The collagen type II (CII) 263–272 peptide is a predominant T and B cell antigenic peptide in rheumatoid arthritis.1 Crystallographic data showed that 263F and 264K of CII263–272 are mainly responsible for binding with HLA‐DR, and that 267Q and 270K were the major T cell receptor (TCR)‐contact residues.2,3 We have shown that the CII altered peptide ligand (APL) with individual or consecutive substitutions of the TCR‐contact amino acids could inhibit T cell activation induced by wild‐type CII peptide in the context of HLA‐DRB1, among which the most potent T cell activation suppressor is sub268–270, in which the amino acids of wild‐type CII263–272 at positions 268, 269 and 270 were substituted with glycine or alanine (FKGEQAGAGE, substitutions underlined).4,5 Collagen‐induced arthritis (CIA) is induced by wild‐type CII in susceptible rodent strains. It has been regarded as the best rheumatoid arthritis model.6 In this study, we examined whether sub268–270 could show inhibitory effect in CIA.
Arthritis was induced in female Lewis rats weighing 120–140 g. Each rat was injected intradermally with 200 μg bovine CII emulsified with Freund's incomplete adjuvant. A booster immunisation with 100 μg emulsified CII was given 7 days later.6 The treatment with APL was initiated 24 h after arthritis onset. APL (sub268–270) at a dose of 90 µg was given intravenously once a week, with a total of three injections. The irrelevant peptide (EGKPGQEGKF) and phosphate‐buffered saline applied at the same dose were used as irrelevant and blank control, respectively. Arthritis scores of all four paws were made by two independent observers, with a scale of 0–4 for each paw: 0 = no change, 1 = swelling and erythema of the digit, 2 = mild swelling and erythema of the limb, 3 = gross swelling and erythema of the limb, 4 = gross deformity and inability to use the limb.7 Radiographic films of both hind paws were taken at the 21st day of treatment and subjected to radiographic scoring by two independent radiologists according to the degree of cartilage and bone erosions: 0 = no obvious abnormality, 1 = tissue swelling and oedema, 2 = joint space narrowing, and 3 = bone erosion and osteophyte formation.8 After killing the rats on treatment day 21, both ankle joints of rats with CIA were detached, embedded in paraffin, stained with haematoxylin–eosin, and pathological scores were made by two independent pathologists according to the following scale: 0 = normal synovium, 1 = synovial membrane hypertrophy and inflammatory cell infiltrates, 2 = pannus formation and cartilage erosion, 3 = major erosion of cartilage and subchondral bone, and 4 = loss of joint integrity and ankylosis.9 These assessments were performed by two independent people who were blinded to groups of the animals. These measures might reduce the selection bias to the least level.
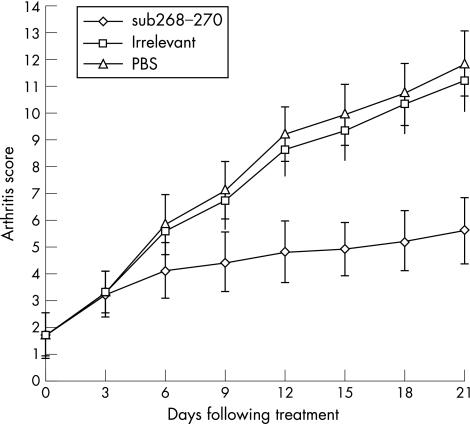
As shown in figure 1, the APL did not reduce the baseline arthritis (p>0.05). However, from day 6 to day 21, sub268–270 effectively reduced the inflammation in CIA as compared with irrelevant peptide and blank control (p<0.01). Radiological bone erosion and pathological lesions were the hallmark of rheumatoid arthritis/CIA. As shown in table 1, APL treatment significantly decreased the radiological score of CIA, implying its bone‐protection potential (p<0.01). APL decreased the pathological score of CIA, suggesting its role in inhibition of pathological lesions (p<0.01).
Figure 1 Inhibition of arthritis in rats with collagen induced arthritis by treatment with APL sub268–270. The arthritis score in sub268–270 treated rats was much lower than that of phosphate buffered saline and irrelevant peptide from day 6 to day 21 (n = 8, p<0.01). PBS, phosphate‐buffered saline.
Table 1 The effect of sub268–270 on radiological and pathological lesions of CIA.
| Groups | sub268–270 | Irrelevant peptide | PBS |
|---|---|---|---|
| Radiological score | 1.32 (0.49)* | 2.63 (0.51) | 2.69 (0.53) |
| Pathological score | 1.38 (0.53)* | 2.94 (0.63) | 3.06 (0.65) |
CIA, collagen‐induced arthritis; PBS, phosphate‐buffered saline.
Data were expressed as mean (SD). *p<0.01
In conclusion, APL sub268–270 could effectively reduce the joint inflammation and also decrease the joint injuries in CIA. These results suggest that this APL might be potentially useful in HLA‐DRB1‐positive patients with rheumatoid arthritis.
Footnotes
Funding: This work was supported by a grant from the National Science Foundation of China (30271223) and the Key Project grant of Beijing Municipal Science Foundation (7041005).
Competing interests: None.
References
- 1.Cope A P, Patel S D, Hall F, Congia M, Hubers H A, Verheijden G F.et al T cell responses to a human cartilage autoantigen in the context of rheumatoid arthritis‐associated and nonassociated HLA‐DR4 alleles. Arthritis Rheum 1999421497–1507. [DOI] [PubMed] [Google Scholar]
- 2.Rosloniec E F, Whittington K B, Zaller D M, Kang A H. HLA‐DR1 (DRB1* 0101) and DR4 (DRB1*0401) use the same anchor residues for binding an immunodominant peptide derived from human type II collagen. J Immunol 2002168253–259. [DOI] [PubMed] [Google Scholar]
- 3.Andersson E C, Hansen B E, Jacobsen H, Madsen L S, Andersen C B, Engberg J.et al Definition of MHC and T cell receptor contacts in the HLA‐DR4 restricted immunodominant epitope in type II collagen and characterization of collagen‐induced arthritis in HLA‐DR4 and human CD4 transgenic mice. Proc Natl Acad Sci USA 1998957574–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, Cheng Y J, Lu H S, Zhou W H, Li Z G. Inhibition of T‐cell activation with HLA‐DR1/DR4 restricted non‐T‐cell stimulation peptides. Hum Immunol 200364857–865. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y J, Zhou Q, Li Z G. The effect of altered CII peptides on HLA‐DRB1 restricted T cell response. Scand J Immunol 200561260–265. [DOI] [PubMed] [Google Scholar]
- 6.Trentham D E, Townes A S, Kang A H. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med 1977146857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K S, Choi Y H, Kim K H, Lee Y C, Kim C H, Moon S H.et al Protective and anti‐arthritic effects of deer antler aqua‐acupuncture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions‐mediated chondrocyte apoptosis and rat collagen‐induced arthritis. Int Immunopharmacol 20044963–973. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, Yoshikawa H. Prevention of the onset and progression of collagen‐induced arthritis in rats by the potent p38 mitogen‐activated protein kinase inhibitor FR167653. Arthritis Rheum 2003482670–2681. [DOI] [PubMed] [Google Scholar]
- 9.Shiozawa S, Shimizu K, Tanaka K, Hino K. Studies on the contribution of c‐fos/AP‐1 to arthritic joint destruction. J Clin Invest 1997991210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]