Abstract
Background
Increasing age at onset has been associated with worse outcome in rheumatoid arthritis, although there are few data from unselected inception cohorts.
Hypothesis
Increasing age is associated with a higher risk of erosions at presentation, and this increase is not explained by age‐related disease confounders.
Subjects and methods
222 subjects (median onset age 59 years) were studied from a primary‐care‐based register of new‐onset inflammatory polyarthritis. Patients had hand and feet radiographs taken within 12 months from symptom onset. Films were scored by two readers using the Larsen score. The risk of erosions in those aged 50–69 and ⩾70 years at onset was compared with the risk in those aged <50 years both before and after adjustment for possible age‐related disease confounders.
Result
The prevalences of erosions were 22%, 52% and 71% in those aged <50, 50–69 and ⩾70 years at onset equivalent to odds ratios (ORs) (95% confidence intervals (CIs)) of 3.5 (2.2 to 5.7) and 7.4 (4.5 to 12.1), respectively, in the two older age groups. Excluding those with proximal interphalangeal (PIP) erosions alone (due to possible osteoarthritis) did not alter these findings. Adjustments for disease characteristics using logistic regression did not attenuate these findings: adjusted ORs (95% CIs) 3.6 (2.1 to 6.1) and 6.9 (3.8 to 12.2) for age groups 50–69 and ⩾70 years, respectively. The influence of age was stronger than most of the disease‐related variables in predicting erosions in this cohort.
Conclusion
Increasing age at symptom onset is strongly associated with higher occurrence of erosions within the first year unexplained by greater disease severity.
Increasing age is a risk factor for the development of inflammatory polyarthritis in general, as well as for rheumatoid arthritis in particular,1 with evidence that there has recently been a shift towards an older age at onset.2,3 There are also age differences in the strength of the association with risk factors such as the human leucocyte antigen (HLA) DRB1,4 which might suggest that age has an effect on disease phenotype. Some reports have even suggested that late‐onset rheumatoid arthritis is a “different” condition in terms of both aetiology and outcome,5,6,7 although it seems more likely that the influence of age is more complex than a simple dichotomy of early versus late disease.
In general, most studies that have discussed the influence of age on disease outcome have concluded that the older the age at onset, the worse the outcome.5,6,7,8,9 For example, in a nested case–control study, women aged ⩾55 years at onset had more disability and more erosive disease than those with a younger age at onset.10 Further, in a prospective radiographic study comparing older (age >60 years at onset) with younger patients with early rheumatoid arthritis, the older group had radiographic damage greater by 2 years, including, but not restricted to, a greater score for joint space narrowing.11
There are, however, several issues to be discussed when interpreting such data on the effect of age on outcome in inflammatory polyarthritis and rheumatoid arthritis. Firstly, the prevalence of osteoarthritis increases substantially with age, with consequences for attributing the origin of joint‐related pain and disability. Secondly, although radiographic erosions may be a more specific marker of damage in inflammatory polyarthritis, erosions do occur in osteoarthritis and may be confused with those due to rheumatoid arthritis, particularly in the PIP joints. Thirdly, there may be referral bias with respect to age. Thus, elderly patients may be referred only if they have severe disease, whereas disease severity does not influence the referral of younger patients. Finally, it is unclear whether any effect of age is direct or can be explained by the influence of age on identifiable disease characteristics.
We have therefore used the opportunity afforded by studying a primary‐care‐derived cohort of subjects with new‐onset inflammatory polyarthritis to examine the effect of age at disease onset on radiological outcome. Specifically, we have investigated the effect of age at onset on the presence and extent of erosions at presentation and compared this with other disease‐related risk factors. We have also examined whether any effect of age can be explained by underlying associations between age and pattern of disease at presentation.
