Abstract
Objectives
To calculate the probabilities for rheumatoid arthritis in a consecutive cohort of patients during diagnostic investigation. Different logistic regression models evaluating the value of human leucocyte antigen (HLA)‐shared epitope determination and testing for rheumatoid factor and anti‐citrullinated protein/peptide antibodies (ACPA) were fitted.
Methods
1003 consecutive patients were included in the study, presenting a new diagnostic problem for which rheumatoid arthritis was included in the differential diagnosis. All patients were tested for ACPA, rheumatoid factor and HLA‐shared epitope.
Results
After 1 year, diagnoses were established: 153 patients had definite rheumatoid arthritis and 629 patients had rheumatoid arthritis excluded. Rheumatoid factor, used as a continuous marker, is useful in evaluating the probability for rheumatoid arthritis. Combined rheumatoid factor and shared epitope testing may provide additional predictive information, but combined ACPA and rheumatoid factor testing is superior. The redundancy of shared epitope testing in a model that includes ACPA testing can be explained by the high association between ACPA and shared epitope both in patients with rheumatoid arthritis and in those with non‐rheumatoid arthritis. The value of rheumatoid factor testing increased if patients presented with at least one swollen joint at baseline.
Conclusion
Valid probabilities for rheumatoid arthritis during routine diagnostic investigation were calculated, and showed that the potential additional value of shared epitope testing disappears when ACPA testing is available. Combined rheumatoid factor and ACPA testing is useful, especially when rheumatoid factor is considered as a continuous parameter reflecting an increasing probability for rheumatoid arthritis at higher rheumatoid factor titres. The value of (continuous) rheumatoid factor testing increases when the a priori chance is higher.
Diagnosis and intensive treatment at an early stage in rheumatoid arthritis is an important factor in slowing its radiological progression.1 Although the diagnosis of rheumatoid arthritis is mainly based on clinical features, these might be insufficient in an early stage of the disease, hampering clinical diagnosis. Therefore, additional serological and genetic tests may be useful. The oldest and best‐known serological antibody test is the rheumatoid factor, which is part of the revised American College of Rheumatology criteria for rheumatoid arthritis.2 More recently, anti‐citrullinated protein/peptide antibodies (ACPA) have been described. These are highly specific markers for rheumatoid arthritis and combine a good sensitivity (45–80%) with a high specificity (89–100%).3,4,5 Detection of ACPA can be achieved using the antigenic substrates citrullinated peptide A (pepA) and citrullinated peptide B (pepB) incorporated in a line immunoassay (LIA),6 or by ELISA tests that are available for cyclic citrullinated peptides7 and deiminated fibrinogen.8 All these last‐generation assays display comparable sensitivities and specificities.4,9 Genetic markers might also have a role in the diagnosis of rheumatoid arthritis; much attention has been given to the human leucocyte antigen (HLA)‐shared epitope, which is found more often in patients with rheumatoid arthritis than in controls.10,11 As rheumatoid factor was the only available serological marker until relatively recently, although not recommended, an additional assessment for the presence of the shared epitope was sometimes performed.
Combinations of rheumatoid factor, shared epitope and ACPA have been used as such, or with other clinical or radiological measures, in models to predict rheumatoid arthritis or radiological progression.12,13,14 The predictive value of these models depends on (1) the characteristics of the investigated population and (2) the prevalence (or a priori chance) of rheumatoid arthritis or persistent erosive disease, varying from 24.2% to 68% between different early arthritis cohorts.12,15
Although most of these models have been applied in early arthritis cohorts, few data are available about the combined value of rheumatoid factor, shared epitope and ACPA testing for the diagnosis of rheumatoid arthritis in a routine clinical diagnostic set up. The aims of the study were threefold: to test the value of rheumatoid factor, ACPA and shared epitope profiles in different models to evaluate the probability for rheumatoid arthritis; to assess the added value of shared epitope and rheumatoid factor testing now that ACPA testing is widely available; and to investigate the optimal combination of these three parameters.
Patients and methods
Patients
This analysis is based on a prospective study in which 1003 consecutive patients from three academic and non‐academic centres were enrolled16: the Department of Rheumatology, Ghent University Hospital (Ghent, Belgium); the Locomotor Center, Elisabeth Hospital (Sijsele‐Damme, Belgium); and the Department of Rheumatology, St Augustinus Hospital (Wilrijk, Belgium). The local ethics committees approved this study, and informed consent was obtained from all patients.
