Abstract
Objective
To compare dedicated low‐field MRI (lfMRI) with conventional MRI (cMRI) in the detection and scoring of synovitis, tenosynovitis and erosions in patients with rheumatoid arthritis.
Patients and methods
The wrist and finger joints of 17 patients with rheumatoid arthritis (median (range) disease duration 8 years (7–12); Disease Activity Score 3.3 (2.6–4.5)) were examined by 0.2 T lfMRI and 1.5 TcMRI. The protocols comprised coronal spin‐echo and three‐dimensional gradient‐echo sequences before and after contrast medium administration. Synovitis of the metacarpophalangeal and proximal interphalangeal joints 2–5 and the wrist joints was scored according to Outcome Measures in Rheumatology recommendations. Tenosynovitis and erosions were scored using 4‐point and 6‐point scales, respectively. The results were analysed by calculating κ values and performing McNemar's test intra‐individually on a joint‐by‐joint basis.
Results
Agreement between the two MRI techniques was good to excellent for synovitis and erosions, and moderate for tenosynovitis. Of the 306 joints evaluated, 245 and 200 joints showed synovitis in lfMRI and cMRI, respectively. Scoring of synovitis of the finger joints yielded κ values from 0.69 to 0.94. Of the 68 flexor tendons evaluated, tenosynovitis was diagnosed by lfMRI in 24 and by cMRI in 33 instances. Of the 391 bones evaluated, 154 and 139 showed erosions in lfMRI and cMRI, respectively. κ values for erosion scores were between 0.65 and 1.
Conclusion
Dedicated, lfMRI shows high agreement with cMRI in diagnosing and scoring synovitis, tenosynovitis and erosions in rheumatoid arthritis when using standardised scoring systems.
Rheumatoid arthritis is an inflammatory systemic autoimmune disease of unknown aetiology that predominantly affects the synovial membrane of joints. It is characterised by polyarticular manifestation, typically with a symmetrical pattern of involvement. In most cases, the finger and toe joints and the wrist are affected.1 The earliest lesion visible on conventional radiographs is juxta‐articular osteoporosis.2,3 Conventional radiography is the most widely used imaging modality for the diagnosis of rheumatoid arthritis. Its advantages are its wide availability, good standardisation and validated evaluation scales.4
Synovitis is among the earliest abnormalities in early arthritis, but is identified on radiographs only indirectly or not at all. Other processes such as tenosynovitis and bone marrow oedema are also not detected by conventional x ray.5,6,7 Destructive lesions such as erosions become visible on radiographs only at later stages of the disease.2,8,9,10
Studies have shown that MRI demonstrates synovitis and minute erosive lesions shortly after initial clinical manifestation.11,12,13 Moreover, MRI is also able to identify cartilaginous defects14,15 and bone marrow oedema.9,16
Dedicated low‐field MRI (lfMRI) systems with a field strength of 0.2 T belong to a new generation of magnetic resonance scanners with new imaging options. These scanners provide excellent patient comfort, which makes the examination much more acceptable.17 Cost effectiveness is another point that must be considered. Not much data are available regarding direct comparison of conventional MRI (cMRI) and extremity MRI in patients with rheumatoid arthritis. Only a few studies have investigated whether lfMRI and cMRI are comparable with regard to the detection of erosions and synovitis.17,18,19 Only one of them attempted to compare pathology scoring results between the two units.18 Although low‐field scanners have not been available for long, the limited data obtained so far suggest that they are equal or only slightly inferior to conventional magnetic resonance scanners in terms of image quality in the evaluation of patients with rheumatoid arthritis, and that there is no loss of diagnostic information.20
We present a cross‐sectional study comparing 1.5 T cMRI and 0.2 T low‐field extremity MRI in terms of their ability to detect and score synovitis, tenosynovitis, and erosions of the wrist and finger joints in patients with rheumatoid arthritis.
Patients and methods
Patients
Seventeen patients (7 men and 10 women with a mean age of 58 years (range 26–75 years)) with rheumatoid arthritis according to the criteria of the American College of Rheumatology21 were enrolled in the study by a university‐based rheumatological outpatient service. Median disease duration was 8 years (range 5–41 years). Rheumatoid factor, antinuclear antibodies, antibodies against cyclic citrullinated peptides and the Disease Activity Score of 28 joints22 were determined in all patients. The study was approved by the local ethics committee and all patients gave written informed consent.