Subjects and methods
Subjects
Subjects were recruited from the Norfolk Arthritis Register (NOAR)—a primary‐care‐based cohort of subjects with new‐onset inflammatory polyarthritis. Details of NOAR have been described elsewhere.1 In brief, NOAR aims to recruit all new adult attenders at primary care with inflammatory polyarthritis, defined as swelling of two or more joints, lasting for ⩾4 weeks. Subjects who are later given a diagnosis other than non‐specific inflammatory polyarthritis, rheumatoid arthritis or psoriatic arthritis by a rheumatologist are then excluded.
Baseline assessment
Subjects are interviewed and examined within 2 weeks of referral by trained research nurses using a standardised approach. Data are gathered on joint symptoms, and joints are systematically examined for tenderness, swelling and deformity. Subjects complete the Health Assessment Questionnaire (HAQ).12 Blood is taken for rheumatoid factor and C reactive protein (CRP) estimation, and DNA extracted for HLA DRB1 assessment as described elsewhere.13
Radiographic assessment
Radiographs of hands and feet were obtained from all consenting individuals following a policy described previously.14 Since January 2000 all subjects have undergone x ray examination at presentation and are the subject of this report.
Radiographs were read by two investigators using Larsen's method,15 with adjudication by a third in case of disagreement. In all, 30 joints were assessed radiologically: all PIP joints (n = 8), the thumb inflammatory polyarthritis joint (n = 2), all the metacarpophalangeal (MCP) joints (n = 10), both wrists (n = 2) and metatarsophalangeal joints 2–5 (n = 8). Subjects were scored as erosive if any individual joint receiving a Larsen score >1 with the wrist weighted by a factor of five.
Statistical analysis
Age at symptom onset was considered using two approaches. Firstly, it was stratified into three groups: (1) <50 years, (2) 50–70 years and (3) >70 years. The prevalence of erosions and the distribution of the Larsen scores were compared between these age groups. A subgroup analysis was performed to allow for the possible influence of erosive osteoarthritis. Thus, subjects with erosions confined to the PIP joints were identified as a “possible osteoarthritis group” and excluded, with the prevalence of erosions in each age group recalculated. Secondly, the influence of age at onset on Larsen score was modelled using the actual age to determine whether any effect of age was linear.
To identify the possible independent effect of age on any increase in prevalence of erosions, a multivariate model was fitted including the following baseline characteristics: sex, symptom duration at the time of radiograph being obtained, maximal duration of morning stiffness, number of swollen, tender and both swollen and tender joints, HAQ, rheumatoid factor, CRP and the presence of the HLA DRB 1 shared epitope. Two approaches to modelling were then used. Firstly, logistic regression was used to model the influence of age on the presence or absence of erosions, and then negative binomial regression to model the influence of age on the actual Larsen score. All analyses were undertaken using STATA V.8.3. In performing these models, the relative contribution of the other disease factors studied was also evaluated.
Results
There were 222 eligible subjects in the cohort. Table 1 shows their baseline characteristics in the whole cohort, and stratified into the three age groups. Overall, they had mild disease with a baseline prevalence of rheumatoid factor of only 30%. The three age groups had similar disease characteristics at baseline, with the exception of a higher mean HAQ in those aged >70 years at onset and a higher prevalence of the shared epitope in both older age groups compared with those aged <50 years at symptom onset. The time from symptom onset to x ray was not different in the three age groups.
Table 1 Baseline characteristic by age group.
