Abstract
Objective
To investigate whether rheumatoid factor isotypes and anti‐cyclic citrullinated peptide (anti‐CCP) antibodies are related to clinical response in patients with rheumatoid arthritis treated with tumour necrosis factor α (TNFα) inhibitors.
Methods
The study was carried out on 132 patients with advanced rheumatoid arthritis refractory to disease‐modifying antirheumatic drugs. Patients were treated with infliximab (n = 63), etanercept (n = 35) or adalimumab (n = 34). All patients completed 1 year of follow‐up, and 126 were evaluable for clinical response according to the disease activity score (DAS) criteria. IgM, IgA and IgG rheumatoid factors and anti‐CCP antibodies were assessed by ELISA both before anti‐TNFα treatment and 1 year later.
Results
The DAS response was reached in 66% of evaluable patients (61% infliximab, 65% etanercept and 76% adalimumab; p = 0.354). A significant reduction in the rheumatoid factor level was reported by all treatment groups after 1 year. The frequency of positive tests for the different antibodies did not differ between responders and non‐responders at baseline; however, significantly higher IgA rheumatoid factor levels were reported by the non‐responder group (130.4 U/ml (interquartile range 13.8–276.7) v 24.8 U/ml (10.2–90.8); p = 0.003). A significant decrease (p<0.001) in the levels of all rheumatoid factor isotypes in the responder group was reported after 1 year of treatment, whereas anti‐CCP antibody levels were not significantly affected.
Conclusions
According to the clinical response, anti‐TNFα agents seem to reduce IgM, IgG and IgA rheumatoid factor levels. More interestingly, high pretreatment levels of IgA rheumatoid factor are associated with a poor clinical response to TNFα inhibitors.
Rheumatoid factor and antibodies to citrullinated proteins are usually regarded as serological markers of rheumatoid arthritis. Classic (IgM) rheumatoid factor is currently assessed in clinical practice; however, the combined detection of additional isotypes may improve this marker's diagnostic and prognostic value.1,2,3 In particular, several studies have already shown that IgA rheumatoid factor may be strongly linked to a more severe disease.4,5,6
Anti‐citrullinated peptide antibodies recognise different citrulline‐containing proteins derived from a post‐translational modification of arginine residues from peptidyl‐arginine deiminase.7 Recently developed tests allow the detection of antibodies recognising cyclic citrullinated peptides (anti‐CCP) in the serum of most patients with rheumatoid arthritis. Anti‐CCP have proved to be highly specific for rheumatoid arthritis and strongly associated with development of radiographic erosions in the early stages of disease.8,9,10,11,12,13,14
The role of these antibodies as markers of response to treatment is not yet fully understood. Some studies reported a drop in rheumatoid factor level after effective treatment with both the traditional disease‐modifying antirheumatic drugs (DMARDs) and anti‐tumour necrosis factor (TNF)α treatment.15,16,17,18,19,20 However, data confirming a definite relationship between decreased rheumatoid factor levels and clinical response are scarce.20 Few data exist regarding IgA and IgG rheumatoid factor subtypes, and studies dealing with changes in anti‐CCP levels have yielded conflicting results.19,21,22
Three different TNFα‐inhibiting agents are currently used to treat active rheumatoid arthritis, all of which effectively reduce the signs and symptoms of the disease and inhibit radiographic joint damage progression.23,24,25,26 Even though these drugs have dramatically changed the treatment of rheumatoid arthritis, almost one third of patients are still poor responders, and no definite serological predictors of lack of response have as yet been reported.27,28 This paper deals with the relationship between serum levels of anti‐CCP or different rheumatoid factor isotypes and clinical response to TNFα blockers.
Methods
Patients
In all, 132 patients with definite rheumatoid arthritis were included in the study and were prospectivally followed up for at least 1 year according to the guidelines of the Italian National Registry for the treatment of severe rheumatoid arthritis with anti‐TNF agents in rheumatoid arthritis therapy.29,30 All patients had active disease despite having previously received treatment with ⩾2 DMARDs, including methotrexate, and gave their informed consent in accordance with the local ethics committee recommendations. A total of 63 patients were treated with infliximab (3 mg/kg intravenously at 0, 2 and 6 weeks and then every 8 weeks) and methotrexate (15–20 mg/week), 35 patients were treated with etanercept (25 mg subcutaneously twice weekly) with or without methotrexate and 34 patients were treated with adalimumab (40 mg subcutaneously every other week) with or without methotrexate or leflunomide. Non‐steroidal anti‐inflammatory drugs and oral prednisone (<10 mg/day) were allowed. Six patients dropped out because of adverse events a few weeks after beginning treatment and were not eligible for clinical response evaluation. Six additional patients discontinued treatment between 14 and 38 weeks because of inefficacy; these patients were included in the clinical response evaluation, but were excluded from the analysis of antibody profile changes.
