Abstract
Background
Rare cases of vasculitis restricted to the lower limbs have been reported, but the characteristics, outcome and response to treatment of this entity are not well known.
Objective
To describe the clinical, complementary examinations and response to treatment of this rare entity in the first retrospective series, and to compare data with historical pooled cases.
Methods
Retrospective analysis of all biopsy‐proven cases observed over a 10‐year period in four French tertiary medical units. Diagnosis of vasculitis restricted to the lower limb required the absence of any clinical symptom and complementary test finding, suggesting major extramuscular visceral involvement.
Results
11 patients were included. Vasculitis restricted to the lower limb was associated with disabling muscle pain of the calves. Fever was present in 50% of cases; ankle arthralgia in 50% and skin involvement in 40%. MRI was the cornerstone of the diagnosis, showing hyperintense signal in T2 weight and in T1 weight after gadolinium injection. MRI findings correlated well with clinical outcome and were useful in guiding biopsy. Muscle biopsy was consistent with a polyarteritis nodosa‐type vasculitis in only 40% cases, whereas a leucocytoclastic vasculitis was seen for all other cases. Treatment with corticosteroids was effective in all cases, but there were relapses requiring immunosuppressive agents in 54% of cases.
Conclusion
Vasculitis of the calf muscles must be considered for patients with calf pain and with a biological inflammatory syndrome.
Most cases of systemic vasculitis include multiorgan involvement and systemic manifestations; occasionally, only one organ is affected.1 Rare cases of polyarteritis nodosa (PAN) vasculitis mostly affecting the skeletal leg muscles have been reported. We report the first series of 11 cases of vasculitis restricted to the lower limbs and compare their characteristics and outcome with the few cases reported previously.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16
Patients and methods
Data for all patients, with a histologically proven vasculitis restricted to lower leg muscles followed in four French tertiary referral medical centres over a 10‐year period, were retrospectively analysed. Only cases with a histological proof of muscle vasculitis were included. The diagnostic criteria for restricted muscle vasculitis were (1) proven histological vasculitis on muscle biopsy and (2) absence of clinical symptoms and complementary test findings suggesting major extramuscular visceral involvement (ie, heart, central nervous system, mononeuropathy multiplex, kidney, gastrointestinal, or upper and lower respiratory tract manifestations).
We looked for similar cases in the literature using the PubMedline Database on the web and the following keywords: “localised polyarteritis nodosa”, “localised vasculitis”, “calf muscle vasculitis” or “limb‐restricted vasculitis”.
Results
Clinical characteristics at onset
Eleven patients fulfilled the inclusion criteria. In all patients except for one, pain was confined to calf muscles and was the presenting symptom (table 1). In one patient, calf‐muscle pain was associated with quadriceps myalgia. Pain was initially moderate and worsened during physical exercise in nine patients. Onset of symptoms was sudden in two patients, with pain causing rapid impotence of the limbs. An induration of the painful muscles of between 2 cm and involvement of the whole calf was present in 8 of 11 patients. Four patients had cutaneous lesions in the affected calf areas, the lesions being vascular purpura, erythema around a muscular nodule, superficial dermitis and/or pseudo erythema nodosa of the calf.
Table 1 Clinical, biological and histological features of the 11 patients compared with the literature.
| Clinical, biological and histological features | New cohort | Pooled cases of the literature |
|---|---|---|
| Median (range) age (years) | 45 (25–72) | 48 (23–90) |
| Sex ratio (F/M) | 1/1.2 | 1.3/1 |
| Median (range) delay to diagnosis (months) | 14 (2–35) | 2 |
| Calf pain | 100% | 100% |
| Bilateral pain | 63% | 80% |
| Calf tumefaction | 72% | 72% |
| Skin abnormalities associated | 36% | ND |
| Fever | 63% | 62% |
| Weight loss | 45% | nd |
| Median (range) ESR (mm/h) | 46 (23–134) | 74 (24–103) |
| Median (range) C reactive protein (mg/l) | 35 (9–125) | ND |
| Muscular biopsy | ||
| Leucocytoclastic vasculitis | 64% | 0% |
| PAN histology | 36% | 100% |
ESR, erythrocyte sedimentation rate; ND, no data; PAN, polyarteritis nodosa.