| Characteristic | Whole cohort n = 222 | Age (years) | ||
|---|---|---|---|---|
| <50 n = 63 | 50–69 n = 91 | ⩾70 n = 68 | ||
| Females, n (%) | 150 (68%) | 46 (73%) | 62 (68%) | 42 (62%) |
| Early morning stiffness, median (IQR) (min) | 60 (30–300) | 120 (60–360) | 60 (30–300) | 60 (15–225) |
| Swollen joint count, median (IQR) | 3 (1–8) | 2 (0–6) | 3 (1–9) | 3 (1.5–9) |
| Tender joint count, median (IQR) | 3 (1–9) | 4 (1–8) | 2 (0–10) | 3 (1–7.5) |
| Swollen and tender joint count, median (IQR) | 1(0–3) | 0(0–3) | 1(0–2) | 1 (0–4) |
| HAQ score, median (IQR) | 0.88 (0.38–1.5) | 0.69 (0.25–1.38) | 0.75 (0.13–1.38) | 1.13 (0.63–1.88) |
| Median time in months from onset to x ray (IQR) | 4.8 (2.5–7.3) | 5.3 (2.8–7.5) | 4.2 (2.5–7.0) | 4.7 (2.5–7.4) |
| CRP mg/dl, median (IQR) | 8 (3–21) | 7 (2.5–21) | 8 (3–29) | 10(4–20) |
| Shared epitope, n (%)* | 89 (52%) | 19 (31%) | 38 (52%) | 32 (59%) |
| Rheumatoid factor positive, n (%)† | 64 (31%) | 19 (33%) | 25 (28%) | 20 (32%) |
CRP, C reactive protein; HAQ, Health Assessment Questionnaire; IQR, interquartile range.
*Shared epitope data available for only 173 subjects.
†Rheumatoid factor available only for 208 subjects.
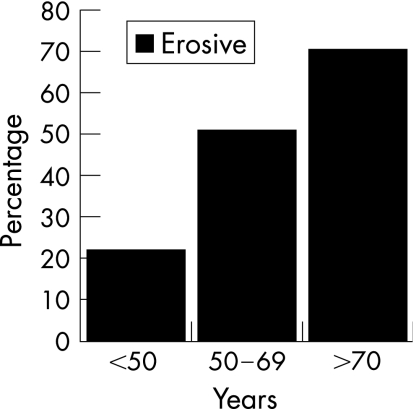
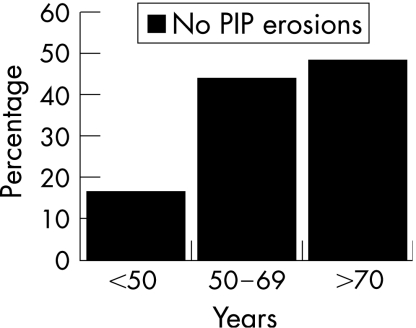
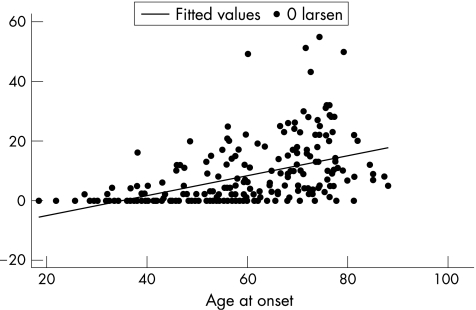
The prevalence of erosions rose with increasing age, from 22% in those aged <50 years to 52% in those aged 50–69 years and 71% in those aged ⩾70 years (fig 1). The prevalence of erosions in the three groups recalculated after excluding subjects with PIP erosions alone also shows a rise from 16% in the youngest age group to 49% in the oldest age group (fig 2). When age was plotted as a continuous variable against the Larsen score, in those subjects with erosions (score>0), the older the patient at presentation, the higher the Larsen score, without any obvious threshold for changing risk (fig 3).
Figure 1 The prevalence of erosions in the three age groups.
Figure 2 The prevalence of erosions after excluding patients with erosions confined to the proximal interphalangeal (PIP) joints.
Figure 3 Scatter plot of Larsen's scores with age using a fitted regression line.
Table 2 shows the distribution of Larsen's scores in the three age groups, both in the group as a whole and in the subset, excluding those with PIP erosions alone. The median Larsen score was 0 in the youngest age group, reflecting the low frequency of erosions. The median score rose with age. Further, excluding those with PIP disease alone did not alter these findings.