Clinical response was evaluated after 1 year (or at drop‐out) in accordance with the European League Against Rheumatism criteria using the modified disease activity score that includes 28 joints (DAS 28).31 The American College of Rheumatology 20 criteria were also evaluated for all cases.32 Table 1 reports the main demographic and clinical characteristics of the cohort.
Table 1 Demographic and clinical characteristic of patients included in the study.
| Total patients (n = 132) | Infliximab (n = 63) | Etanercept (n = 35) | Adalimumab (n = 34) | |
|---|---|---|---|---|
| Age (years) | 57.28 (12.46) | 59.12 (10.86) | 58.4 (11.99) | 52.57 (14.87) |
| Female/male | 101/31 | 46/17 | 28/7 | 27/7 |
| Disease duration (years) | 8.3 (6.93) | 9.08 (8.11) | 6.15 (4.72) | 9.58 (6.65) |
| Tender joints (68) | 15.96 (9.9) | 18.1 (10.3) | 16.65 (9.7) | 11.03 (7.36) |
| Swollen joints (66) | 9.59 (5.7) | 7.9 (4.88) | 13.17 (7.46) | 9.36 (3.21) |
| Erosions (%) | 100 | 100 | 100 | 100 |
| DAS 28 | 5.87 (0.99) | 5.93 (1.08) | 5.95 (1.08) | 5.52 (0.7) |
| HAQ | 1.598 (0.69) | 1.73 (0.57) | 1.74 (0.77) | 1.11 (0.66) |
| Responders (%)* | 66 | 61 | 65 | 76 |
| Previous DMARDs, n of patients (%) | ||||
| Methotrexate | 131 (99.24) | 63 (100) | 34 (97.14) | 34 (100) |
| Hydroxycloroquine | 93 (70.45) | 53 (84.12) | 22 (62.85) | 19 (55.88) |
| Sulfasalazine | 61 (46.21) | 32 (50.79) | 16 (47.71) | 13 (38.23) |
| Ciclosporin A | 48 (36.36) | 29 (46.03) | 12 (34.28) | 7 (20.58) |
| Others | 34 (25.75) | 14 (22.22) | 9 (25.71) | 11 (32.35) |
| DMARDs associated, n of patients (%) | ||||
| Methotrexate | 115 (87.12) | 63 (100) | 29 (82.85) | 23 (67.64) |
| Leflunomide | 2 (1.51) | 0 | 0 | 2 (5.88) |
| None | 15 (11.36) | 0 | 6 (17.14) | 9 (26.47) |
DAS 28, disease activity score including 28 joint counts; DMARD, disease‐modifying antirheumatic drug; HAQ, Health Assessment Questionnaire.
Where applicable, the values are expressed as mean (SD).
*126 patients evaluable for clinical response.
Autoantibody analysis
Serum samples for autoantibody assessment were collected and stored at −70°C immediately before the first administration of the TNF‐blocking drugs and then 1 year later during physical examination for the clinical response analysis. Testing for the different autoantibodies was carried out on all serum samples at the end of the study.
Rheumatoid factors
Classic rheumatoid factor was measured by immunonephelometry using the quantitative N Latex rheumatoid factor system (Dade Behring, Marburg, Germany). According to the manufacturer's recommendations, rheumatoid factor concentrations >15 IU/ml were considered positive.
The different rheumatoid factor isotypes (IgM, IgA and IgG) were assessed using an indirect solid‐phase enzyme immunoassay (ELISA; Orgentec Diagnostika, Mainz, Germany) involving the binding of Fc fragments of highly purified human IgG to the microwells. The quantitative test system for IgM, IgG and IgA rheumatoid factor is calibrated in relative arbitrary units related to the 1st British Standard Preparation 64/2 as reported in the kit insert. The procedure was carried out in triplicate. According to the manufacturer's recommendations, the test's upper normal limit was 20 U/ml. Serum samples from 30 healthy controls were tested for rheumatoid factor (nephelometry), and IgM, IgG and IgA rheumatoid factor (ELISA). All samples tested negative by ELISA and one proved borderline (15 IU/ml) using nephelometry.17
Anti‐cyclic citrullinated peptides
Anti‐CCP were tested using a commercially available second‐generation ELISA kit (Axis‐Shield, Dundee, UK) as described previously.17 Serum samples were evaluated in triplicate, and the upper normal limit (5 U/ml ) was set in accordance with the manufacturer's recommendations. All sera from controls gave negative results. All serum samples patient showing high concentration (⩾100 U/ml) were evaluated after a further 10× dilution and then corrected for this additional dilution factor.
