transformants were then screened for the large-colony phenotype. As shown in Fig. 6A, fragments containing the region between cps2D and cps2T could restore the parental phenotype in two independent mutants, suggesting that the suppressor mutations were located in this region. We therefore PCR amplified and sequenced this region from three independent cps2K deletion mutants. For each, a different point mutation was identified in cps2E. This gene encodes a 455-amino-acid protein previously demonstrated to be the glycosyltransferase responsible for the addition of Glc-1-P onto a polyprenol carrier to initiate repeat unit synthesis (15). The cps2E mutations contained in the Δcps2K mutants BX511, BX512, and BX533 were G303V, G292R, and D425R, respectively.
FIG. 6.
Localization of suppressor mutations in Δcps2K mutants. (A) Restriction fragments of BX535 (D39 containing an Em marker upstream of the capsule locus) were used to transform BX518, a cps2K-repaired strain. Em-resistant transformants were screened for large, smooth colonies to denote repair of the suppressor mutation. Vertical lines indicate the region expected to contain the mutation based on the ability of the fragments to restore the parental phenotype. Numbers are Emr-smooth transformants/total Emr transformants examined in two independent transformations. (B) Indirect capsule ELISAs of intact cells for derivatives in which both cps2K and cps2E have been repaired. Results are shown for BX545, the cps2K- and cps2E-repaired derivative of BX511. Identical results were obtained for BX544, the doubly repaired derivative of BX512. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of three experiments. (C) Indirect capsule ELISAs of culture supernatants, as in panel B. (D) Indirect ELISAs for surface accessibility using a polyclonal antiserum to surface antigens. Results are the means (± standard errors) from two independent cultures assayed in the same experiment and are representative of two experiments. (E) Capsule immunoblots reacted with type 2-specific polyclonal antiserum. CW, cell wall fraction; P, protoplast fraction. BX544, cps2K and cps2E repair of BX512; BX545, cps2K and cps2E repair of BX511.
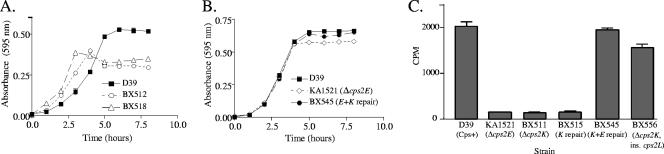
To confirm that the mutations in cps2E were responsible for the altered capsule phenotype observed in the repaired cps2K mutants, allelic exchange of a parental copy of cps2E for the mutated cps2E was performed with two of the independent mutants. Clones containing the entire cps2E gene and the 500-bp flanking regions were used to transform the repaired cps2K strains. Transformations were plated in the absence of selection, and colonies were screened for the large, glossy parental phenotype. Approximately 10% of the colonies were large. Sequence analyses confirmed the repair of the cps2E mutations in several large-colony transformants. ELISA, surface accessibility, and immunoblot analyses demonstrated parental levels of capsule in these isolates (Fig. 6B to E). Consistent with the reduced numbers of bacteria on agar plates, the cps2K mutants and the repaired cps2K mutants (both of which contained a cps2E mutation) exhibited altered growth patterns (Fig. 7A). In contrast, growth of the cps2E deletion mutant KA1521 and the doubly repaired cps2K cps2E mutant BX545 was like that of the parent D39 (Fig. 7B). Thus, the capsule and growth phenotypes in the cps2K-repaired mutants were due to the cps2E suppressor mutations, and repair of both the cps2K deletion and the cps2E point mutation restored the full parental phenotypes. These results confirmed that no other mutations or downstream effects of the cps2K deletions were responsible for the observed phenotypes.
FIG. 7.