Magnetic resonance imaging
All patients underwent cMRI and lfMRI on the same day. Both MRI examinations were performed with the administration of the paramagnetic contrast medium, gadolinium diethylenetriaminepentaacetic acid (Gd‐DTPA; Magnevist, Schering, Berlin, Germany), which was administered at a dose of 0.2 mmol/kg body weight for lfMRI23 and 0.1 mmol/kg body weight for cMRI. Both MRI examinations were performed with a minimum delay of 7 h.
MRI protocols
Low‐field MRI
The patients were examined on a 0.2 T, dedicated low‐field magnetic resonance scanner (C‐scan, Esaote Biomedica, Genoa, Italy) using a dedicated hand coil. The patients were examined in a semi‐sitting position with the arm abducted and the hand in the coil.
The imaging protocol was chosen in accordance with the guidelines of the MRI in rheumatoid arthritis study group of the Outcome Measures in Rheumatology (OMERACT) initiative.24,25,26
The following sequences were acquired: gradient‐echo short‐tau inversion‐recovery sequence in coronal orientation, T1‐weighted spin‐echo sequence in axial and coronal orientations and T1‐weighted three‐dimensional gradient‐echo sequence in coronal orientation before and after bolus administration of contrast medium. Table 1 summarises the sequence parameters.
Table 1 Parameters of the MRI sequences used.
| TR (ms) | TE (ms)/TI (ms) | Flip angle (deg) | In‐plane resolution (mm2) | Slice thickness/ gap (mm) | FOV (mm) | Matrix size | Time (min:s) | |
|---|---|---|---|---|---|---|---|---|
| Low‐field MRI (0.2 T) | ||||||||
| GE‐STIR | 700 | 16/75 | 90 | 1.13×0.54 | 3/0.3 | 180 | 192×256 | 5:19 |
| T1 SE | 520 | 26 | 90 | 0.56×0.56 | 3.5/0.3 | 180 | 320×512 | 5:35 |
| T1 GRE | 35 | 16 | 65 | 0.83×0.83 | 0.86/0 | 160 | 256×256 | 8:00 |
| Conventional MRI (1.5 T) | ||||||||
| STIR | 5000 | 65/150 | 90 | 0.70×0.70 | 3/0.3 | 180 | 256×256 | 1:57 |
| T1 SE | 500 | 21 | 90 | 0.35×0.35 | 3.0/0.3 | 180 | 512×512 | 4:21 |
| T1 GRE | 8.8 | 3.5 | 8 | 0.47×0.47 | 1.0/0 | 180 | 384×384 | 3:43 |
FOV, field of view; GE‐STIR, gradient recalled echo STIR; STIR, short‐tau inversion‐recovery; T1 SE, T1‐weighted spin‐echo sequence; T1 GRE, T1‐weighted gradient recalled echo sequence; TE, echo time; TI, inversion time; TR, repetition time.
The dataset acquired with the T1‐weighted three‐dimensional gradient‐echo sequence was used for reconstruction of axial views.
Conventional MRI
The high‐field examination was performed on a 1.5‐T, whole‐body magnetic resonance scanner (Sonata, Siemens Medical Solutions, Erlangen, Germany) using a large flexible surface coil. The patient was positioned prone with the hand extended over the head.
The following sequences were acquired: short‐tau inversion‐recovery sequence in coronal orientation, T1‐weighted spin‐echo sequence in axial and coronal orientations, three‐dimensional gradient recalled echo sequences in coronal orientation before and after bolus administration of the contrast medium, and T1‐weighted, fat‐saturated spin‐echo sequence. Table 1 summarises the sequence parameters. The three‐dimensional dataset was reconstructed in axial orientation.