Statistical analysis
Data were described as mean and standard deviation (SD), or median and 25th–75th centiles if continuous, and as counts and percentages if categorical. Rheumatoid factors were dichotomised according to their cut‐off for positivity. The χ2 test was used to compare the percentages of DAS 28 responder patients in the infliximab, etanercept and adalimumab groups. The Fisher exact test and the Mann–Whitney U test were run to compare the characteristics of the responder and non‐responder groups. The Wilcoxon sign test was used to compare rheumatoid factor over time. Furthermore, the association of each of the baseline rheumatoid factors with the clinical response was assessed, while controlling for age, sex, disease duration, Health Assessment Questionnaire (HAQ), serum C reactive protein (CRP) levels, DAS, positivity for anti‐CCP and type of treatment, by means of a multivariable logistic model. Rheumatoid factors were log transformed to be fitted into the model. Odds ratios (ORs) and their 95% confidence intervals (95% CI) were computed to measure the increase in risk of non‐response for 1 log unit increase in the rheumatoid factor.
Stata V.9.2 was used for computation. A two‐sided p value <0.05 was considered significant.
Results
Baseline autoantibody profile and clinical response
Clinical response was obtained in 83 (66%) patients, with minor differences between the different anti‐TNFα agents (p = 0.354; table 1). Table 2 summarises the main clinical and serological characteristics according to treatment response. As for the serological variables, the frequency of positive cases for rheumatoid factors was similar for both responders and non‐responders. Anti‐CCP‐positive cases were slightly more common in the non‐responder group, but the difference did not reach statistical significance.
Table 2 Baseline characteristics of patients included in the study according to clinical response.
| Responders (n = 83) | Non‐responders (n = 43) | p Value | |
|---|---|---|---|
| Age (years) | 58.35 (48.02–65.55) | 62.75 (50.5–69.7) | 0.131 |
| Female/male | 57/26 | 38/5 | 0.017 |
| Disease duration (years) | 7 (4–12.72) | 5.5 (2.62–8.93) | 0.075 |
| Tender joints | 12 (7–21.5) | 17 (9.5–24.5) | 0.106 |
| Swollen joints | 9 (6–12) | 8 (5–15) | 0.457 |
| DAS 28 | 5.7 (5–6.42) | 6.13 (5.32–6.6) | 0.022 |
| HAQ | 1.5 (0.87–1.87) | 1.9 (1.33–2.37) | 0.002 |
| Previous DMARD, n | 3 (2–4) | 3 (2–4) | 1 |
| Associated DMARD, % | 92.77 (77/83) | 83.72 (36/43) | 0.13 |
| RF (nephelometry), % positive | 83.13 (69/83) | 83.72 (36/43) | 1 |
| Level* | 86 (15–268.4) | 97 (28–338) | 0.769 |
| IgM RF, % positive | 78.31 (65/83) | 83.72 (36/43) | 0.638 |
| Level* | 41.5 (22.1–120) | 67.6 (32.9–295) | 0.035 |
| IgA RF, % positive | 61.44 (51/83) | 69.76 (30/43) | 0.434 |
| Level* | 24.8 (10.2–90.8) | 130.4 (13.8–276.7) | 0.003 |
| IgG RF, % positive | 57.83 (48/83) | 69.76 (30/43) | 0.246 |
| Level* | 24.3 (7.2–94.9) | 46.1 (13.2–210.6) | 0.057 |
| Anti‐CCP, % positive | 72.28 (60/83) | 81.39 (35/43) | 0.285 |
| Level* | 25.27 (2.93–74.27) | 35.28 (7.32–114.99) | 0.233 |
Anti‐CCP, anti‐cyclic citrullinated peptide antibodies; DAS 28, disease activity score including 28 joint counts; DMARD, disease‐modifying antirheumatic drug; RF, rheumatoid factor.
The values of continuous variables are expressed as median and interquartile range.
*The negative RF samples were included and counted with the measured values.
There was a trend for the non‐responders to have higher levels of rheumatoid factor and anti‐CCP. This trend was most evident for IgA rheumatoid factor levels, which were significantly higher in the non‐responder group than in the responder group. Similar results were obtained when responders were defined according to American College of Rheumatology 20 criteria (81/126 points: 64.28%). In particular, IgA rheumatoid factor levels were significantly higher in non‐responders (134.7 (56.1–293.25)) than in responders (25.2 (10.27–114.25); p = 0.007).