Patients were seen by one of the participating rheumatologists, and consecutively entered the study if they presented with a new diagnostic problem for which rheumatoid arthritis was included in the differential diagnosis. This setting typically reflects the case where a rheumatologist would request rheumatoid factor or ACPA testing. Blood was taken at inclusion; serum samples obtained were aliquoted and frozen at −20°C, and whole blood was stored at −80°C. Participating rheumatologists were asked to fill in a file at baseline and after 1 year of follow‐up asking for the clinical diagnosis established by the treating rheumatologist by ticking a box containing one of the following diagnoses (in ascending probability for rheumatoid arthritis): definite non‐rheumatoid arthritis, potential rheumatoid arthritis, probable rheumatoid arthritis and non‐rheumatoid arthritis. To improve the comparability of our results, further classification of all patients with rheumatoid arthritis was performed after 1 year by systematically checking the (cumulative) rheumatoid arthritis classification criteria by an independent investigator.2 Patients fulfilling both the clinical diagnosis for definite rheumatoid arthritis and the classification criteria were further taken into account as patients with rheumatoid arthritis. Patients in whom rheumatoid arthritis was excluded were taken as the non‐rheumatoid arthritis control group.
Rheumatoid factor
Rheumatoid factor was determined by the latex fixation test. A suspension of uniform polystyrene particles sensitised in glycine buffer with heat‐altered human IgG (Difco Laboratories, Detroit, Michigan, USA) was diluted 1:20 and incubated with progressive dilutions of human sera in microtitre wells. The reagents were mixed and incubated at 37°C for 2 h. The plates were then shaken gently and inspected for observable agglutination. Titres were converted to U/ml using a reference serum, to correct for interassay variation. A low (>95% specificity level, 25 U/ml) and a high (>98% specificity level, 100 U/ml) cut‐off was defined on the basis of a previously described cohort.4
Detection of anti‐pepA antibodies by LIA
Anti‐pepA antibodies were detected by a research LIA containing the synthetic citrullinated peptide referred to as pepA (INNO‐LIA RA ‐ for research use only; Innogenetics, Ghent, Belgium).4,6 Air‐dried strips were scanned using a HP Scanjet 5P scanner. A reference sample was included in each test run. To minimise test‐to‐test variability, the scan values of individual samples were corrected by dividing them by the scan values of the cut‐off sample. Anti‐pepA positivity was defined as a corrected scan value of ⩾1, at which the test had a specificity of ⩾98.5% and a sensitivity of 63.6% in an independent cohort of patients.4
HLA typing by INNO‐line probe assay technology
DNA was extracted from whole‐blood samples and amplified using the INNO‐line probe assay HLA‐DRB1 or HLA‐DRB decoder amplification kits (Innogenetics) as instructed by the manufacturer. HLA typing was performed with the INNO‐line probe assay HLA‐DRB1 or HLA‐DRB decoder kits (Innogenetics), which are based on the reverse hybridisation principle; specific oligonucleotide probes are immobilised as parallel lines on membrane‐based strips. The amino acid sequences QRRAA, QKRAA and RRRAA at positions 70–74 constitute the rheumatoid arthritis‐shared epitope sequence. Patients were classified into two groups according to the inheritance of zero versus one or two copies of the shared epitope.
Statistical methods
The dataset described previously,16 was analysed by logistic regression techniques. Different models with all possible combinations of anti‐pepA antibodies, shared epitope and rheumatoid factor results as explanatory variables, and rheumatoid arthritis diagnosis as explained variable were fitted with logistic regression. The validity of the models was confirmed by fitting a full model and performing a backward elimination of the interaction terms. Logistic regression models are an extension of the general linear regression and fit S‐shaped curves by modelling the logit [ = log(x/(1/x))] of the probabilities for a dichotomous outcome, in this case the probability for rheumatoid arthritis ( = π), using the formula logit(π) = log(π/(1−π)) = α+β1x1+β2x2+ …. The predicted probabilities thus obtained can be plotted against the different explanatory variables x.17 This method allows us to evaluate the variables in a continuous manner and to visualise the models so that they are easier to interpret.