Table 2 Larsen scores in each age group and excluding proximal interphalangeal erosions.
| Cohort | Age (years) | |||
|---|---|---|---|---|
| All | <50 | 50–69 | ⩾70 | |
| All, median score (IQR) | 4 (0–13) | 0 (0–2) | 4 (0–13) | 11 (5–22) |
| Excluding those with PIP erosions alone, median score (IQR) | 3 (0–10) | 0 (0–2) | 3 (0–10) | 10 (5–17) |
IQR, interquartile range; PIP, proximal interphalangeal.
Table 3 shows the result of modelling the influence of age and the other risk factors on both the presence of erosions and on Larsen score. Both approaches used patients aged <50 years at onset as the referent group. The logistic regression can be interpreted as the increased likelihood of being erosive at baseline. The negative binomial regression coefficients can be interpreted as the average increase in Larsen score between the age categories. The odds of erosions and the increase in Larsen score increased dramatically with increasing age at symptom onset using logistic regression. This influence of age was much stronger than any of the other variables analysed.
Table 3 Predictors of erosions and Larsen score.
| Characteristic | Risk of erosions OR (95% CI) | Increase in Larsen score multiplier (95% CI) |
|---|---|---|
| Age (years) | ||
| <50 | 1 (referent) | 1 (referent) |
| 50–69 | 3.50 (2.24 to 5.74) | 3.74 (1.82 to 7.70) |
| ⩾70 | 7.39 (4.51 to 12.10) | 8.40 (3.81 to 18.52) |
| Sex | ||
| Male | 1 (referent) | 1 (referent) |
| Female | 0.87 (0.50 to 1.53) | 1.17 (0.75 to 1.84) |
| Swollen joints | ||
| Lowest tertile | 1 (referent) | 1 (referent) |
| Middle tertile | 1.98 (1.03 to 3.82) | 1.41 (0.85 to 2.33) |
| Highest tertile | 2.55 (1.31 to 4.95) | 2.00 (1.21 to 3.32) |
| Tender joints | ||
| Lowest tertile | 1 (referent) | 1 (referent) |
| Middle tertile | 2.23 (1.11 to 4.48) | 1.25 (0.74 to 2.09) |
| Highest tertile | 1.10 (0.67 to 1.82) | 1.34 (0.72 to 2.49) |
| Rheumatoid factor* | ||
| −ve | 1 (referent) | 1 (referent) |
| +ve | 1.65 (0.91 to 2.99) | 1.01 (0.63 to 1.63) |
| HAQ score | ||
| <1 | 1 (referent) | 1 (referent) 1.60 |
| ⩾1 | 1.51 (1.05 to 2.17) | (1.19 to 2.14) |
| CRP | ||
| Lower tertile | 1 (referent) | 1 (referent) |
| Middle tertile | 0.92 (0.47 to 1.82) | 1.21 (0.70 to 2.08) |
| Highest tertile | 1.33 (0.68 to 2.60) | 1.38 (0.81 to 2.37) |
| Shared epitope† | ||
| Number of copies | 1 (referent) | 1 (referent) |
| Any copy | 1.28 (0.71 to 2.30) | 1.26 (0.80 to 1.98) |
CRP, Creative Protein; HAQ, Health Assessment Question
*Shared epitope data available only in 173 subjects.
†Rheumatoid factor available only in 208 subjects.
Multivariate analysis was then undertaken to assess the effect of age after adjustment was made for all the other baseline variables. The results suggest only a modest attenuation of these age‐related risks (table 4); it was seen that the effect of age remained the same.
Table 4 Multivariate analysis of influence of age on erosion status and Larsen score.
| Risk of erosions* OR (95% CI) | Increase in Larsen score multiplier† (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | |
| Age (years) | ||||
| <50 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 50–69 | 3.50 (2.24 to 5.74) | 4.01 (1.80 to 8.94) | 3.74 (1.82 to 7.70) | 4.09 (2.39 to 6.99) |
| ⩾70 | 7.39 (4.51 to 12.10) | 6.90 (4.89 to 16.47) | 8.40 (3.81 to 18.52) | 6.92 (3.90 to 12.29) |
*Using logistic regression.