The significant association between the baseline IgA rheumatoid factor level (log transformed) and the lack of response was confirmed (OR 1.44; 95% CI 1.09 to 1.9; p = 0.009) on multivariable analysis with adjustment for confounders such as age, sex, disease duration, HAQ, CRP, DAS, positivity for anti‐CCP and type of treatment.
Using the same analysis, no significant association was reported between lack of response and the other rheumatoid factor isotypes (IgM rheumatoid factor (OR 1.25; 95% CI 0.89 to 1.76; p = 0.194); IgG rheumatoid factor (OR 1.17; 95% CI 0.9 to 1.52; p = 0.22)).
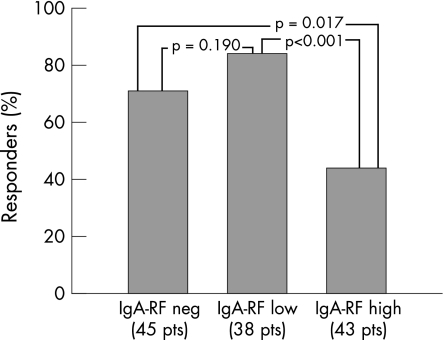
When patients were divided into three groups according to IgA rheumatoid factor levels, 45 patients were negative (<20 U/ml), 38 were low positive (between 20 and 100 U/ml) and 43 were high positive (>100 U/ml). High‐positive cases showed a significantly lower response rate with respect to either low‐positive cases or negative cases, whereas no obvious difference was found between IgA rheumatoid factor low‐positive and negative cases (fig 1). Previous DMARD treatment was identical in patients with either high or low and negative IgA rheumatoid factor levels. Co‐medication with DMARD during the anti‐TNFα treatment was given to 39/43 (90.06%) patients with high IgA rheumatoid factor levels, and to 74/83 (89.15%) patients with low or negative levels of IgA rheumatoid factor (p = 1).
Figure 1 Percentage of responders according to IgA rheumatoid factor (RF) levels at baseline. The patients were stratified into three different groups: IgA rheumatoid factor negative (level <20 U/ml), IgA rheumatoid factor low (level between 20 and 100 U/ml) and IgA rheumatoid factor high (level >100 U/ml). Pt, Patient
Treatment‐induced changes in autoantibody profile
In all, 120 patients completed 1 year of treatment. The frequency of the different antibodies tested after treatment did not differ significantly from the pretreatment values in either group (table 3).
Table 3 Frequency of occurrence of different autoantibodies assessed at baseline and after 1 year of treatment with anti‐tumour necrosis factor α agents according to clinical response.
| Autoantibody | Responders (83 patients) | Non‐responders (37 patients) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 year | p Value | Baseline | 1 year | p Value | |
| RF (nephelometry) | 69 | 61 | 0.186 | 33 | 32 | 1 |
| IgM‐RF | 65 | 59 | 0.372 | 31 | 30 | 1 |
| IgA‐RF | 51 | 41 | 0.159 | 25 | 26 | 1 |
| IgG‐RF | 48 | 41 | 0.350 | 26 | 23 | 0.623 |
| Anti‐CCP | 60 | 58 | 0.864 | 32 | 31 | 1 |
Anti‐CCP, anti‐cyclic citrullinated peptide antibodies; RF, rheumatoid factor.
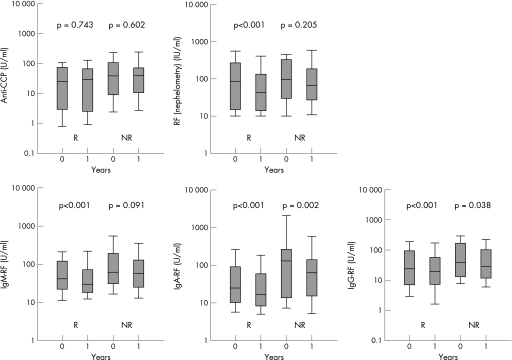
As for the antibody levels, there was no significant difference in the anti‐CCP levels after 1 year of treatment, whereas a significant drop in classic rheumatoid factor was noted in the responder group. A significant reduction in rheumatoid factor levels was observed for all isotypes in the responder group. In the non‐responder group, only a small decrease was found in IgA rheumatoid factor and IgG rheumatoid factor (fig 2).
Figure 2 Changes in the serum levels of anti‐cyclic citrullinated peptide antibodies (anti‐CCP), rheumatoid factor (RF; nephelometry) and IgM, IgA and IgG rheumatoid factor in patients with rheumatoid arthritis after 1 year of treatment with anti‐tumour necrosis factor (TNF)α agents according to clinical response. (Data are shown as box plots. Each box represents the 25th–75th centiles; lines inside the boxes represent the medians. Lines outside the box represent the 10th and 90th centiles.)