Image analysis
The magnetic resonance images were evaluated by KGH and CS who were blinded to the clinical data. Discrepant results were solved by consensus. The images of the lfMRI and cMRI examinations were reviewed with an interval of 3 months in between. Images were evaluated for synovitis of the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints 2–5, the radiocarpal joint, the distal radioulnar joint, the styloid process of ulna, the proximal and distal intercarpal joints, and carpometacarpal joints 1–5 and for tenosynovitis of the flexor tendons of fingers 2–5. The presence of erosions was evaluated at the MCP and PIP joints 2–5 of the fingers, all carpal bones, including the distal radius and ulna, and the base of the metacarpal bones 1–5. The presence of bone marrow oedema was not included in the analysis because the gradient‐echo short‐tau inversion‐recovery sequence of the lfMRI system did not yield artefact‐free images in all cases, which might have biased the results.
Definition of lesions
Erosions and synovitis were defined as suggested by the OMERACT group.26 According to these definitions, synovitis is an area in the synovial compartment that shows above‐normal enhancement after Gd‐DTPA administration of a thickness greater than the width of the normal synovium. A published and validated definition for tenosynovitis does not exist. On the basis of the description of synovitis, we defined tenosynovitis as follows: an area adjacent to a tendon with an above‐normal enhancement and an abnormal thickening of the tendon sheath. An erosion is defined by the OMERACT group as a sharply marginated bone lesion with correct juxta‐articular localisation. T1‐weighted magnetic resonance images depict an erosion as a lesion with low signal intensity in at least two planes, with cortical disruption seen in at least one plane.
Scoring
Images were analysed using the semiquantitative synovitis scale recommended by the OMERACT group (Rheumatoid Arthritis Magnetic Resonance Imaging Score, RAMRIS)26 and a system based on the OMERACT scale for evaluation of tenosynovitis.
The synovitis scale assigns one of four scores: 0 for no synovitis, 1 for mild synovitis, 2 for moderate synovitis and 3 for severe synovitis. Tenosynovitis was evaluated in the same fashion (0, no; 1, mild; 2, moderate; and 3, severe tenosynovitis). Only the flexor tendons of digits 2–5 were evaluated. The extensor tendons were deemed too small for semiquantitative scoring.
Erosions were assessed using a semiquantitative scoring system based on the radiographic scoring system according to Larsen et al27 modified for analysis of magnetic resonance images. Points are assigned as follows: 0, no erosions; 1, no erosions but narrowing of the joint cleft or contour irregularities; 2, erosions involving up to 25% of the joint area; 3, erosions of up to 50% of the area; 4, erosions of up to 75% of the area; 5, >75% of the joint area damaged by erosions or mutilation. The joint area is defined as the sum of the joint surfaces of the metacarpal head and the base of the proximal phalanx for the MCP joint and as the sum of the head of the proximal phalanx and the base of the middle phalanx for the PIP joint.
Inter‐reader agreement
A subset of 20 MRI examinations (10 lfMRI, 10 cMRI) was selected to test for inter‐reader agreement as a separate analysis 8 months after the original image analysis. Here, both the readers scored the MRI images separately.
Statistical analysis
Neither of the imaging techniques evaluated in this study was regarded as the gold standard. Therefore, the κ coefficient was used to describe agreement between the two techniques. A poor agreement was assumed at κ<0.4, moderate to good agreement at values 0.4–0.75 and excellent agreement at values >0.75.28 Systematical differences between both methods were tested by applying McNemar's test as a significance test on a joint‐by‐joint or bone‐by‐bone basis. Significance was assumed at p<0.05. The scores of the individual joints assigned with both MRI techniques were represented in contingency tables. StatXact with Cytel Studio, V.6.1 (Cytel, Cambridge, Massachusetts, USA; software for exact non‐parametric inference) was used to calculate weighted κ coefficients and to perform the McNemar tests and SPSS for Windows, V.11.0, to establish the contingency tables. Inter‐reader agreement was calculated using both weighted κ values as well as intraclass correlation coefficients.
Results
Seventeen patients were evaluated for synovitis, tenosynovitis and erosions of the clinically dominant hand (13 right and 4 left); 12 of the patients (71%) had a positive rheumatoid factor. Disease activity was moderate (median disease Activity Score of 28 joints was 3.3 (quartiles 2.6; 4.5)). The antinuclear antibody test was positive in 13 patients (76%) and antibodies against cyclic citrullinated peptides were identified in 10 patients (59%).