Growth and Cps2E glycosyltransferase activity. (A and B) Growth curves of BX512 (Δcps2K), BX518 (cps2K repair), BX545 (cps2K and cps2E repair), and KA1521 (Δcps2E) compared to that of the parent D39. Cultures were diluted 1/10 from a THY culture and grown in THY. Absorbance readings were taken at the indicated time points. (C) Isolated membranes were used to measure incorporation of [3H]Glc from UDP-[3H]Glc to an organically soluble product, indicative of transfer of Glc-1-P to a polyprenol carrier (15). Membranes contained 10 μg of total protein and equivalent levels of Cps2E, as determined by Western blotting (not shown). Similar results were obtained for BX512 and its respective repaired derivatives.
To determine the effects of the suppressor mutations on Cps2E activity, isolated S. pneumoniae membranes were used as a source of enzyme activity for in vitro assays. As shown in Fig. 7C, Cps2E activity for the cps2K and repaired cps2K mutants BX511 and BX515, respectively, was not detectable above the background level observed with the cps2E deletion strain KA1521. In contrast, parental levels of activity were observed with the doubly repaired cps2K cps2E mutant BX545. Cps2E protein levels of the mutant and repaired strains were unchanged from that of the parent (data not shown). cps2E deletion mutants do not make capsular polysaccharide that is detectable by ELISA (15) or immunoblotting (data not shown). Thus, capsule synthesis in the cps2K and repaired cps2K mutants, each of which contains a cps2E mutation, must be due to a low level of in vivo Cps2E activity.
cps2K deletion mutants consistently contain suppressor mutations, which are located in cps2E or elsewhere within or near the capsule locus.
To determine whether suppressor mutations were necessary for the isolation of cps2K deletion mutants, we generated 18 additional cps2K mutants in independent reactions. For all, the colony morphologies and capsule ELISA analyses were similar to those for the original cps2K mutants (data not shown). Sequence analyses of these mutants revealed cps2E mutations in 15 of the strains. All 15 mutations differed from those isolated originally. The locations of the mutations for these 15 strains are given in Table 1 (strains BX532, BX539, BX547 to -549, BX554, BX555, BX605 to -607, BX609 to -612, and BX635). The three remaining cps2K mutants could be restored to the parental phenotype by transformation with the complete capsule locus, indicating the presence of suppressor mutations in this region. The mutations in two of the mutants were localized by linkage and sequence analyses. One of these mutants, BX551, contained a transition mutation located 4 base pairs downstream of the −10 sequence in the predicted capsule promoter located upstream of cps2A. A twofold reduction in the amount of capsule transcript was observed for this mutant by RNA slot blot analysis (data not shown). The second mutant had a 1-kb insertion located in cps2L. This gene encodes the Glc-1-P thymidylyltransferase that converts Glc-1-P to TDP-Glc in the first step of TDP-rhamnose synthesis (34, 43). The insertion is expected to be polar on cps2MNO, the remaining genes in the capsule locus that are required for the final three steps in TDP-Rha synthesis. Cps2E activity in the cps2L suppressor mutant was similar to that in the parent strain (Fig. 7B, strain BX556). The location of the suppressor mutation in the third non-cps2E mutant has not been determined.
In both the original and subsequent experiments to construct cps2K deletions in the parent D39 strain, the number of isolates obtained was small (≤1 Km-resistant isolate per 106 recipients). This result was consistent with the necessity to transform the rare spontaneous mutants that contained cps2E or other suppressor mutations that allowed for survival in the presence of a cps2K deletion. To determine whether the frequency of obtaining cps2K deletion mutants could be enhanced, we used as recipients isolates already containing cps2E mutations, which had been derived by repair of a cps2K deletion. Here, ∼500 Km-resistant isolates were obtained per 106 recipients. Both D39 and the recipients already containing cps2E mutations were transformed with donor DNA containing an Em resistance marker unlinked to the capsule locus at high efficiency (∼500 to 800 Em-resistant isolates per 106 recipient for each strain), indicating that they were equally competent for transformation. Thus, deletion of cps2K is detrimental to the cell, and such mutants can be isolated only in the presence of suppressor mutations that reduce or eliminate capsule synthesis.