†Using negative binomial regression.
‡Adjusted for sex, joint counts, symptom duration, Health Assessment Questionnaire score, rheumatoid factor, C reactive protein and shared epitope status.
Discussion
In this study, the prevalence of erosions at presentation was assessed in a primary‐care‐derived cohort, with a specific focus on the influence of age. The data have shown a low frequency and severity of erosions in those presenting below the age of 50 years, but with a strong influence of increasing age on both prevalence and severity. These effects were not explained by older subjects having baseline disease characteristics that are associated with radiological damage. Further, in an attempt to exclude those with erosive osteoarthritis, restricting the analysis to those without disease confined to the PIP joints did not considerably alter these results.
These are the first published data derived from a primary‐care‐based inception cohort on the influence of age on erosions during the first 12 months of disease. Using this approach, we aimed to minimise left censorship and referral bias (whereby the severity threshold for referring older patients to hospital may be higher). Interestingly, these results are different from our previously published analysis on erosion risk in cohorts of NOAR subjects recruited 10 years previously, which did not show an influence of age.16 It is possible, though unlikely, that there has been such a large secular change in the influence of age on disease severity. It is also possible that there may have been changes either in the pattern of patient attendance or in primary care referral by age. In our previous analyses, only selected subjects were invited for x rays on the basis of their satisfying the ACR criteria for rheumatoid arthritis, whereas in the current cohort all subjects were analysed, who were hence less selected.
Our data are compatible with the observations of others5,8,10,11 in clinic‐derived cohorts that older age groups are more likely to be erosive, although the magnitude of the effect in the current study is greater than that previously observed. The increasing influence of age across the three age groups on radiological damage does not suggest that late‐onset arthritis should be considered as a distinct disease entity. Indeed, as shown (fig 3), there is no obvious age threshold, but rather the effect of age seems to be a continuous one, with the age strata used to show the relative effect of age in the different groups. Not all studies have shown an influence of age on erosions,17,18,19 although in all such studies differences in age‐related selection for participation might be important. The fundamental basis behind NOAR is that, by widening recruitment to be based in primary care should militate against such section biases.
Thus, other subject selection factors are to be considered in interpreting data from all such studies. We deliberately chose to include all subjects with inflammatory polyarthritis and not restrict inclusion to only patients who satisfy the ACR criteria.20 We have shown that, in early disease, ACR criteria assignment is unstable, with the proportion satisfying the criteria increasing with duration of observation.2,21 Further, as erosion is one of the key criteria for classification, it induces circularity if both study inclusion criteria and outcome assessment are based on the same data. Indeed, this circularity makes it impossible to interpret studies on the incidence of erosions in newly diagnosed rheumatoid arthritis, particularly in patient populations referred to specialist centres.
Firstly, we could not exclude all selection factors. Thus, it is possible that even in primary care, at the same level of symptoms, younger patients present earlier to medical care. The median interval between recalled onset of joint swelling and baseline assessment was 4.8 months (interquartile range 2.5–7.3 months), and if at all anything was lower in the older age group, this was not significant. Another possibility is that older subjects with milder joint symptoms either do not present to primary care or were “missed” as having inflammatory synovitis by their primary care doctor, and hence not referred to NOAR. As a consequence, NOAR might have interpreted elderly subjects as having more severe inflammatory polyarthritis. This is difficult to exclude, although one piece of evidence against this comes from our previous observation that incidence rises with age.1 A third explanation is that older patients could have had prior episodes of synovitis, which could have subsided but have led to erosions. Such individuals then present after an interval with a new episode of synovitis and do not recall the previous symptoms. As a consequence, the current radiographic appearance is wrongly attributed to current synovitis. In this regard, several years ago, Kellgren and Lawrence22 showed that, in unselected normal population samples, there is an increasing prevalence of erosions with age.