Treatment‐induced changes were roughly similar for all anti‐TNFα agents. Infliximab, adalimumab and etanercept did not induce a significant reduction in anti‐CCP levels. IgM rheumatoid factor levels by nephelometry and ELISA were significantly reduced by all three treatments. However, the reduction in IgA rheumatoid factor and IgG rheumatoid factor levels was more marked with infliximab (table 4).
Table 4 Changes in levels of rheumatoid factor and anti‐cyclic citrullinated peptide antibodies assessed before and after 1 year of treatment according to the anti‐tumour necrosis factor agent used.
| Treatment | Level* | |||
|---|---|---|---|---|
| Baseline | 1 year | p Value | ||
| Anti‐CCP | Infliximab | 30.48 (14.08–73.21) | 30.25 (6.43–64.86) | 0.071 |
| Etanercept | 26.82 (3.7–87.72) | 28.98 (3.46–104.87) | 0.084 | |
| Adalimumab | 35.64 (2.23–75.02) | 36.78 (2.23–67.6) | 0.426 | |
| All | 31.18 (6.22–78.95) | 30.89 (4.56–66.25) | 0.589 | |
| RF (nephelometry) | Infliximab | 115 (43.3–290.3) | 65 (25.65–166.25) | <0.001 |
| Etanercept | 61 (17.67–367) | 54 (17.25–200) | 0.036 | |
| Adalimumab | 64 (15–259.75) | 30 (11.5–130.75) | 0.003 | |
| All | 90 (22.5–304.5) | 51.7 (17–163) | <0.001 | |
| IgM‐RF | Infliximab | 41.3 (17.07–117.05) | 30.9 (15.82–67.42) | <0.001 |
| Etanercept | 23.55 (7.25–86.25) | 22.35 (5.2–70.95) | 0.034 | |
| Adalimumab | 17.6 (6.67–84.97) | 11.7 (5.07–31.77) | 0.048 | |
| All | 29.9 (10.8–104.5) | 23.45 (9.15–64) | <0.001 | |
| IgA‐RF | Infliximab | 64.2 (22.1–179.4) | 41.8 (17.42–92.42) | <0.001 |
| Etanercept | 23.7 (6.45–109) | 22.7 (8.3–33.47) | 0.004 | |
| Adalimumab | 16.9 (8.8–33.47) | 12.3 (7.37–30.35) | 0.067 | |
| All | 30.1 (10.85–144.35) | 28.6 (9.45–83.5) | <0.001 | |
| IgG‐RF | Infliximab | 61.6 (28.87–156.8) | 42.6 (24.72–75.95) | <0.001 |
| Etanercept | 58.35 (33.35–181.1) | 53.5 (28.45–127.6) | 0.144 | |
| Adalimumab | 27.5 (12.5–76.1) | 20.5 (12.8–28.62) | 0.096 | |
| All | 48 (24.2–132.9) | 38.8 (19.7–86.3) | <0.001 | |
Anti‐CCP, anti‐cyclic citrullinated peptide antibodies; RF, rheumatoid arthritis.
*Median value (interquartile range).
Discussion
The aim of this study was to investigate whether rheumatoid factor, rheumatoid factor isotypes and anti‐CCP could be useful for predicting and monitoring the clinical response to anti‐TNFα treatment in advanced rheumatoid arthritis. Our results indicate that (a) high levels of IgA rheumatoid factor are significantly associated with a poor response rate and (b) the clinical response is associated with decreased rheumatoid factor levels during treatment.