Deletion of cps2H or cps2J also selects for isolates that contain cps2E mutations.
To determine whether other mutations that affected polymer assembly would be detrimental to the cell, in-frame deletions of cps2J and cps2H, which encode the putative Wzx flippase and Wzy polymerase, respectively, were constructed as described in Materials and Methods. These mutants should synthesize complete repeat units that are either retained on the cytoplasmic face of the membrane (flippase mutants) or translocated to the outer face of the membrane but not polymerized (polymerase mutants). The Km-resistant transformants obtained exhibited the small, rough colony morphology indicative of nonencapsulated mutants. Based on the results for the cps2K mutants, we sequenced cps2E in independent mutants from each construction. All contained mutations. The Δcps2H mutants BX552 and BX505 contained a point mutation resulting in an amino acid change (L199F) and a 1-base-pair deletion resulting in a premature stop at residue 244, respectively. The Cps2E alterations in the Δcps2J mutants BX667, BX668, and BX669 were G411R, I265S, and G371A, respectively. For the Δcps2H mutant BX552, the level of Cps2E protein was similar to that of the parent strain, whereas Cps2E activity was not detectable in the in vitro assay, as observed for the original cps2K mutants (data not shown). As discussed below, the suppressor mutation in the cps2H mutant is located in an extracytoplasmic loop of Cps2E, whereas the suppressor mutations of the original cps2K mutants are located in a cytoplasmic region.
DISCUSSION
In both gram-positive and gram-negative bacteria, capsule synthesis by the Wzy-dependent mechanism likely initiates on the C55 lipid undecaprenyl-phosphate (Und-P), the same lipid acceptor that is used to initiate synthesis of peptidoglycan, LPS O-antigen repeat units in gram-negative bacteria, and teichoic acids in gram-positive bacteria. In S. pneumoniae, synthesis initiates by transfer of Glc-1-P to a polyprenyl-P whose size and properties are consistent with Und-P (15). By analogy with peptidoglycan synthesis, the Und-P acceptor is expected to be recycled from the outer to the inner face of the cytoplasmic membrane following transfer of the linked polymer to another lipid-linked subunit or acceptor. The cellular levels of Und-P are low (40), and thus the amounts and ratios of different polymers on the cell surface may be limited by the pool of available Und-P. The results of the present study lead to several conclusions regarding Wzy-dependent capsule synthesis in S. pneumoniae, as discussed below.
Lack of the terminal GlcUA of the side chain alters the ability to transfer the type 2 capsule to the cell wall.
The lack of cell wall polymer in the Cps2K mutants could reflect a requirement for recognition of GlcUA by one or more enzymes in the capsule pathway, an alteration in the secondary structure of the polymer such that it no longer serves as a substrate for one or more enzymes, or an insufficient level of polymer substrate for transfer. The shift to predominantly high-molecular-weight polymer in the Cps2K mutants is consistent with continued polymerase activity in the absence of chain termination and suggests that both the flippase and polymerase are active in the absence of the GlcUA residue, although we cannot exclude the possibility that their activities are not optimal. Low levels of polymer substrate do not inherently preclude transfer to the cell wall, as we have shown previously that deletion of cps2C or cps2D results in the synthesis of very small amounts of mainly low-molecular-weight polymer that is effectively transferred (6). Although it has been reported that Cps2C has a role in transfer of polymer to the cell wall (42), cps2C and cps2D deletion mutants exhibit parental ratios of cell wall to membrane-associated polymer (6), demonstrating that it is not required for this function. The Cps2K mutants were unchanged with regard to Cps2D production and tyrosine phosphorylation, and thus this system was not responsible for the observed reduction in capsule levels or the failure to transfer polymer to the cell wall. The absolute requirement for GlcUA may therefore lie with the enzyme or enzymes necessary for transfer of the polymer from Und-P to the cell wall. Such enzymes have not been identified in any gram-positive bacteria, and not enough genes are present in the capsule loci to encode enzymes unique to this function.