Seven patients presented with prolonged fever, three having temperature peaks >39°C (102°F). Significant weight loss (2–9 kg) was observed in 5 of 11 patients at presentation. Arthralgia without arthritis limited to the ankles affected half of the cases. No patient had major organ involvement, consistent with the inclusion criteria. The median time between the onset of symptoms and the final diagnosis was 14 months (range 2–35).
Complementary examinations
A biological inflammatory pattern was present in all patients (table 1). Creatine kinase, aldolase, liver function markers and serum creatinine were in the normal range in all cases. None of the patients had severe kidney involvement.
Tests for antinuclear antibodies, anti‐neutrophil cytoplasmic antibodies and cryoglobulinaemia were negative in all but one patient who had perinuclear anti‐neutrophil cytoplasmic antibodies by indirect immunofluorescence test without anti‐myeloperoxidase or anti‐proteinase 3 specificity by ELISA. Antibody tests for HIV were negative, and no patient had active hepatitis B virus or hepatitis C virus infections.
An electromyogram of the legs was performed in four patients and was found to be normal.
Bone x ray of the lower limbs was normal in all patients except for one, with new periosteal bone formation bordering the internal part of the peroneal diaphysis. Technetium scintigraphy was performed in two patients and showed localised uptake with new periosteal bone formation.
Magnetic resonance imaging
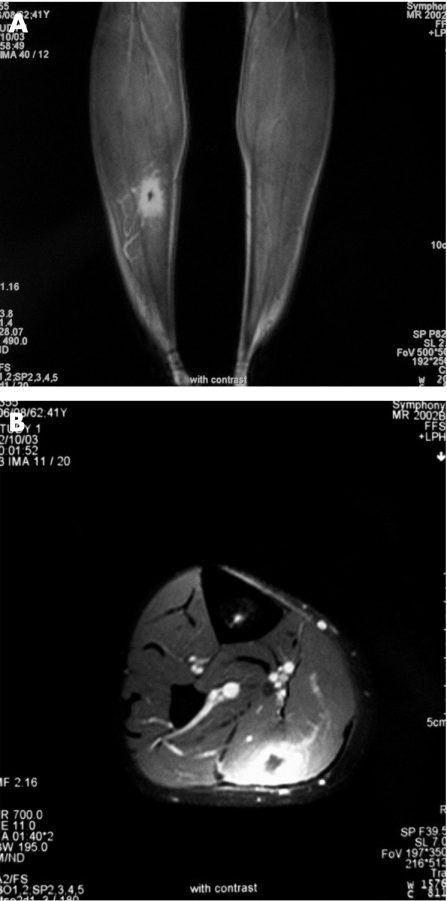
MRI of the calves was performed in all patients, and showed normal unenhanced T1‐weighted sequences; however, hyperintense T2‐weighted signals in the involved areas indicated increased muscle fluid content. Gadolinium in T1‐weighted sequences showed an enhanced signal. Six patients had several muscle groups involved and nine patients had bilateral lesions. The main muscle affected was the gastrocnemius muscle (n = 8; fig 1). Other affected muscles were quadriceps (n = 1), tibialis anterior (n = 2) and peroneus longus (n = 2). MRI features correlated well with clinical evolution; subsequent MRI showed disappearance of the abnormal signal from six patients who initially responded well to treatment. A new MRI was performed in eight patients who relapsed, and showed in all cases a recurrence of the hyperintense signal in the painful area.
Figure 1 (A) MRI of the limb sequence T1 with gadolinium. (B) Enhancement of the internal part of the gastronecmius.