We calculated odds ratios (ORs) and their 95% confidence intervals as a measure of the correlation between dichotomous variables. Homogeneity between marginal ORs was calculated with the Breslow–Day statistic. Common ORs were calculated by the Mantel–Haenszel test.17
The analyses were performed using two classical statistical packages: SPSS V.12.0 and S‐Plus V.6.1 (Insightful Corporation, Seattle, Washington, USA).
Results
Patients' characteristics
After 1 year of follow‐up, the treating rheumatologist diagnosed each patient according to the following categories: definite rheumatoid arthritis (n = 153), probable rheumatoid arthritis (n = 72), potential rheumatoid arthritis (n = 75), non‐rheumatoid arthritis (n = 629) or lost to follow‐up (n = 74). Only patients diagnosed by their treating rheumatologist as definite rheumatoid arthritis and fulfilling the revised American College of Rheumatology criteria for rheumatoid arthritis2 were further considered in the rheumatoid arthritis‐group (n = 144). The control population with non‐rheumatoid arthritis (n = 629) had the following diagnoses: osteoarthritis (38%), abarticular rheumatic symptoms (including peri‐arthritis scapulohumeralis, non‐rheumatic tendinopathies, etc; 14%), spondyloarthropathy (10%), connective tissue diseases (including polymyalgia rheumatica (15%), psoriatic arthritis (7%), crystal induced arthritis (1%), fibromyalgia and aspecific arthralgias (5%)), and other and undifferentiated diseases (including infections, malignancies and neurological disorders (10%)). We lost nine patients with non‐rheumatoid arthritis and three patients with rheumatoid arthritis, because of lack of serum or DNA samples of good quality. The mean age of the patients with rheumatoid arthritis and non‐rheumatoid arthritis was 58 and 51 years, respectively. In both populations, 66% of patients were women. The mean duration of symptoms was 19.3 months in the rheumatoid arthritis group and 15.9 months in the non‐rheumatoid arthritis group. We further identified a subpopulation of 498 patients with at least one swollen joint at baseline, including 134 patients with rheumatoid arthritis and 230 patients with non‐rheumatoid arthritis.
Test results
A total of 620 patients with non‐rheumatoid arthritis and 141 patients with rheumatoid arthritis were tested for anti‐pepA antibodies, rheumatoid factor and shared epitope. A positive anti‐pepA result was observed in 78 (55.3%) patients with rheumatoid arthritis and 13 (2.1%) patients with non‐rheumatoid arthritis. Median pepA scan values were 2.15 (range 0–9.62) for patients with rheumatoid arthritis and 0.04 (range 0–6.07) for patients with non‐rheumatoid arthritis. In all, 90 (63.8%) patients with rheumatoid arthritis and 264 (42.6%) patients with non‐rheumatoid arthritis carried at least one copy of the shared epitope. Two copies were found in 32/141 (21.1%) patients with rheumatoid arthritis and in 30/620 (5.1%) patients who did not have rheumatoid arthritis. The median rheumatoid factor titres were 50 U/ml (range 0–1600 U/ml) for the patients with rheumatoid arthritis and 0 U/ml (range 0–1600 U/ml) for the patients with non‐rheumatoid arthritis.
Comparison between positive predictive values and predicted probabilities
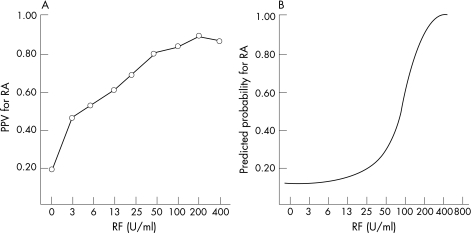
Figure 1 shows the plots of the positive predictive values (PPVs) and predicted probabilities as a function of rheumatoid factor titres. PPVs were calculated by defining different cut‐offs at different titre steps and plotted against rheumatoid factor or anti‐pepA antibody titres (fig 1A). Figure 1B shows the predicted probabilities, calculated by logistic regression.
Figure 1 Plots of positive predictive values (PPV) and predicted probability for rheumatoid arthritis (RA) against rheumatoid factor (RF). PPVs were calculated by defining different cut‐offs at different titre (A). Predicted probabilities were calculated by logistic regression (B).