Mutations eliminating side chain assembly, transport, or polymerization are obtained only in the presence of suppressor mutations.
The lethality of the cps2K, cps2J, and cps2H mutations may have resulted from sequestration of Und-P in the capsule pathway and either preclusion of its turnover for utilization in essential pathways or destabilization of the membrane due to an accumulation of lipid-linked intermediates. This effect is most easily explained for the Wzx flippase (cps2J) mutants, which would be expected to accumulate single-repeat units on the inner face of the cytoplasmic membrane. For the cps2K mutants, the effect appears to reflect either directly or indirectly the inability to transfer polymer to the cell wall. It has not been established whether polymer transfer from Und-P to the cell wall occurs directly or via an intermediate acceptor or whether membrane-bound polymer in the parent strain is retained on Und-P or transferred to another acceptor. The high levels of membrane-bound polymer that accumulate in the parent strain apparently without harm (Fig. 2D) (6) indicate that either this level of Und-P sequestration is not lethal or the membrane-bound polymer is not linked to Und-P. The lethality of the cps2K mutations and the severe reductions in membrane-bound polymer in these mutants is consistent with the latter and a failure to transfer the polymer from Und-P to another membrane acceptor in the mutants. In the Wzy polymerase (cps2H) mutants, lipid-linked intermediates should accumulate only if single-repeat units cannot be transferred from Und-P to the cell wall or another acceptor. The fact that isolation of these mutants required suppressor mutations suggests that transfer of single-repeat units either did not occur or was very inefficient. Our previous studies demonstrated that short polymers can be transferred to the cell wall (6). The present results therefore suggest either that the linking enzyme cannot efficiently recognize and/or transfer a single, lipid-linked repeat unit or that the missing polymerase is involved in the transfer.
Secondary mutations, some of which were localized to the initiating glycosyltransferase, have similarly been noted in studies examining Pseudomonas aeruginosa LPS flippase (wzx) mutants (12), Xanthomonas campestris xanthan gum mutants (35), and Salmonella enterica serovar Typhimurium LPS mutants that failed to polymerize O-antigen subunits due to the lack of an abequose branch (74). Effects on cell viability resulting from the accumulation of lipid-linked subunits were also observed in these studies and in the characterization of E. coli LPS mutants (12, 49, 74). In contrast, mutations in S. agalactiae that resulted in lack of the side chain terminal sialic acid in the type III capsule led to reductions in capsule amount (∼20% of parental levels) that could be fully restored by complementation (17). Thus, either these mutations were not lethal or any secondary mutations that occurred did not have an apparent phenotype in the complemented strain. In contrast to our observations, essentially all of the S. agalactiae polymer was transferred to the cell wall for both the parent and mutant strains, possibly precluding the necessity of a secondary mutation.
Cps2E may have functions in addition to the initiation of repeat unit formation.