A total of 306 joints were evaluated for synovitis. Overall agreement between both magnetic resonance techniques was good to excellent. Table 2 summarises the findings with regard to synovitis at the finger joints. There was excellent agreement of synovitis scoring (κ = 0.81–0.94) for all joints except for PIP joint 5, for which agreement was good (0.74). The contingency tables for the joints evaluated show that higher scores are assigned with lfMRI. Significantly different scores were identified for MCP joint 5 and PIP joint 5. Table 3 summarises the κ values and results of McNemar's test for the wrist joints. Agreement was good to excellent (κ = 0.69–0.93), as fig 1 shows. Significant differences were seen for the distal radioulnar joint, the proximal and distal rows of intercarpal joints, the styloid process and carpometacarpal joint 3, with higher scores being assigned with lfMRI.
Table 2 Detection and scoring of synovitis at metacarpophalangeal and proximal interphalangeal joints (n = 136).
| Joint | Synovitis | κ† | CI† | p Value† (McNemar) | |
|---|---|---|---|---|---|
| Low‐field MRI (n)* | Conventional MRI (n)* | ||||
| 2nd MCP | 17 | 16 | 0.94 | 0.88 to 0.99 | 0.359 |
| 3rd MCP | 15 | 13 | 0.93 | 0.87 to 0.99 | 1.000 |
| 4th MCP | 12 | 10 | 0.81 | 0.62 to 0.99 | 1.000 |
| 5th MCP | 15 | 12 | 0.89 | 0.80 to 0.98 | 0.007 |
| 2nd PIP | 12 | 10 | 0.90 | 0.82 to 0.98 | 0.726 |
| 3rd PIP | 14 | 10 | 0.94 | 0.87 to 0.99 | 0.062 |
| 4th PIP | 16 | 13 | 0.92 | 0.86 to 0.97 | 0.179 |
| 5th PIP | 14 | 8 | 0.74 | 0.53 to 0.94 | 0.005 |
| Total | 115 | 92 | |||
MCP, metacarpophalangeal joint; PIP, proximal interphalangeal joint.
*Number of affected joints.
†Based on scoring results of individual joints.
Table 3 Detection and scoring of synovitis at wrist joints (n = 170).
| Joint | Synovitis | κ† | CI† | p Value† (McNemar) | |
|---|---|---|---|---|---|
| Low‐field MRI (n)* | Conventional MRI (n)* | ||||
| Radiocarpal | 11 | 8 | 0.77 | 0.56 to 0.98 | 0.187 |
| Distal radioulnar | 12 | 10 | 0.72 | 0.58 to 0.86 | 0.007 |
| Styloid process | 13 | 12 | 0.83 | 0.71 to 0.95 | 0.031 |
| Proximal intercarpal | 16 | 13 | 0.89 | 0.79 to 0.98 | 0.015 |
| Distal intercarpal | 16 | 12 | 0.90 | 0.81 to 0.99 | 0.031 |
| CMC 1 | 16 | 14 | 0.92 | 0.87 to 0.98 | 0.453 |
| CMC 2 | 13 | 9 | 0.69 | 0.40 to 0.98 | 0.062 |
| CMC 3 | 11 | 9 | 0.73 | 0.58 to 0.87 | 0.035 |
| CMC 4 | 11 | 10 | 0.77 | 0.62 to 0.92 | 0.109 |
| CMC 5 | 11 | 11 | 0.83 | 0.68 to 0.98 | 0.312 |
| Total | 130 | 108 | |||
CMC, carpometacarpal joint.
*Number of affected joints.
†Based on scoring results of individual joints.
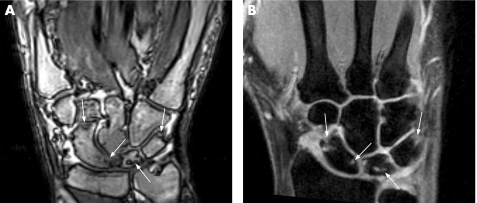
Figure 1 Comparison of erosions at the carpal bones in a 60‐year‐old patient with rheumatoid arthritis since 5 years. Gradient‐echo sequence after contrast injection acquired by low‐field MRI (A) and contrast‐enhanced gradient‐echo sequence with fat suppression (B) of the wrist show the same erosions at the scaphoid, lunate and triquetrum bones (arrows). In addition, mild synovitis is seen in both examinations, partly also within the erosions.