Logistic regression models in the global population
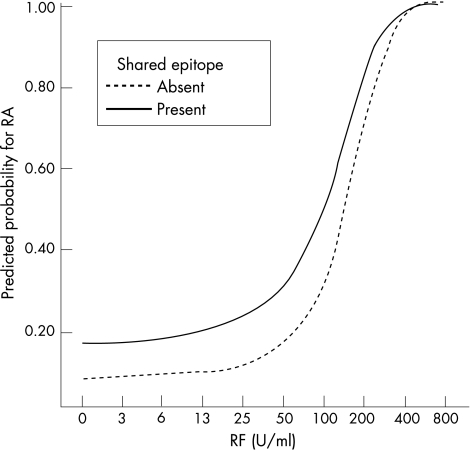
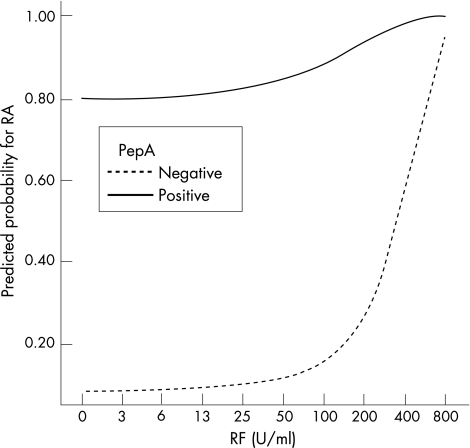
Different logistic regression models were fitted with different combinations of anti‐pepA, rheumatoid factor and shared epitope testing. These analyses showed that ACPA testing in combination with shared epitope has no additional value. This resulted in two final models: (1) a model with combined rheumatoid factor and shared epitope testing and (2) a model with combined rheumatoid factor and ACPA testing. The results of these two models are visualised in the predicted probability plots in figs 2 and 3, showing that the added value of combined rheumatoid factor and shared epitope testing is limited compared with combined rheumatoid factor and ACPA testing.
Figure 2 Plot of the predicted probabilities as a function of rheumatoid factor (RF) titres and shared epitope testing. This figure illustrates that shared epitope testing has a small additional value to RF testing for the diagnosis of rheumatoid arthritis (RA).
Figure 3 Plots of the predicted probabilities as a function of rheumatoid factor (RF) titres and anti‐citrullinated protein/peptide antibodies (ACPA) testing. This figure illustrates that ACPA testing has a big additional value to RF testing, especially in the low RF range. PepA, citrullinated peptide A; RA, rheumatoid arthritis.
Logistic regression models in the subpopulation of patients with at least one swollen joint at baseline
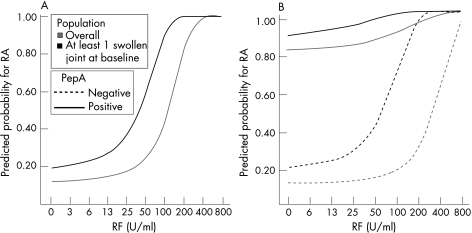
As observed in the global population (continuous), rheumatoid factor testing has additional value to ACPA testing alone. Moreover, in this subpopulation with at least one swollen joint, additional (continuous) rheumatoid factor testing seems to add more value than in the global population: lower rheumatoid factor titres become more relevant. The predicted probability curves are shifted up and have steeper slopes (fig 4).
Figure 4 Plots of the predicted probabilities as a function of rheumatoid factor (RF) titres in patients with at least one swollen joint at baseline. In this figure, we evaluated the effect of the baseline presence of at least one swollen joint on the interpretation of RF results. (A) We evaluated single continuous RF testing in patients with at least one swollen joint compared with the model from fig 3 (grey). (B) We evaluated a model of combined RF–anti‐citrullinated protein/peptide antibody testing calculated in the global population (grey, fig 3) compared with a model calculated in the subgroup of patients with at least one swollen joint at baseline (black). PepA, citrullinated peptide A; RA, rheumatoid arthritis.