Muscle biopsy
A calf muscle biopsy was performed in the abnormal area localised with MRI in all patients. Of the 11 patients, four had a surgical biopsy because of the depth of the muscle lesions, and biopsy was non‐surgical in seven patients. All biopsy specimens were analysed by a trained anatomopathologist of the French Vasculitis Study Group. Histological analysis revealed leucocytoclastic vasculitis in seven patients and a non‐granulomatous necrotising vasculitis in four.
Treatment and outcome
One patient did not receive any treatment because of spontaneous improvement, followed by a transient relapse after a 30‐month follow‐up.
One patient was treated with 2 mg/day colchicine alone with a rapid improvement. This patient was, however, dependent on the treatment, which is still continuing at 32 months of follow‐up.
Prednisone at a median dose of 1 mg/kg body weight per day (range 0.5–1) was used as a preferred treatment for nine patients, leading to substantial improvement in all patients. However, 8/9 (90%) patients suffered a relapse after a median of 11 months (range 3–34) when the median prednisone dose was 8 mg/day (range 4–11). The severity of the relapse and/or the dependence on corticosteroids led to administration of one or several immunosuppressive agents to six patients, associated in one patient with plasma exchange (methotrexate, n = 4; azathioprine, n = 2; cyclophosphamide, n = 1). In one patient, the relapse was successfully managed by a transient increase of the daily dose of steroids. One patient received 100 mg dapsone daily after recurrence with no success and then 400 mg hydroxychloroquine daily with a better outcome.
None of the 11 patients developed systemic extramuscular vasculitis during follow‐up (median 48 months; range 16–120). At the time of writing this report, four patients were receiving no treatment, one patient was receiving colchicine, four patients a low dose of prednisone (3–15 mg/day) and two patients prednisone associated with immunosuppressive agents (azathioprine or methotrexate).
Discussion
Our series confirms the clinical characteristics of vasculitis restricted to the lower limb: disabling muscle pain, frequently associated with fever, alteration of general status and inflammatory syndrome. The most frequently involved muscles are those in the calf, but other lower limb areas, particularly the quadriceps, may be involved. The nosology of this syndrome is unclear. Some authors postulate that such localised forms of vasculitis are cases of systemic PAN diagnosed early and treated.6 However, the absence of extra‐musculoskeletal major organ involvement in our series even after prolonged follow‐up argues against this hypothesis.
Like other authors,3,5 we highlight the importance of MRI for diagnosis. In all our patients, MRI revealed the affected area, confirming the inflammatory process in the muscles and providing useful guidance for the pathologist or the surgeon for the muscular biopsy. The very long delay before diagnosis in our series confirms the poor awareness of this kind of vasculitis among doctors. MRI should be performed earlier in the course of the disease in patients with persistent and unexplained calf pain associated with biological markers of inflammation. Radiological abnormalities correlated well with the clinical outcome, so serial MRI might be helpful for monitoring the course of the disease and response to treatment.
The histology of the previously reported cases of vasculitis confined to calf muscles was always typical of a medium‐vessel vasculitis consistent with a PAN. By contrast, leucocytoclastic vasculitis was the most frequent finding on muscular biopsy in our series, and PAN medium‐vessel vasculitis was found in only 40% of cases. The clinical and biological manifestations, outcomes and responses to treatment did not differ between these two histological presentations.
Eighteen cases2,3,4,5,6,7,8,9,10,11,12,13,14,15,16 of lower limb‐restricted vasculitis have been reported in the literature: corticosteroids seem to be very effective, and the course and prognosis of the disease were considered benign. Sensitivity to steroids was confirmed in our series, and all patients initially responded to steroid treatment. However, 90% of patients relapsed when the treatment was tapered and in >50% of patients, immunosuppressive agents were needed for a better control and as a steroid‐sparing strategy. So the reputation of “benign disease” attributed to the localised vasculitis of the calf should be reconsidered, and patients must be informed of the risk of relapse despite initially successful corticotherapy. In contrast with the high risk of relapse, inflammatory phenomena in the vessels remained localised in muscles in our series, and evolution towards severe multiorgan vasculitis seems unlikely.