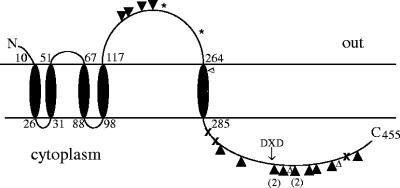
The high frequency of suppressor mutations in cps2E is perhaps surprising considering the other potential targets where mutations could theoretically abolish capsule production. In vitro, Cps2E catalyzes the addition of Glc-1-P to Und-P as well as the reverse reaction (15). The retention of Cps2E activity in a cps2L suppressor mutant, which would lack the ability to synthesize the TDP-Rha precursor and therefore fail to add Rha to Und-P-P-Glc, suggests that either the Cps2E reverse reaction occurs in vivo or the accumulation of Und-P-P-Glc is not toxic. Thus, mutations in the glycosyltransferase that catalyzes addition of the first Rha to the repeat unit, as well as mutations affecting TDP-Rha synthesis [cps2LMNO] or polar mutations in essentially any part of the locus, could be effective in relieving the stress induced by the cps2K, cps2J, or cps2H mutations. Yet, only 3 of our 26 suppressor mutations occurred outside cps2E. Mutations in other genes may therefore not be sufficient to prevent lethality, or Cps2E may provide many effective targets for disrupting capsule synthesis if it has roles beyond that of repeat unit initiation. Consistent with this possibility is the presence of a large extracytoplasmic domain in addition to the cytoplasmic region that contains the glycosyltransferase activity necessary for repeat unit initiation (Fig. 8). Approximately 20% of the suppressor mutations were located in the extracytoplasmic loop, which exhibits no conserved domains suggestive of putative functions. Most of the suppressor mutations were located in the cytoplasmic domain in residues conserved among Cps2E homologues in S. pneumoniae and other bacteria. However, none were in residues known to be important in glycosyltransferase activity, such as the DXD motif characteristic of UDP-sugar binding sites (10, 19).
FIG. 8.
Predicted topology of Cps2E determined using the TMpred program from the ExPASy Proteomics website (http://www.expasy.org/tools). Numbers denote amino acid number. ×, locations of mutations in original Δcps2K mutants. ▴ or ▾, locations of mutations contained in additional Δcps2K mutants. Numbers in parentheses represent the numbers of mutants with the same mutation. * and ▵, amino acid changes found in Δcps2H and Δcps2J mutants, respectively. The DXD motif in the cytoplasmic domain is indicated.
Cps2E belongs to a family of proteins that is structurally similar and includes initiating glycosyltransferases used for the syntheses of LPS O antigens, xanthan gum exopolysaccharide, and capsules (62). The extracytoplasmic loop is present in Salmonella enterica WbaP, which initiates LPS O-antigen synthesis by the addition of galactose-1-P to Und-P, but is absent in the Cps2E homologues of all S. pneumoniae serotypes that lack Glc in their polymers and therefore must initiate repeat unit formation with other sugars (8). This domain appears not to be essential for transfer of the S. pneumoniae capsule to the cell wall, as it is lacking in the CpsE homologue of serotype 4, which exhibits cell wall-associated capsule (6, 54). We noted, however, that the repaired cps2K mutants that retained cps2E suppressor mutations failed to release capsule from the cell. It is not yet know whether this observation is a direct effect of the cps2E mutation or relates to the low level of capsule produced.
The S. enterica WbaP protein is bifunctional, with the C-terminal cytoplasmic domain containing the glycosyltransferase activity and the N-terminal domain proposed to be important in releasing Und-P-P-galactose from WbaP and preferentially allowing the release of completed subunits (62). Such a role could fit with the phenotypes observed for the Cps2K mutants and the frequent occurrence of suppressor mutations in Cps2E; i.e., if the repeat unit remains associated with Cps2E until complete, the lack of GlcUA would block synthesis, resulting in the accumulation of lipid-linked repeat units on the inner face of the cytoplasmic membrane. Suppressor mutations in Cps2E that relaxed the requirement for a complete repeat unit could allow some synthesis to continue. As discussed above, however, the lack of GlcUA would still be an impediment to capsule synthesis due to its requirement for transfer to the cell wall.
Conclusions.
The results of these studies demonstrate that the inability to properly assemble the capsule can be detrimental to the cell, and mutants affected in the assembly process may carry suppressor mutations that affect the phenotypes observed. Although we began the studies with a focus on the role of the side chain, the results strongly point toward Cps2E, the initiating glycosyltransferase, as a central player in the control of polymer assembly. Identifying the further roles of Cps2E and determining the requirements for cell wall association of the polymer are essential to fully understanding the capsule assembly process. The use of cps2K and other deletions to readily generate mutations in cps2E provides a unique means for potentially identifying proteins with which Cps2E interacts and for characterizing a class of glycosyltransferases that is widespread in nature. In addition, the ability to block capsule synthesis at intermediate stages by targeting functionally equivalent enzymes present in many bacteria could provide a novel therapeutic approach to bacterial infections that would be effective because of loss of an important virulence factor and detrimental effects on cell viability.