Correlation between shared epitope and anti‐pepA antibody positivity
When the dataset was split into patient groups with rheumatoid arthritis and with non‐rheumatoid arthritis, we obtained the following marginal ORs for the association of anti‐pepA antibody positivity with shared epitope positivity: 4.63 (95% confidence interval (CI) 1.26 to 17; p = 0.011) for patients with non‐rheumatoid arthritis and 4.21 (95% CI 2.03 to 8.74; p<0.001) for patients with rheumatoid arthritis. Application of the Breslow–Day test indicated that the ORs for the association between shared epitope and anti‐pepA antibody positivity were not significantly different (which also means that there is no interaction between shared epitope and anti‐pepA antibody positivity). This allowed us to calculate an overall common OR for the relationship between anti‐pepA antibody positivity and shared epitope positivity, which was 4.31 (95% CI 2.28 to 8.17; p<0.001).
Correlation between the shared epitope and rheumatoid factor positivity
The ORs for rheumatoid factor positivity with shared epitope positivity at the low cut‐off were 0.72 (95% CI 0.42 to 1.24; p = NS) for the patients with non‐rheumatoid arthritis and 3.1 (95% CI 1.5 to 6.5; p = 0.002) for the patients with rheumatoid arthritis. Using the high rheumatoid factor cut‐off, the ORs were 0.5 (95% CI 0.165 to 1.716; p = NS) for the patients with non‐rheumatoid arthritis and 2.8 (95% CI 1.3 to 5.8; p = 0.001) for the patients with rheumatoid arthritis. However, when corrected for anti‐pepA antibody positivity, the significant association between rheumatoid factor and shared epitope, initially observed in the population with rheumatoid arthritis, disappeared. The ORs thus obtained were 1.7 (95% CI 0.5 to 3.1; p = NS) at the low rheumatoid factor cut‐off and 1.7 (95% CI 0.7 to 3.8, p = NS) using the high rheumatoid factor cut‐off.
Discussion
The set‐up of this study represents real‐life clinical practice by the inclusion of consecutive patients seen by a rheumatologist (two non‐academic centres and one university centre), for a new diagnostic problem in which rheumatoid arthritis was included in the differential diagnosis. After 1 year, diagnoses were established as rheumatoid arthritis, non‐rheumatoid arthritis and persistent undifferentiated disease. To avoid misclassifications, the patients with persistent undifferentiated disease were further excluded from this analysis. Such a study design allows the calculation of representative predictive values and the estimation of representative probabilities for rheumatoid arthritis. This contrasts with other types of case–control studies, where the calculated predictive values should not be extrapolated, as predictive values depend on the prevalence (or the a priori chance) of the disease.16,18,19 In contrast with the classical exploration by means of sensitivity, specificity and predictive value, we evaluated the (predicted) probabilities for disease (figs 1B, 2, 3, 4 and 6). The use of probabilities is especially helpful when a continuous marker known to express higher titres in patients with diseased than in those without disease is considered (which is the case for rheumatoid factor testing). The use of PPVs requires the definition of a cut‐off point (eg, a rheumatoid factor cut‐off at 25 U/ml, which corresponds to a PPV of 61.5% in this study). This cut‐off has the disadvantage that a patient who displays a titre of 100 U/ml still has a greater chance of having rheumatoid arthritis than a patient with a titre of only 25 U/ml. Therefore, the use of PPVs may overestimate the probability for disease in patients displaying low positive titres (fig 1A, compared with fig 1B). In this analysis, we used logistic regression to calculate these probabilities. Although logistic regression is a parametric regression technique, and therefore provides only an estimate for the probabilities by extrapolation, it has the great advantage that it easily allows the exploration of combinations of different markers in a continuous and categorised manner. This is in contrast with the commonly used “and/or combinations” at preset sensitivity or specificity levels for which dichotomisation by defining a cut‐off is needed.15,16,20
Plotting (predicted) probabilities by means of logistic regression, as performed in these analyses, should not be confused with the cumulative probability plots, described by Landewé and van der Heijde.20
We thus calculated (predicted) probabilities for rheumatoid arthritis by means of logistic regression, using different combinations of rheumatoid factor, the presence of shared epitope and anti‐pepA antibody testing. These analyses showed that shared epitope testing did not significantly contribute to a model where anti‐pepA antibody testing is already present. In contrast, shared epitope testing contributed significantly when only rheumatoid factor testing was performed (fig 2). The redundancy of shared epitope testing when ACPA testing is available was also shown in a model for prediction of radiological progression and persisting erosive disease.12,14 This redundancy of shared epitope testing when ACPA testing is available can be explained by the high association (without interaction for diagnosis of rheumatoid arthritis) between shared epitope and ACPA in patients with rheumatoid arthritis and non‐rheumatoid arthritis (OR 4.3). It is important to highlight that this association between ACPA and shared epitope is also observed in patients who did not have rheumatoid arthritis. The association between ACPA and shared epitope has also been described in patients with rheumatoid arthritis and undifferentiated arthritis, and has led to hypotheses about the induction of ACPA.3,22,23,24,25,26,27,28,29,30 Interestingly, this association between ACPA and shared epitope might also be the reason for the observed association between rheumatoid factor and shared epitope, which disappears after correction for ACPA positivity. Berglin et al29 showed that combined testing for ACPA and shared epitope had an additional value for predicting rheumatoid arthritis in healthy blood donors. This can be explained by the fact that some shared epitope‐positive blood donors who later developed rheumatoid arthritis had APCA that were not yet detectable at the time of blood sampling: only 16/34 (47%) of the shared epitope‐positive patients who later developed rheumatoid arthritis were ACPA positive (in contrast with 78% in this study).