A total of 68 flexor tendons were evaluated. Tenosynovitis was diagnosed by lfMRI in 24 instances and by cMRI in 33 instances (table 4). There was moderate to good agreement of the tenosynovitis scores (κ = 0.51–0.65) without any significant differences. In general, more tendons were detected positive for tenosynovitis in cMRI (fig 2).
Table 4 Detection and scoring of tenosynovitis of flexor tendons (n = 68).
| Flexor tendon | Tenosynovitis | κ† | CI† | p Value† (McNemar) | |
|---|---|---|---|---|---|
| Low‐field MRI (n)* | Conventional MRI (n)* | ||||
| 2nd finger | 6 | 8 | 0.60 | 0.25 to 0.95 | 0.109 |
| 3rd finger | 8 | 10 | 0.51 | 0.21 to 0.80 | 0.492 |
| 4th finger | 5 | 8 | 0.59 | 0.36 to 0.82 | 0.687 |
| 5th finger | 5 | 7 | 0.65 | 0.26 to 1.00 | 0.226 |
| Total | 24 | 33 | |||
*Number of affected tendons.
†Based on scoring results of individual tendons.
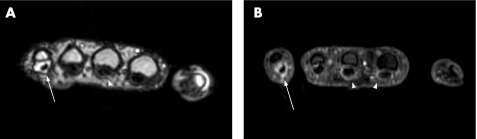
Figure 2 Comparison of tenosynovitis of the flexor tendons in a 26‐year‐old patient with rheumatoid arthritis since 7 years. Transverse reconstructions of the 0.2 T three‐dimensional gradient‐echo sequence after contrast injection (A) and the 1.5 T fat‐suppressed three‐dimensional gradient‐echo sequence after contrast injection (B) both show severe flexor tenosynovitis at the fifth finger (arrow) and mild tenosynovitis at the third finger (arrowhead). Tenosynovitis at the second finger (second arrowhead in B) is only seen in the 1.5 T examination.
A total of 391 regions (finger joints, bases of metacarpal bones, carpal bones, radius and ulna) were scored for the presence of erosions. There was good to excellent agreement for the finger joints (κ = 0.65–0.95) and there were no significant differences (table 5, fig 3). Altogether, erosions affected the MCP joints more frequently than the PIP joints. Agreement in the detection of erosions was slightly poorer for the PIP joints. κ values for the wrist joints showed wide variation from good agreement (lowest κ of 0.65) to full agreement of both magnetic resonance systems (κ = 1.0; table 6). McNemar's test did not identify any bone of the wrist, with significantly higher erosion scores.
Table 5 Detection and scoring of erosions at metacarpophalangeal and proximal interphalangeal joints (n = 136).
| Joint | Erosions | κ† | CI† | p value† (McNemar) | |
|---|---|---|---|---|---|
| Low‐field MRI (n)* | Conventional MRI (n)* | ||||
| 2nd MCP | 11 | 12 | 0.96 | 0.90 to 1.01 | 1.000 |
| 3rd MCP | 10 | 8 | 0.89 | 0.78 to 1.00 | 0.125 |
| 4th MCP | 6 | 6 | 0.92 | 0.80 to 1.00 | 1.000 |
| 5th MCP | 9 | 8 | 0.77 | 0.59 to 0.95 | 0.812 |
| 2nd PIP | 2 | 1 | 0.65 | 0.35 to 0.96 | 0.500 |
| 3rd PIP | 3 | 2 | 0.73 | 0.50 to 0.97 | 0.500 |
| 4th PIP | 1 | 1 | 0.79 | 0.78 to 0.80 | 1.000 |
| 5th PIP | 1 | 1 | 0.79 | 0.78 to 0.80 | 1.000 |
| Total | 43 | 39 | |||
MCP, metacarpophalangeal joint; PIP, proximal interphalangeal joint.
