Abstract
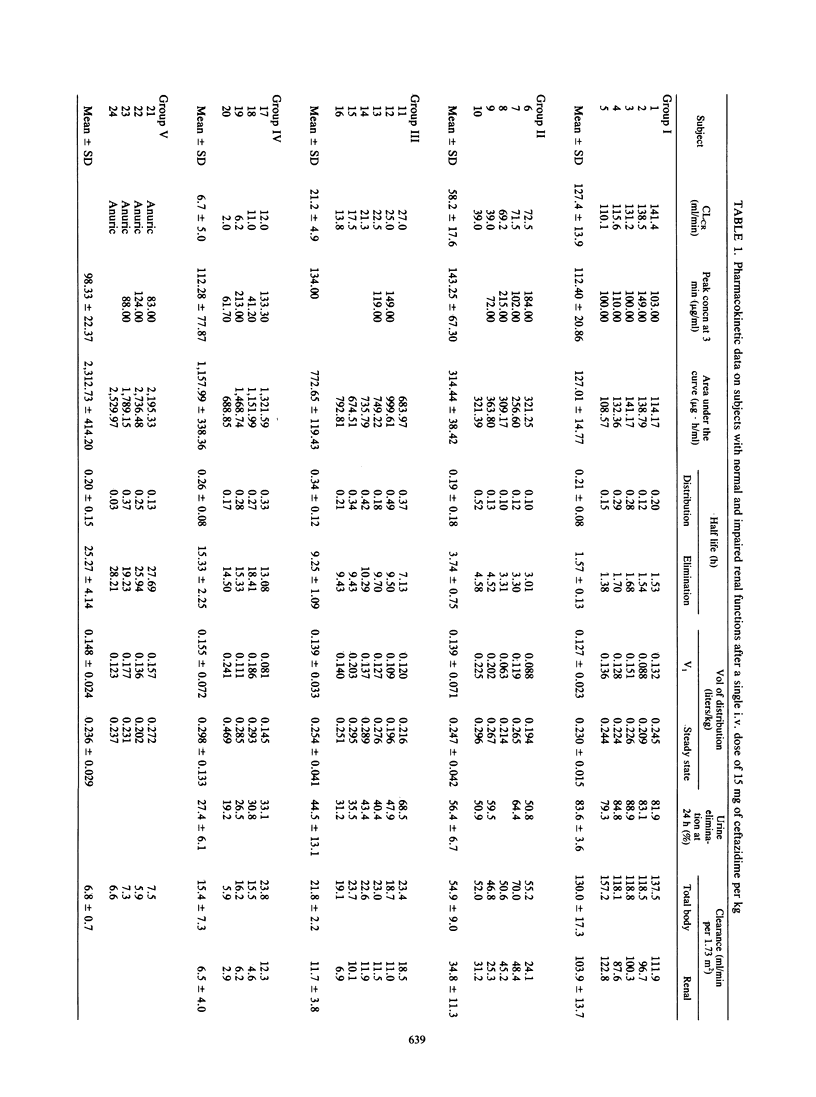
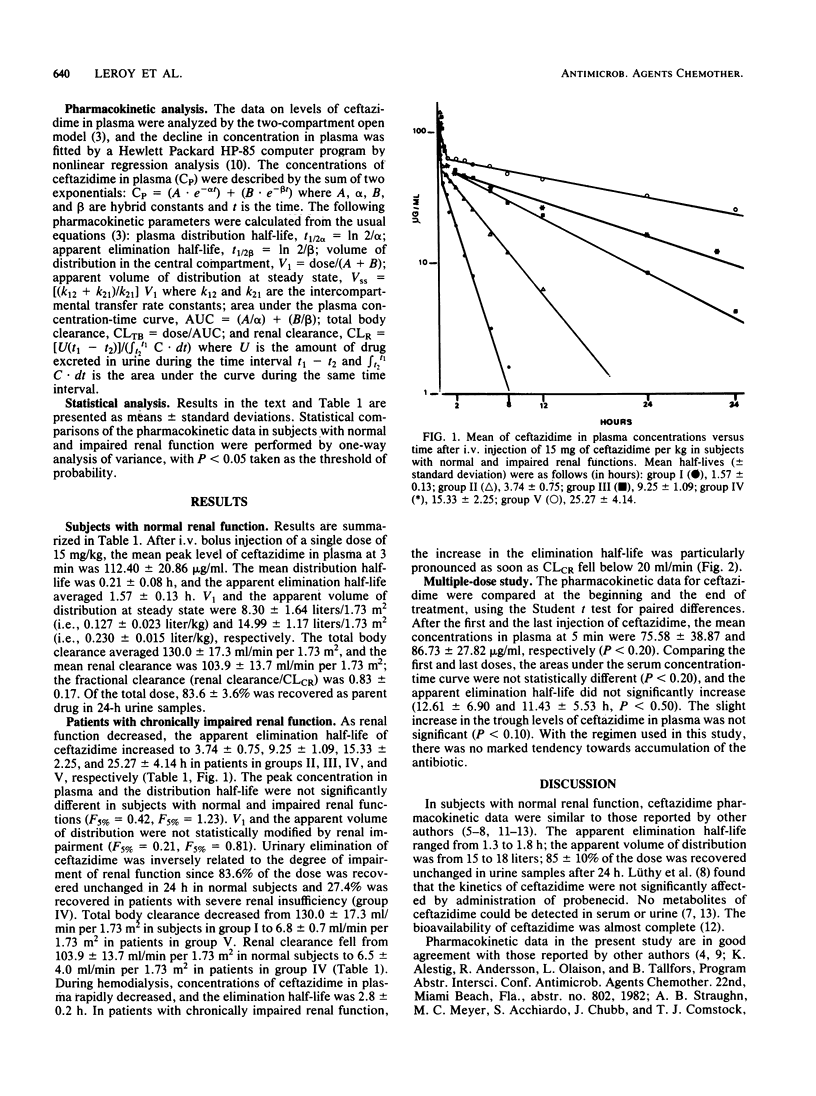
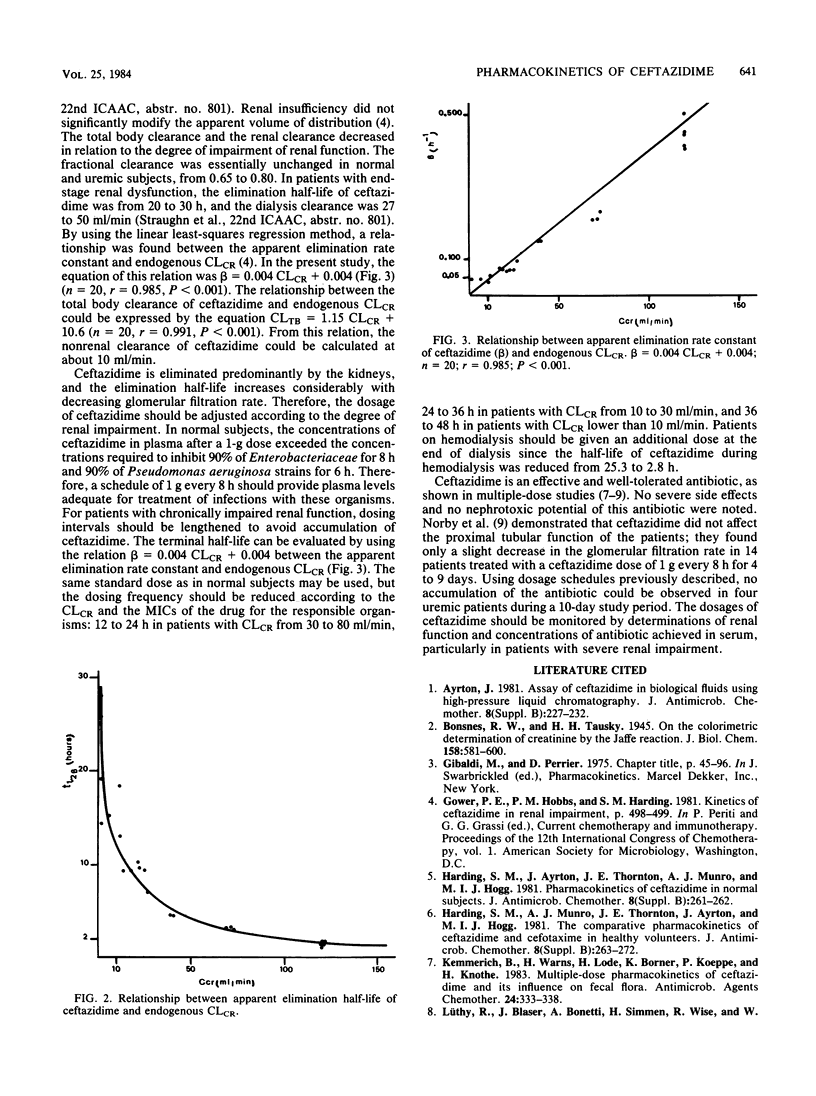
The pharmacokinetics of ceftazidime, administered as a single intravenous dose of 15 mg/kg given in a bolus injection over 3 min, were investigated in 5 normal subjects and in 19 uremic patients. The subjects studied were divided into five groups according to values for endogenous creatinine clearance (CLCR): group I, five subjects with CLCR greater than 80 ml/min; group II, five patients with CLCR = 30 to 80 ml/min; group III, six patients with CLCR = 10 to 30 ml/min; group IV, four patients with CLCR = 2 to 10 ml/min; and group V, four anuric patients on hemodialysis. A two-compartment open model was used to calculate the pharmacokinetic parameters. In normal subjects, the mean apparent elimination half-life was 1.57 +/- 0.13 h. The central distribution volume and the apparent volume of distribution were 0.127 +/- 0.023 and 0.230 +/- 0.015 liter/kg, respectively. Of the injected dose, 83.6 +/- 3.6% was eliminated in the urine as parent drug within 24 h. The terminal half-life increased with impairment of renal function to about 25 h in severely uremic patients. Impairment of function did not significantly modify the half-life at alpha phase, central distribution volume, or apparent distribution volume. A 6- to 8-h hemodialysis procedure reduced concentrations of ceftazidime in plasma by approximately 88%, and the elimination half-life was 2.8 +/- 0.2 h. There was no evidence of accumulation of ceftazidime in four patients with severe and chronic impairment of function who received doses of 0.5 to 1.0 g every 24 h for 10 days.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eng R. H., Gorski S., Person A., Mangura C., Chmel H. Clindamycin elimination in patients with liver disease. J Antimicrob Chemother. 1981 Oct;8(4):277–281. doi: 10.1093/jac/8.4.277. [DOI] [PubMed] [Google Scholar]
- Kemmerich B., Warns H., Lode H., Borner K., Koeppe P., Knothe H. Multiple-dose pharmacokinetics of ceftazidime and its influence on fecal flora. Antimicrob Agents Chemother. 1983 Sep;24(3):333–338. doi: 10.1128/aac.24.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., Blaser J., Bonetti A., Simmen H., Wise R., Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Nov;20(5):567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Burman L. A., Linderholm H., Trollfors B. Ceftazidime: pharmacokinetics in patients and effects on the renal function. J Antimicrob Chemother. 1982 Sep;10(3):199–206. doi: 10.1093/jac/10.3.199. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., Walters L., Van Wyk M., Harding S. M., Paton A. M., Ayrton J. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother. 1983 Jun;23(6):892–896. doi: 10.1128/aac.23.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Van Hecken A., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Comparative pharmacokinetics of ceftazidime and moxalactam. Antimicrob Agents Chemother. 1982 Aug;22(2):237–241. doi: 10.1128/aac.22.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]