In contrast with the clear association between ACPA and the shared epitope, there seems to be no (or only a weak) correlation between rheumatoid factor and the shared epitope.4,22,23 This explains why combined shared epitope and rheumatoid factor testing displayed a statistically significant additional value to rheumatoid factor testing alone, as displayed in fig 2. However, this additional value is limited when compared with the important additional value provided by the combination of ACPA and rheumatoid factor testing (fig 3).
Interestingly, these models also show that the probability for rheumatoid arthritis, given a positive ACPA test, is <80% when rheumatoid factor is negative, but increases when higher rheumatoid factor titres are present. At high rheumatoid factor titres, the value of additional ACPA testing seems to be reduced (fig 3). In contrast, ACPA testing has more added value when only intermediate positive rheumatoid factor titres are displayed. The models from figs 1–3 have been calculated in a population of patients in whom the treating rheumatologist would routinely ask for a rheumatoid factor test. We also evaluated the value of rheumatoid factor and ACPA testing in the subgroup of patients with at least one swollen joint at baseline (fig 4). These figures show that intermediate rheumatoid factor titres become more relevant when the a priori chance for rheumatoid arthritis is higher.
These findings might be used to propose multistep testing models in which different rheumatoid factor cut‐offs are defined. In such models, additional ACPA testing might not be required in patients with a high positive rheumatoid factor test or with an intermediate high positive rheumatoid factor but with a high clinical probability for rheumatoid arthritis.13 In contrast, additional ACPA testing seems to add much value in cases with low positive rheumatoid factor. The value of the combined testing of rheumatoid factor and ACPA has also been shown in models of early or undifferentiated arthritis to predict rheumatoid arthritis or erosive disease.12,32,33 Logistic regression can be useful in evaluating the diagnostic value of different laboratory tests by different models and plots. The conditions for the generalisation of those models and plots are similar to those for generalised predictive values (PPV, negative predictive value): populations and a priori chance for disease should be similar.34 Logistic regression can also be used to construct prediction models including variables from medical history and physical examination, but generalisation of such models may be more difficult when more variables are used.12,35,36 To conclude, this study provides unbiased models to calculate the probabilities for the development of rheumatoid arthritis in a diagnostic set‐up. Although not recommended in daily clinical practice,37 we showed that, if ACPA results are not available, shared epitope testing may add some diagnostic information in addition to rheumatoid factor results. However, as ACPA testing has become available, additional shared epitope testing seems no longer appropriate in a diagnostic investigation, because of the high correlation between ACPA and the shared epitope. Finally, a diagnostic strategy that combines ACPA testing and rheumatoid factor testing in a single‐step or multistep method would seem superior to single rheumatoid factor or single ACPA testing alone. Rheumatoid factor testing is especially useful when it is considered as a continuous parameter that reflects a higher probability for rheumatoid arthritis when higher titres are displayed.
Acknowledgements
We thank Luc De Clercq, Lieve Schatteman and Stefaan Poriau for their collaboration in the recruitment and follow‐up of the patients.