Earlier or more aggressive therapy may influence erosion risk, as we have shown in other subjects in NOAR,23 and an influence of age on treatment choice could be one explanation for our findings. However, the radiographs were taken shortly after the first NOAR visit, and at that stage very few of the subjects in any of the age groups had been started on disease‐modifying antirheumatic drugs.
We were able to consider, in part, whether the higher prevalence of erosions in the elderly may reflect underlying osteoarthritis rather than erosive inflammatory polyarthritis. Distinguishing the aetiology of erosions is not easy, and the Larsen assessment method does not distinguish between the causes. As one test for this, we undertook a subgroup analysis excluding subjects with PIP joint erosions alone, a much more frequent site for osteoarthritis than the MCP joints,24 and the results were little altered. We also analysed the data, restricting consideration just to the MCP joints with similar findings, although the prevalence of erosions was much less.
We attempted to investigate by adjusting for baseline variable whether the age effect could be explained by, for example, evidence of increased inflammatory activity in the older groups. In fact, the latter was not the case (table 1), and, as a consequence, adjusting for baseline status did not alter the results. Thus, some aspect of the ageing process seems to be associated with an increased erosion risk. A possible pathway for this is that older subjects have less cartilage and hence are more susceptible to inflammation‐mediated cartilage degradation. Alternatively, elderly subjects may also have lower bone mineral density, and therefore bone loss in the elderly would occur quicker.
In conclusion, we have shown that increasing age at symptom onset seems to be an independent risk factor for the initial development of erosions, although the exact mechanism is unclear. The effects were surprisingly strong and not easily explained. It is important that other studies with unselected new cohorts verify these findings. The immediate practical consequences of these observations is that the threshold for referring older patients to hospital and starting aggressive disease‐modifying therapy should be the same as, if not lower than, that for younger patients.
Acknowledgements
We thank the substantial support of Professor David Scott and clinical colleagues at the Norfolk and Norwich Hospital and that of the local primary care physicians in recruiting patients and providing access to clinical data.
Abbreviations
CRP - C reactive protein
HAQ - Health Assessment Questionnaire
HLA - human leucocyte antigen
MCP - metacarpophalangeal
NOAR - Norfolk Arthritis Register
PIP - proximal interphalangeal
Footnotes
Funding: The Norfolk Arthritis Register (NOAR) is funded by the UK Arthritis Research Campaign.
Competing interests: None declared.
References
- 1.Symmons D P, Barrett E M, Bankhead C R, Scott D G, Silman A J. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol 199433735–739. [DOI] [PubMed] [Google Scholar]
- 2.Wiles N, Symmons D P, Harrison B, Barrett E, Barrett J H, Scott D G.et al Estimating the incidence of rheumatoid arthritis: trying to hit a moving target? Arthritis Rheum 1999421339–1346. [DOI] [PubMed] [Google Scholar]
- 3.Kaipiainen‐Seppanen O, Aho K. Incidence of chronic inflammatory joint diseases in Finland in 1995[review]. J Rheumatol 20002794–100. [PubMed] [Google Scholar]
- 4.MacGregor A, Ollier W, Thomson W, Jawaheer D, Silman A. HLA‐DRB1*0401/0404 genotype and rheumatoid arthritis: increased association in men, young age at onset, and disease severity. J Rheumatol 1995221032–1036. [PubMed] [Google Scholar]
- 5.Pease C T, Bhakta B B, Devlin J, Emery P. Does the age of onset of rheumatoid arthritis influence phenotype? a prospective study of outcome and prognostic factors. Rheumatology 199938228–234. [DOI] [PubMed] [Google Scholar]