*Number of affected joints.
†Based on scoring results of individual joints.
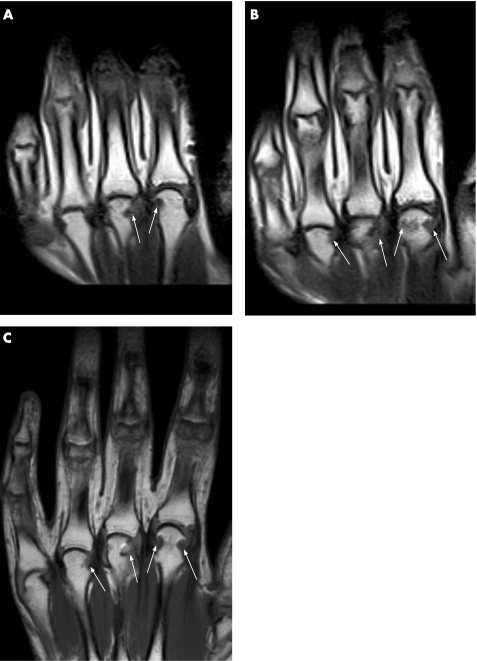
Figure 3 Comparison of erosions at metacarpophalangeal (MCP) joints 2–4 (same patient as in fig 2). Spin‐echo sequence (two adjacent slices) (A, B) acquired by low‐field MRI and fast spin‐echo sequence (C) of the MCP joints acquired by conventional MRI show the same marginal erosions at the metacarpal heads (arrows).
Table 6 Detection and scoring of erosions at wrist bones (n = 255).
| Bone | Erosions | κ† | CI† | p Value† (McNemar) | |
|---|---|---|---|---|---|
| Low‐field MRI (n)* | Conventional MRI (n)* | ||||
| Radius | 4 | 4 | 1.00 | 1.00 to 1.00 | NA |
| Ulna | 10 | 7 | 0.88 | 0.74 to 1.00 | 0.250 |
| Pisiform | 2 | 3 | 0.73 | 0.49 to 0.97 | 1.000 |
| Triquetrum | 11 | 10 | 0.92 | 0.82 to 1.00 | 1.000 |
| Lunate | 11 | 9 | 0.85 | 0.71 to 0.99 | 0.375 |
| Scaphoid | 8 | 8 | 0.84 | 0.68 to 1.00 | 0.562 |
| Hamate | 12 | 11 | 0.78 | 0.68 to 0.89 | 0.062 |
| Capitate | 11 | 10 | 0.94 | 0.88 to 1.00 | 0.500 |
| Trapezoid | 5 | 3 | 0.68 | 0.49 to 0.87 | 0.125 |
| Trapezium | 8 | 8 | 0.65 | 0.51 to 0.79 | 0.531 |
| 1st MC base | 5 | 5 | 0.95 | 0.87 to 1.00 | 1.000 |
| 2nd MC base | 8 | 7 | 0.83 | 0.65 to 1.00 | 0.625 |
| 3rd MC base | 7 | 7 | 0.97 | 0.93 to 1.00 | 1.000 |
| 4th MC base | 5 | 4 | 0.87 | 0.62 to 1.00 | 1.000 |
| 5th MC base | 4 | 4 | 1.00 | 1.00 to 1.00 | NA |
| Total | 111 | 100 | |||
MC, metacarpal; NA, not applicable (no discordant pairs).
*Number of affected bones.
†Based on scoring results of individual bones.
Inter‐reader agreement was excellent both for lfMRI and for cMRI for scoring synovitis and erosions, and good for scoring tenosynovitis (table 7).
Table 7 Inter‐reader agreement.
| ICC | CI | Weighted κ | SE | |
|---|---|---|---|---|
| Low‐field MRI | ||||
| Erosions | 0.84 | 0.80 to 0.87 | 0.78 | 0.05 |
| Synovitis | 0.86 | 0.79 to 0.90 | 0.78 | 0.07 |
| Tenosynovitis | 0.76 | 0.60 to 0.86 | 0.65 | 0.12 |
| Conventional MRI | ||||
| Erosions | 0.81 | 0.76 to 0.85 | 0.78 | 0.05 |
| Synovitis | 0.88 | 0.81 to 0.92 | 0.77 | 0.08 |
| Tenosynovitis | 0.74 | 0.56 to 0.86 | 0.74 | 0.12 |
ICC, intraclass correlation coefficient.