Abbreviations
ACPA - anti‐citrullinated protein/peptide antibodies
HLA - human leucocyte antigen
LIA - line immunoassay
PepA - citrullinated peptide A
PepB - citrullinated peptide B
PPV - positive predictive value
Footnotes
Funding: BVC was supported by a concerted action grant GOA 2001/12051501 from Ghent University, Ghent, Belgium.
Competing interests: AU and LM are employees of Innogenetics, Ghent, Belgium.
References
- 1.Jones G, Halbert J, Crotty M, Shanahan E M, Batterham M, Ahern M. The effect of treatment on radiological progression of rheumatoid arthritis: a systematic review of randomised placebo‐controlled trials. Rheumatology (Oxford) 2003426–13. [DOI] [PubMed] [Google Scholar]
- 2.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 3.Vossenaar E R, van Venrooij W J. Anti‐CCP antibodies, a highly specific marker for (early) rheumatoid arthritis. Clin Appl Immunol Rev 20044239–262. [Google Scholar]
- 4.De Rycke L, Peene I, Hoffman I E A, Kruithof E, Union A, Meheus L.et al Rheumatoid factor and anti‐citrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra‐articular manifestations. Ann Rheum Dis 2004631587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Söderlin M K, Kastbom A, Kautiainen H, Leirisalo‐Repo M, Strandberg G, Skogh T. Antibodies against cyclic citrullinated peptide (CCP) and levels of cartilage oligomeric matrix protein (COMP) in very early arthritis: relation to diagnosis and disease activity. Scand J Rheumatol 200433185–188. [DOI] [PubMed] [Google Scholar]
- 6.Union A, Meheus L, Humbel R L, Conrad K, Steiner G, Moereels H.et al Identification of citrullinated rheumatoid arthritis‐specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum 2002461185–1195. [DOI] [PubMed] [Google Scholar]
- 7.Schellekens G A, Visser H, de Jong B A W, van den Hoogen F H J, Hazes J M W, Breedveld F C.et al The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 200043155–163. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira L, Chapuy‐Regaud S, Constantin A, Clavel C, Sebbag M, Cantagrel A.et al Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for early rheumatoid arthritis diagnosis. Arthritis Res Ther 20035S6 [Google Scholar]
- 9.Nielen M, van der Horst A, van Schaardenburg D, van der Horst‐Bruinsma I, van de Stadt R, Aarden L.et al Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and protnostic value in early arthritis. Ann Rheum Dis 2005641199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winchester R, Dwyer E, Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheum Dis Clin North Am 199218761–783. [PubMed] [Google Scholar]
- 11.Visser H, le Cessie S, Vos K, Breedveld F C, Hazes J M. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 200246357–365. [DOI] [PubMed] [Google Scholar]
- 12.Nell V, Machold K P, Stamm T A, Eberl G, Heinzl H, Uffmann M.et al Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 13.Kroot E J, De Jong B A W, Van Leeuwen M A, Swinkels H, Van den Hoogen F H J, van't Hof M.et al The prognostic value of anti‐cyclic citrullinated peptide antibody in patients with recent onset rheumatoid arthritis. Arthritis Rheum 2000431831–1835. [DOI] [PubMed] [Google Scholar]
- 14.Jansen A, van der Horst‐Bruinsma I E, van Schaardenburg D, van de Stadt R J, de Koning M H M T, Dijkmans B A C. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol 2002292074–2076. [PubMed] [Google Scholar]
- 15.Hoffman I E A, Peene I, Pottel H, Union A, Hulstaert F, Meheus L.et al Diagnostic performance and predictive value of rheumatoid factor, anti‐citrullinated peptide antibodies and the HLA shared epitope for the diagnosis of rheumatoid arthritis. Clin Chem 200551261–263. [DOI] [PubMed] [Google Scholar]
- 16.Agresti A.An introduction to categocial data analysis. New York: John Wiley and Sons, 1996
- 17.Halpern E F, Gazelle G S. Probability in radiology. Radiology 200322612–15. [DOI] [PubMed] [Google Scholar]
- 18.Kamoun M. Diagnostic performance and predictive value of anti‐citrullinated peptide antibodies for diagnosis of rheumatoid arthritis: toward more accurate detection? Clin Chem 20055112–13. [DOI] [PubMed] [Google Scholar]