Acknowledgments
We thank Leigh Millican from the University of Alabama at Birmingham electron microscopy core facility for assistance with the electron microscopy and Robert Cartee for advice regarding membrane isolations and glycosyltransferase assays.
This study was supported by Public Health Service grants AI28457 and T32 AI07041 from the National Institutes of Health.
Footnotes
Published ahead of print on 23 February 2007.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrecubieta, C., E. Garcia, and R. Lopez. 1996. Demonstration of UDP-glucose dehydrogenase activity in cell extracts of Escherichia coli expressing the pneumococcal cap3A gene required for the synthesis of type 3 capsular polysaccharide. J. Bacteriol. 178:2971-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrecubieta, C., E. Garcia, and R. Lopez. 1995. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene 167:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell, G. 1966. New colorimetric methods of sugar anaylsis. Meth Enzymol. 8:85-95. [Google Scholar]
- 5.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender, M. H., R. T. Cartee, and J. Yother. 2003. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J. Bacteriol. 185:6057-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 8.Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitter, T., and H. M. Muir. 1962. A modified uronic acid carbazole reaction. Anal. Biochem. 4:330-334. [DOI] [PubMed] [Google Scholar]
- 10.Breton, C., L. Snajdrova, C. Jeanneau, J. Koca, and A. Imberty. 2006. Structures and mechanisms of glycosyltransferases. Glycobiology 16:29R-37R. [DOI] [PubMed] [Google Scholar]
- 11.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows, L. L., and J. S. Lam. 1999. Effect of wzx (rfbX) mutations on A-band and B-band lipopolysaccharide biosynthesis in Pseudomonas aeruginosa O5. J. Bacteriol. 181:973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caimano, M. J., G. G. Hardy, and J. Yother. 1998. Capsule genetics in Streptococcus pneumoniae and a possible role for transposition in the generation of the type 3 locus. Microb. Drug Resist. 4:11-23. [DOI] [PubMed] [Google Scholar]
- 14.Campbell, R. E., R. F. Sala, I. van de Rijn, and M. E. Tanner. 1997. Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible inhibition by UDP-chloroacetol. J. Biol. Chem. 272:3416-3422. [DOI] [PubMed] [Google Scholar]
- 15.Cartee, R. T., W. T. Forsee, M. H. Bender, K. D. Ambrose, and J. Yother. 2005. CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation. J. Bacteriol. 187:7425-7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartee, R. T., W. T. Forsee, J. S. Schutzbach, and J. Yother. 2000. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J. Biol. Chem. 275:3907-3914. [DOI] [PubMed] [Google Scholar]
- 17.Chaffin, D. O., L. M. Mentele, and C. E. Rubens. 2005. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsK and is required for optimal capsule polymerization and expression. J. Bacteriol. 187:4615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328:307-317. [DOI] [PubMed] [Google Scholar]
- 20.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng, L., D. L. Kasper, T. P. Krick, and M. R. Wessels. 2000. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J. Biol. Chem. 275:7497-7504. [DOI] [PubMed] [Google Scholar]
- 22.Dillard, J. P., M. W. Vandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillard, J. P., and J. Yother. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol. Microbiol. 12:959-972. [DOI] [PubMed] [Google Scholar]
- 24.Dische, Z., and L. B. Shettles. 1951. A new spectrophotometric test for the detection of methylpentose. J. Biol. Chem. 192:579-582. [PubMed] [Google Scholar]
- 25.Dougherty, B. A., and I. van de Rijn. 1993. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity. J. Biol. Chem. 268:7118-7124. [PubMed] [Google Scholar]