Our data show that a positive rheumatoid factor status does not correlate with clinical response, but high IgA rheumatoid factor levels may predict a poor response rate. Patients with low‐positive IgA rheumatoid factor and those with negative IgA rheumatoid factor had a good response rate, whereas patients with high positive IgA rheumatoid factor were poor responders. IgA rheumatoid factor has been reported to be more specific for rheumatoid arthritis than classic (IgM) rheumatoid factor and more specifically associated with radiographic erosions in early disease.2,6,33,34 Our data suggest that the IgA isotype may be also useful in advanced disease for predicting the response to treatment with TNF inhibitors. This might be relevant for the choice of suitable treatment, as biological agents targeting the late precursors of the (auto)antibody‐producing cells are now available.35
There was also a trend for non‐responders to have high levels of IgG and IgM rheumatoid factors, and we cannot exclude the possibility that the lack of statistical significance for these isotypes might simply reflect a type II error. The anti‐CCP antibodies in our patients also showed a similar trend. Two previous studies on smaller series suggested a poor response rate in the patients with high levels of anti‐CCP.20,36 Further multicentre studies are needed to ascertain whether and how anti‐CCP and IgM or IgG rheumatoid factors may be predictive of clinical response with anti‐TNF‐blocking agents. Additional parameters associated with poor response at univariate analysis were high values of DAS 28, HAQ and female sex. A reference to the difference in response to TNFα blockers between men and women has also recently been made in the national registers for Italy and Norway.37 The paper suggested that blockage of TNF‐induced upregulation of aromatase would particularly increase the level of (anti‐inflammatory) androgens in men, leading to a better clinical outcome.37
A drop in rheumatoid factor levels during treatment with infliximab has been found by many authors, whereas the changes induced in anti‐CCP levels still remain a controversial issue.17,18,19,20,22,38,39,40 In a recent study, anti‐CCP have been reported to be reduced by treatment only in early disease.16 Data regarding etancercept and adalimumab are also conflicting; two studies have reported the reduction of both anti‐CCP and rheumatoid factor levels with either etanercept or adalimumab, whereas a third study on a small series of patients failed to show any such reduction.19,21,41
In this study, we have analysed the effects of three different anti‐TNFα agents after 1 year of treatment. It seems clear that each of the anti‐TNF agents are able to reduce rheumatoid factor levels, but not that of anti‐CCP. These data are in keeping with the previous observations made on infliximab‐treated patients who we followed up for 78 weeks and with other studies carried out on patients with advanced disease.16,17,22
With respect to previous studies, we are now able to show a significant link between decreased IgM rheumatoid factor levels and the clinical response to TNFα blockade. Decreased levels of all rheumatoid factor isotypes were observed in the responder group, whereas only the IgA and IgG rheumatoid factor levels experienced a drop in the non‐responders. The latter finding may be because of the high pretreatment levels in the non‐responder group, as it has been reported that the degree of rheumatoid factor reduction after treatment is higher in the patients with high pretreatment values.16
Viewed as a whole, our findings indicate that anti‐TNF treatment may have different effects on rheumatoid factors and anti‐CCP. As all rheumatoid factor isotypes are considerably reduced, this cannot be ascribed to different clearance related to the Ig class—that is, IgM for rheumatoid factor and IgG for anti‐CCP. A preferential reduction in all rheumatoid factor isotypes has recently been reported after treatment with rituximab, a B‐cell‐targeting monoclonal antibody.42,43 Also, in these cases, a dramatic reduction in serum rheumatoid factor levels was associated with good clinical response.44,45 A marked reduction in rheumatoid factor levels (but not that of anti‐CCP) was also reported after effective treatment with traditional DMARDs in advanced rheumatoid arthritis.16 The reasons for the different effect of treatment on rheumatoid factor and anti‐CCP production in advanced rheumatoid arthritis are as fascinating as elusive. We can only speculate that rheumatoid factor production could be at least partially dependent on inflammation whereas anti‐CCP production might be more “constitutive” even though in loco production of antibodies directed to citrullinated antigens has been reported in rheumatoid synovitis.46,47,48
In conclusion, our study indicates that high pretreatment levels of IgA rheumatoid factor are associated with a poor response rate to the TNFα inhibitors in advanced rheumatoid arthritis refractory to conventional DMARDs. Furthermore, sustained clinical response to anti‐TNF agents is associated with a significant decrease in the serum level of rheumatoid factors.
Abbreviations
CCP - cyclic citrullinated peptide
CRP - C reactive protein
DAS - disease activity score
DMARD - disease‐modifying antirheumatic drug
HAQ - Health Assessment Questionnaire
TNF - tumour necrosis factor
Footnotes
Funding: This work was supported by a grant of IRCCS Policlinico S Matteo Foundation of Pavia, Italy.
Competing interests: None declared.