- 6.Hellier J P, Eliaou J F, Daures J P, Sany J, Combe B. HLA‐DRB1 genes and patients with late onset rheumatoid arthritis. Ann Rheum Dis 200160531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajocchi G, La Corte R, Locaputo A, Govoni M, Trotta F. HLA‐DRB1 genes and patients with late onset rheumatoid arthritis. Ann Rheum Dis 200160531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaarela K. Prognostic factors and diagnostic criteria in early rheumatoid arthritis. Scand J Rheum 198557(Suppl)1–54. [DOI] [PubMed] [Google Scholar]
- 9.Luukkainen R, Kaarela K, Isomaki H, Martio J, Kivinieme P. The prediction of radiological destruction during the early stage of rheumatoid arthritis. Clin Exp Rheum 19831295–298. [PubMed] [Google Scholar]
- 10.Mikuls T, Saag K, Criswell L, Merlino L, Cerhan J R. Health related quality of life in women with elderly onset rheumatoid arthritis. J Rheumatol 200330952–957. [PubMed] [Google Scholar]
- 11.van der Heijde D M, van Riel P L, van Leeuwen M A, van 't Hof M A, van Rijswijk M H, van de Putte L B. Older versus younger onset rheumatoid arthritis: results at onset and after 2 years of a prospective followup study of early rheumatoid arthritis. J Rheumatol 1991181285–1289. [PubMed] [Google Scholar]
- 12.Kirwan J R, Reeback J S. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 198625206–209. [DOI] [PubMed] [Google Scholar]
- 13.Thomson W, Harrison B, Ollier B, Wiles N, Payton T, Barrett J.et al Quantifying the exact role of HLA‐DRB1 alleles in susceptibility to inflammatory polyarthritis: results from a large, population‐based study. Arthritis Rheum 199942757–762. [DOI] [PubMed] [Google Scholar]
- 14.Bukhari M, Harrison B, Lunt M, Scott D G, Symmons D P, Silman A J. Time to first occurrence of erosions in inflammatory polyarthritis: results from a prospective community‐based study. Arthritis Rheum 2001441248–1253. [DOI] [PubMed] [Google Scholar]
- 15.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 197718481–491. [DOI] [PubMed] [Google Scholar]
- 16.Brennan P, Harrison B, Barret E, Chakarvarty K, Scott D, Silman A.et al A simple algorithm to predict the development of radiographic erosions in patients with early rheumatoid arthritis: prospective cohort study. Br Med J 1996313471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos I A, Katsimbri P, Alamanos Y, Voulgari P V, Drosos A A. Early rheumatoid arthritis patients: relationship of age. Rheumatol Int 20032370–74. [DOI] [PubMed] [Google Scholar]
- 18.Peltomaa R, Leirisalo‐Repo M, Helve T, Paimela L. Effect of age on 3 year outcome in early rheumatoid arthritis. J Rheumatol 200027638–643. [PubMed] [Google Scholar]
- 19.van Schaardenburg D, Lagaay A M, Breedveld F C, Hijmans W, Vandenbroucke J P. Rheumatoid arthritis in a population of persons aged 85 years and over. Br J Rheumatol 199332104–109. [DOI] [PubMed] [Google Scholar]
- 20.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 21.Harrison B J, Symmons D P, Barrett E M, Silman A J. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. American Rheumatism Association. J Rheumatol 1998252324–2330. [PubMed] [Google Scholar]
- 22.Kellgren J H, Lawrence J S. Radiological assessment of rheumatoid arthritis. Ann Rheum Dis 195716485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukhari M A, Wiles N J, Lunt M, Harrison B J, Scott D G, Symmons D P.et al Influence of disease‐modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum 20034846–53. [DOI] [PubMed] [Google Scholar]
- 24.Solovieva S, Vehmas T, Riihimaki H, Luoma K, Leino‐Arjas P. Hand use and patterns of joint involvement in osteoarthritis. A comparison of female dentists and teachers. Rheumatology 200544521–528. [DOI] [PubMed] [Google Scholar]