To conclude, doctors should consider the diagnosis of localised vasculitis in middle‐aged patients having persistent and unexplained calf pain associated with fever and/or altered general status and abnormal markers of inflammation. MRI of the legs is best for diagnosis and should be rapidly performed whenever the cause of persistent pain is not obvious. It shows features of the localised inflammatory process and can guide muscle biopsy.
Abbreviations
PAN - polyarteritis nodosa
Footnotes
Competing interests: None.
References
- 1.Kariv R, Sidi Y, Gur H. Systemic vasculitis presenting as a tumorlike lesion. Four case reports and an analysis of 79 reported cases. Medicine (Baltimore) 200079349–359. [DOI] [PubMed] [Google Scholar]
- 2.Carron P, Hoffman I E, De Rycke L, Peene I, Veys E M, De Keyser F.et al Case number 34: relapse of polyarteritis nodosa presenting as isolated and localised lower limb periostitis. Ann Rheum Dis 2005641118–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel C G, Sibbitt R R, Sibbitt W L, Jr, Newell J D, Jr, Narva A., Jr A possible role for MRI in polyarteritis nodosa: the “creeping fat” sign. Magn Reson Imaging 19886713–715. [DOI] [PubMed] [Google Scholar]
- 4.Ferreiro J E, Saldana M J, Azevedo S J. Polyarteritis manifesting as calf myositis and fever. Am J Med 198680312–315. [DOI] [PubMed] [Google Scholar]
- 5.Gallien S, Mahr A, Rety F, Kambachna M, Lhota F, Cohen P.et al Magnetic resonance imaging of skeletal muscle involvement in limb restricted vasculitis. Ann Rheum Dis 2002611107–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia F, Pedrol E, Casademont J, Mellado B, Cordoba R, Cid M. Polyarteritis nodosa confined to calf muscles. J Rheumatol 199219303–305. [PubMed] [Google Scholar]
- 7.Garcia‐Porrua C, Mate A, Duran‐Marino J L, Fernandez‐Martinez C, Gonzalez‐Gay M A. Localized vasculitis in the calf mimicking deep venous thrombosis. Rheumatology (Oxford) 200241944–945. [DOI] [PubMed] [Google Scholar]
- 8.Gardner G C, Lawrence M K. Polyarteritis nodosa confined to calf muscles. J Rheumatol 199320908–909. [PubMed] [Google Scholar]
- 9.Golding D N. Polyarteritis presenting with leg pains. Br Med J 19701277–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall C, Mongey A B. Unusual presentation of polyarteritis nodosa. J Rheumatol 200128871–873. [PubMed] [Google Scholar]
- 11.Hofman D M, Lems W F, Witkamp T D, Putte V D, Bijlsma J W. Demonstration of calf abnormalities by magnetic resonance imaging in polyarteritis nodosa. Clin Rheumatol 199211402–404. [DOI] [PubMed] [Google Scholar]
- 12.Kamimura T, Hatakeyama M, Torigoe K, Nara H, Kaneko N, Satou H. Muscular polyarteritis nodosa as a cause of fever of undetermined origin: a case report and review of the literature. Rheumatol Int 200525394–397. [DOI] [PubMed] [Google Scholar]
- 13.Laitinen O, Haltia M, Lahdevirta J. Polyarteritis confined to lower extremities. Scand J Rheumatol 19821171–74. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Tomoda K, Yamamura Y, Tsukano M, Honda I, Iyama K. Polyarteritis nodosa limited to calf muscles: a case report and review of the literature. Clin Rheumatol 200322149–153. [DOI] [PubMed] [Google Scholar]
- 15.Nash P, Fryer J, Webb J. Vasculitis presenting as chronic unilateral painful leg swelling. J Rheumatol 1988151022–1025. [PubMed] [Google Scholar]
- 16.Soubrier M, Bangil M, Franc S, Dubost J J, Ristori J M, Kemeny J L.et al Vasculitis confined to the calves. Report of a case. Rev Rhum Engl Ed 199764414–416. [PubMed] [Google Scholar]