References
- 1.Johnson P M, Faulk W P. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol 19766414–430. [DOI] [PubMed] [Google Scholar]
- 2.Bas S, Perneger T V, Kunzle E, Vischer T L. Comparative study of different enzyme immunoassays for measurement of IgM and IgA rheumatoid factors. Ann Rheum Dis 200261505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swedler W, Wallman J, Froelich C J, Teodorescu M. Routine measurement of IgM, IgG, and IgA rheumatoid factors: high sensitivity, specificity, and predictive value for rheumatoid arthritis.J Rheumatol 1997241037–1044. [PubMed] [Google Scholar]
- 4.Zlabinger G J, Haberhauer G, Dax K, Menzel E J, Broll H. Rheumatoid factor isotypes and circulating immune complexes in rheumatoid arthritis. Clin Exp Rheumatol 19908113–119. [PubMed] [Google Scholar]
- 5.Jorgensen C, Legouffe M C, Bologna C, Brochier J, Sany J. IgA isotype rheumatoid factor in rheumatoid arthritis: clinical implications. Clin Exp Rheumatol 199614301–304. [PubMed] [Google Scholar]
- 6.Berglin E, Johansson T, Sundin U, Jidell E, Wadell G, Hallmans G.et al Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA‐RF at disease onset. Ann Rheum Dis 200665453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vossenaar E R, Radstake T R, van der Heijden A, van Mansum M A, Dieteren C, de Rooij D J.et al Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 200463373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Cruyssen B, Peene I, Cantaert T, Hoffman I E A, De Rycke L, Veys E M.et al Anti‐citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun Rev 20054468–474. [DOI] [PubMed] [Google Scholar]
- 9.Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B, BARFOT Study Group Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti‐CCP). Ann Rheum Dis 2004631090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti‐CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004631085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronnelid J, Wick M C, Lampa J, Lindblad S, Nordmark B, Klagerskog L.et al Longitudinal analysis of citrullinated protein/peptide antibodies (anti‐CP) during 5 year follow up in early rheumatoid arthritis: anti‐CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005641744–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroot E J, de Jong B A, van Leeuwen M A, Swinkels H, van den Hoogen F H, van't Hof M.et al The prognostic value of anti‐cyclic citrullinated peptide antibody in patients with recent‐onset rheumatoid arthritis. Arthritis Rheum 2000431831–1835. [DOI] [PubMed] [Google Scholar]
- 13.Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V.et al Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 200362427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza K, Breese M, Nightingale P, Kumar K, Potter T, Carruthers D M.et al Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol 200532231–238. [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen N J, Callahan L F, Pincus T. In vitro rheumatoid factor synthesis in patients taking second‐line drugs for rheumatoid arthritis. Independent associations with disease activity. Arthritis Rheum 1988311090–1096. [DOI] [PubMed] [Google Scholar]
- 16.Mikuls T R, O'Dell J R, Stoner J A, Parrish L A, Arend W P, Norris J M.et al Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti‐cyclic citrullinated peptide antibody. Arthritis Rheum 2004503776–3782. [DOI] [PubMed] [Google Scholar]
- 17.Bobbio‐Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long‐term infliximab treatment. Arthritis Res Ther 20046R264–R272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rycke L, Verhelst X, Kruithof E, Van den Bosch F, Hoffman I E, Veys E M.et al Rheumatoid factor, but not anti‐cyclic citrullinated peptide antibodies, is modulated by infliximab treatment in rheumatoid arthritis. Ann Rheum Dis 200564299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atzeni F, Sarzi‐Puttini P, Dell' Acqua D, de Portu S, Cecchini G, Cruini C.et al Adalimumab clinical efficacy is associated with rheumatoid factor and anti‐cyclic citrullinated peptide antibody titer reduction: a one‐year prospective study. Arthritis Res Ther 20058R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessandri C, Bombardieri M, Papa N, Cinquini M, Magrini L, Tincani A.et al Decrease of anti‐cyclic citrullinated peptide antibodies and rheumatoid factor following anti‐TNFα therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis 2004631218–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdani‐Biuki B, Stadlmaier E, Mulabecirovic A, Brezinschek R, Tilz G, Demel U.et al Blockade of tumour necrosis factor {alpha} significantly alters the serum level of IgG‐ and IgA‐rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 2005641224–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zendman A J, van Venrooij W J, Pruijn G J. Use and significance of anti‐CCP autoantibodies in rheumatoid arthritis. Rheumatology 20064520–25. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 24.Genovese M C, Bathon J M, Martin R W, Fleischmann R M, Tesser J R, Schiff M H.et al Etanercept versus methotrexate in patients with early rheumatoid arthritis: two‐year radiographic and clinical outcomes. Arthritis Rheum 2002461443–1450. [DOI] [PubMed] [Google Scholar]