- 19.Vallbracht I, Rieber J, Oppermann M, Förger F, Siebert U, Helmke K. Diagnostic and clinical value of anti‐cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 2004631079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landewe R, van der Heijde D. Radiographic progression depicted by probability plots: presenting data with optimal use of individual values. Arthritis Rheum 200450699–706. [DOI] [PubMed] [Google Scholar]
- 21.van Gaalen F A, van Aken J, Huizinga T W, Schreuder G M, Breedveld F C, Zanelli E.et al Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum 2004502113–2121. [DOI] [PubMed] [Google Scholar]
- 22.De Rycke L, Nicholas A, Cantaert T, Kruithof E, Echols J D, Vandekerckhove B.et al Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase are pathophysiologically relevant antigenic determinants of rheumatoid arthritis‐specific humoral autoimmunity. Arhtiris Rheum 2005522323–2330. [DOI] [PubMed] [Google Scholar]
- 23.Sebbag M, Chapuy‐Regaud S, Auger I, Petit‐Texeira E, Clavel C, Nogueira L.et al Clinical and pathophysiological significance of the autoimmune response to citrullinated proteins in rheumatoid arthritis. Jt Bone Spine 200471493–502. [DOI] [PubMed] [Google Scholar]
- 24.Hill J A, Southwood S, Sette A, Jevnikar A M, Bell D A, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high‐affinity peptide interaction with the rheumatoid arthritis‐associated HLA‐DRB1*0401 MHC class II molecule. J Immunol 200315538–541. [DOI] [PubMed] [Google Scholar]
- 25.Quinn M A, Gough A K, Green M J, Devlin J, Hensor E M, Greenstein A.et al Anti‐CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology (Oxford) 200645478–480. [DOI] [PubMed] [Google Scholar]
- 26.Huizinga T W, Amos C I, van der Helm‐van Mil A H, Chen W, van Gaalen F A, Jawaheer D.et al Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA‐DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 2005523433–3438. [DOI] [PubMed] [Google Scholar]
- 27.Linn‐Rasker S P, van der Helm‐van Mil A H, Van Gaalen F A, Kloppenburg M, de Vries R, le Cessie S.et al Smoking is a risk factor for anti‐CCP antibodies only in RA patients that carry HLA‐DRB1 shared epitope alleles. Ann Rheum Dis 200665366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J.et al A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA‐DR (shared epitope)‐restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 20065438–46. [DOI] [PubMed] [Google Scholar]
- 29.Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, Van Venrooij W J.et al A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA‐DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther 20046R303–R308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gaalen F A, Linn‐Rasker S P, van Venrooij W J, de Jong B A, Breedveld F C, Verweij C L.et al Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arhthritis in patients with undifferentiated arthritis. A prospective cohort study. Arthritis Rheum 200450709–715. [DOI] [PubMed] [Google Scholar]
- 31.Lindqvist E, Eberhardt K, Bendtzen K, Heinegard D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 200564196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glas A S, Lijmer J G, Prins M H, Bonsel G J, Bossuyt P M. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003561129–1135. [DOI] [PubMed] [Google Scholar]
- 33.Bellman R.“Curse of dimensionality”. Adaptive control processes: a guided tour. Princeton, NJ; Princeton University Press 1961
- 34.Van Looy S, Meeus J, Wyns B, Vander Cruyssen B, Boullart L, De Keyser F. “Comparison of Machine Learning models for prediction of dose increase in patients with rheumatoid arthritis.” Proceedings of the Ninth International Conference on Engineering Applications of Neural Networks (EANN05), 24–26 August 2005, Lille, France, 189–96
- 35.Symmons D P, Ollier W E, Brennan P, Silman A J. Should patients with recent onset rheumatoid arthritis be offered genetic screening? Ann Rheum Dis 199655407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Loay S, Meeus J, Wyns B, Vander Conyssen B, Boullart L, De Keyser F. Comparison of machine learning models for prediction of dose increase in patients with rheumatoid arthritis. Proceedings of The Ninth International Conference on Engineering Applications of Neural Networks, (EANN05). Lille, France: 189–96. (24–26 Aug 2006)
- 37.Symmons D P, Ollier W E, Brennan P, Silman A J. Should patients with recent onset rheumatoid arthritis be offered genetic screening? Ann Rheum Dis 199655407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]