- 26.Filisetti-Cozzi, T. M., and N. C. Carpita. 1991. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 197:157-162. [DOI] [PubMed] [Google Scholar]
- 27.Georgellis, D., S. Arvidson, and A. von Gabain. 1992. Decay of ompA mRNA and processing of 9S RNA are immediately affected by shifts in growth rate, but in opposite manners. J. Bacteriol. 174:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves, V. M., M. Takagi, R. B. Lima, H. Massaldi, R. C. Giordano, and M. M. Tanizaki. 2003. Purification of capsular polysaccharide from Streptococcus pneumoniae serotype 23F by a procedure suitable for scale-up. Biotechnol. Appl. Biochem. 37:283-287. [DOI] [PubMed] [Google Scholar]
- 29.Griffith, C. L., J. S. Klutts, L. Zhang, S. B. Levery, and T. L. Doering. 2004. UDP-glucose dehydrogenase plays multiple roles in the biology of the pathogenic fungus Cryptococcus neoformans. J. Biol. Chem. 279:51669-51676. [DOI] [PubMed] [Google Scholar]
- 30.Guidolin, A., J. K. Morona, R. Morona, D. Hansman, and J. C. Paton. 1994. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect. Immun. 62:5384-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy, G. G., A. D. Magee, C. L. Ventura, M. J. Caimano, and J. Yother. 2001. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect. Immun. 69:2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katzen, F., D. U. Ferreiro, C. G. Oddo, M. V. Ielmini, A. Becker, A. Puhler, and L. Ielpi. 1998. Xanthomonas campestris pv. campestris gum mutants: effects on xanthan biosynthesis and plant virulence. J. Bacteriol. 180:1607-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleerebezem, M., R. van Kranenburg, R. Tuinier, I. C. Boels, P. Zoon, E. Looijesteijn, J. Hugenholtz, and W. M. de Vos. 1999. Exopolysaccharides produced by Lactococcus lactis: from genetic engineering to improved rheological properties? Antonie Leeuwenhoek 76:357-365. [PubMed] [Google Scholar]
- 37.Kolkman, M. A., D. A. Morrison, B. A. Van Der Zeijst, and P. J. Nuijten. 1996. The capsule polysaccharide synthesis locus of Streptococcus pneumoniae serotype 14: identification of the glycosyl transferase gene cps14E. J. Bacteriol. 178:3736-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolkman, M. A. B. 1997. Capsular polysaccharide synthesis in Streptococcus pneumoniae. University of Utrecht, Utrecht, The Netherlands.
- 39.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 69:3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengin-Lecreulx, D., L. Texier, M. Rousseau, and J. van Heijenoort. 1991. The murG gene of Escherichia coli codes for the UDP-N-acetylglucosamine:N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase involved in the membrane steps of peptidoglycan synthesis. J. Bacteriol. 173:4625-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2002. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J. Bacteriol. 184:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morona, J. K., R. Morona, and J. C. Paton. 2006. Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease. Proc. Natl. Acad. Sci. USA 103:8505-8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morona, J. K., R. Morona, and J. C. Paton. 1997. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol. Microbiol. 23:751-763. [DOI] [PubMed] [Google Scholar]
- 44.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 45.Niemeyer, D., and A. Becker. 2001. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 183:5163-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paiment, A., J. Hocking, and C. Whitfield. 2002. Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J. Bacteriol. 184:6437-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelosi, L., M. Boumedienne, N. Saksouk, J. Geiselmann, and R. A. Geremia. 2005. The glucosyl-1-phosphate transferase WchA (Cap8E) primes the capsular polysaccharide repeat unit biosynthesis of Streptococcus pneumoniae serotype 8. Biochem. Biophys. Res. Commun. 327:857-865. [DOI] [PubMed] [Google Scholar]