- 25.Genovese M C, Bathon J M, Fleischmann R M, Moreland L W, Martin R W, Whitmore J B.et al Longterm safety, efficacy and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 2005321232–1242. [PubMed] [Google Scholar]
- 26.Keystone E C, Kavanaugh A F, Sharp J T, Tannenbaum H, Hua Y, Teoh L S.et al Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumour necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis Rheum 2004501400–1411. [DOI] [PubMed] [Google Scholar]
- 27.Keystone E C. Tumour necrosis factor‐alpha blockade in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am 200127427–443. [DOI] [PubMed] [Google Scholar]
- 28.Hyrich K L, Watson K D, Silman A J, Symmons D P. Predictors of response to anti‐TNF‐{alpha}therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology 2006451558–1565. [DOI] [PubMed] [Google Scholar]
- 29.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 30.Ministero della Salute (Italian Ministery of Health) Studio osservazionale ANTARES. Protocollo di monitoraggio per il trattamento dei pazienti affetti da artrite reumatoide con farmaci “biologici”. Gazzetta Ufficiale della Repubblica Italiana No 127. 04/06/2001. www.reumatologia.it (accessed 29 Dec 2006)
- 31.Prevoo M L, van't Hof M A, Kuper H H, van leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 32.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 33.Bas S, Genevay S, Meyer O, Gabay C. Anti‐cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology 200342677–680. [DOI] [PubMed] [Google Scholar]
- 34.Combe B, Dougados M, Goupille P, Cantagrel A, Eliaou J F, Sibilia J.et al Prognostic factors for radiographic damage in early rheumatoid arthritis: a multiparameter prospective study. Arthritis Rheum 2001441736–1743. [DOI] [PubMed] [Google Scholar]
- 35.Edwards J C, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close D R.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 36.Braun‐Moscovici Y, Markovits D, Zinder O, Schapira D, Rozin A, Ehrenburg M.et al Anti‐cyclic citrullinated protein antibodies as a predictor of response to anti‐tumour necrosis factor‐alpha therapy in patients with rheumatoid arthritis. J Rheumatol 200633497–500. [PubMed] [Google Scholar]
- 37.Straub R H, Harle P, Sarzi‐Puttini P, Cutolo M. Tumor necrosis factor‐neutralizing therapies improve altered hormone axes. Arthritis Rheum 2006542039–2046. [DOI] [PubMed] [Google Scholar]
- 38.Charles P J, Smeenk R J, De Jong J, Feldmann M, Maini R N. Assessment of antibodies to double‐stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumour necrosis factor alpha: findings in open‐label and randomized placebo‐controlled trials. Arthritis Rheum 2000432383–2390. [DOI] [PubMed] [Google Scholar]
- 39.Caramaschi P, Biasi D, Tonolli E, Pieropan S, Martinelli N, Carletto A.et al Antibodies against cyclic citrullinated peptides in patients affected by rheumatoid arthritis before and after infliximab treatment. Rheumatol Int 20052658–62. [DOI] [PubMed] [Google Scholar]
- 40.Allanore Y, Sellam J, Batteux F, Job Deslandre C, Weill B, Kahan A. Induction of autoantibodies in refractory rheumatoid arthritis treated by infliximab. Clin Exp Rheumatol 200422756–758. [PubMed] [Google Scholar]
- 41.Chen H A, Lin K C, Chen C H, Liao H T, Wang H P, Chang H N.et al The effect of etanercept on anti‐cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 20066535–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambridge G, Leandro M J, Edwards J C, Ehrensein M R, Salden M, Bodman‐Smith M.et al Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 2003482146–2154. [DOI] [PubMed] [Google Scholar]
- 43.Cambridge G, Stohl W, Leandro M J, Migone T S, Hilbert D M, Edwards J C. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum 200654723–732. [DOI] [PubMed] [Google Scholar]
- 44.De Vita S, Zaja F, Sacco S, De Candia, Fanin R, Ferraccioli G. Efficacy of selective B cell blockade in the treatment of rheumatoid arthritis: evidence for a pathogenetic role of B cells. Arthritis Rheum 2002462029–2033. [DOI] [PubMed] [Google Scholar]
- 45.Kramm H, Hansen K E, Gowing E, Bridges A. Successful therapy of rheumatoid arthritis with rituximab: renewed interest in the role of B cells in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol 20041028–32. [DOI] [PubMed] [Google Scholar]
- 46.Reparon‐Schuijt C C, van Esch W J, van Kooten C, Levarht E W, Breedveld F C, Verweij C L. Functional analysis of rheumatoid factor‐producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum 1998412211–2220. [DOI] [PubMed] [Google Scholar]
- 47.Hakoda M, Ishimoto T, Hayashimoto S, Inoue K, Taniguchi A, Kamatani N.et al Selective infiltration of B cells committed to the production of monoreactive rheumatoid factor in synovial tissue of patients with rheumatoid arthritis. Clin Immunol Immunopathol 19936916–22. [DOI] [PubMed] [Google Scholar]
- 48.De Rycke L, Nicholas A P, Cantaert T, Kruithof E, Echols J D, Vandekerckhove B.et al Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis‐specific humoral autoimmunity. Arthritis Rheum 2005522323–2330. [DOI] [PubMed] [Google Scholar]