- 48.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rick, P. D., K. Barr, K. Sankaran, J. Kajimura, J. S. Rush, and C. J. Waechter. 2003. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 278:16534-16542. [DOI] [PubMed] [Google Scholar]
- 50.Robbins, P. W., D. Bray, B. M. Dankert, and A. Wright. 1967. Direction of chain growth in polysaccharide synthesis. Science 158:1536-1542. [DOI] [PubMed] [Google Scholar]
- 51.Selinger, D. S., and W. P. Reed. 1979. Pneumococcal adherence to human epithelial cells. Infect. Immun. 23:545-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sieberth, V., G. P. Rigg, I. S. Roberts, and K. Jann. 1995. Expression and characterization of UDPGlc dehydrogenase (KfiD), which is encoded in the type-specific region 2 of the Escherichia coli K5 capsule genes. J. Bacteriol. 177:4562-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, E. E., G. T. Mills, H. P. Bernheimer, and R. Austrian. 1958. The formation of uridine pyrophosphoglucuronic acid from uridine pyrophosphoglucose by extracts of a nonencapsulated strain of pneumococcus. Biochim. Biophys. Acta 28:211-212. [DOI] [PubMed] [Google Scholar]
- 54.Sorensen, U. B., J. Henrichsen, H. C. Chen, and S. C. Szu. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 55.Strominger, J. L., E. S. Maxwell, and H. M. Kalckar. 1957. Determination of UDPG and UTP by means of UDGPG dehydrogenase. Methods Enzymol. 3:974-977. [Google Scholar]
- 56.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 57.van Dam, J. E., A. Fleer, and H. Snippe. 1990. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek 58:1-47. [DOI] [PubMed] [Google Scholar]
- 58.van der Flier, M., N. Chhun, T. M. Wizemann, J. Min, J. B. McCarthy, and E. I. Tuomanen. 1995. Adherence of Streptococcus pneumoniae to immobilized fibronectin. Infect. Immun. 63:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van de Rijn, I., and R. E. Kessler. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Selm, S., M. A. Kolkman, B. A. van der Zeijst, K. A. Zwaagstra, W. Gaastra, and J. P. van Putten. 2002. Organization and characterization of the capsule biosynthesis locus of Streptococcus pneumoniae serotype 9V. Microbiology 148:1747-1755. [DOI] [PubMed] [Google Scholar]
- 61.Ventura, C. L., R. T. Cartee, W. T. Forsee, and J. Yother. 2006. Control of capsular polysaccharide chain length by UDP-sugar substrate concentrations in Streptococcus pneumoniae. Mol. Microbiol. 61:723-733. [DOI] [PubMed] [Google Scholar]
- 62.Wang, L., D. Liu, and P. R. Reeves. 1996. C-terminal half of Salmonella enterica WbaP (RfbP) is the galactosyl-1-phosphate transferase domain catalyzing the first step of O-antigen synthesis. J. Bacteriol. 178:2598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessels, M. R., R. F. Haft, L. M. Heggen, and C. E. Rubens. 1992. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect. Immun. 60:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 88:8317-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitfield, C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75:39-68. [DOI] [PubMed] [Google Scholar]
- 68.Williams, C. A., and M. W. Chase. 1967. Preparation of capsular polysaccharides. Methods Immunol. Immunochem. 1:51-63. [Google Scholar]
- 69.Winkelstein, J. A. 1984. Complement and the host's defense against the pneumococcus. Crit. Rev. Microbiol. 11:187-208. [DOI] [PubMed] [Google Scholar]
- 70.Wugeditsch, T., A. Paiment, J. Hocking, J. Drummelsmith, C. Forrester, and C. Whitfield. 2001. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 276:2361-2371. [DOI] [PubMed] [Google Scholar]
- 71.Yother, J. 2004. Capsules, p. 30-48. In E. I. Toumanen, T. J. Mitchell, D. A. Morrison, and B. G. Spratt (ed.), The pneumococcus. ASM Press, Washington, DC.
- 72.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuasa, R., M. Levinthal, and H. Nikaido. 1969. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J. Bacteriol. 100:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]