Discussion
The aim of our study was to compare the diagnosis and scoring of pathologies between cMRI and lfMRI in a group of patients with rheumatoid arthritis using semiquantitative scoring systems. To date, not many studies compared lfMRI with cMRI for the diagnosis and monitoring of treatment.
Savnik et al17 were the first to compare the detection of synovitis by lfMRI and cMRI, showing good agreement between both modalities. They used both a dichotomic evaluation system and volume analysis of the inflammatory synovial membrane. Similar results were reported in a study with 18 patients, although limited by the fact that MRI was performed without contrast medium administration.18 Recently, Ejbjerg et al19 reported a high sensitivity of contrast‐enhanced lfMRI in diagnosing synovitis in 37 patients with rheumatoid arthritis, using cMRI as the gold standard. This approach is to be questioned. Despite increasing data on the role of MRI in patients with arthritis,6 there is still no consensus statement regarding the recognition of MRI as the sole gold standard. As a superior diagnostic accuracy of lfMRI cannot be shown in comparison to another method, if that is regarded the gold standard (as sensitivity cannot be calculated >100%), we did not apply this approach in our study. We therefore calculated κ values that confirm a good agreement between both MRI techniques in evaluating synovitis. In most joints, equal scores were determined, whereas only few joints show significantly higher synovitis scores for lfMRI. More joints detected with synovitis for lfMRI were also found by Savnik et al,17 whereas Ejbjerg et al19 reported slightly more joints with synovitis for cMRI. Comparison of the two units for synovitis scoring was performed only by Taouli et al.18 They also used a scoring scale of 0–3 and found no significant differences between both units. However, Taouli et al did not use contrast material in their study and hence the evaluation of synovitis lacks the ability to differentiate between inflamed synovium and fluid.
MRI visualises soft‐tissue lesions in rheumatoid arthritis such as tenosynovitis,29 but only few studies have investigated the evaluation of tenosynovitis by MRI30,31,32 and there is no study that compares cMRI with lfMRI in that respect in rheumatoid arthritis.
Conventional MRI identified more instances of tenosynovitis than lfMRI, however, without statistical significance. We found moderate to good agreement in the scoring of tenosynovitis between both imaging devices. Reference images from the OMERACT group are available only for synovitis, bone marrow oedema and erosions.24,25 An accepted scoring system for evaluating inflammatory tendon processes of the finger and wrist joints in rheumatoid arthritis similar to the RAMRIS does not yet exist. We therefore evaluated tenosynovitis using a modified version of the RAMRIS for synovitis. The relatively lower agreement rate between the two units found for tenosynovits could result from the lack of a standardised scoring system and of a reference atlas. Also, further studies are necessary to investigate the clinical role of inflammatory tendon processes and their possible effect on therapeutic decision making in the course of rheumatoid arthritis, as recently outlined in the research agenda of the OMERACT MRI in rheumatoid arthritis working group.33
Early identification of erosions by means of sensitive imaging modalities has a decisive effect on therapeutic decisions and further disease course.12,34 We found excellent agreement between both MRI techniques in detecting erosions. Both the scoring results of individual joints and the total number of affected joints showed that more erosions were detected by lfMRI than with cMRI (154 vs 139 eroded joints). Savnik et al17 also reported a good overall agreement between both types of scanners. They also identified more erosions with lfMRI (n = 496) than with cMRI (n = 379). Dichotomic evaluation of erosions in the study by Ejbjerg et al19 showed a sensitivity of 94% for lfMRI, which identified a total of 370 joints with erosions. cMRI served as the reference method and detected a total of 318 eroded joints. Like in our study, Taouli et al18 did not find a statistically significant difference between the scores assigned with both MRI techniques (mean score of 28.8 vs 27.5). However, they do not present data on individual bones and joints.
Why do lfMRI scanners detect more erosions than conventional scanners throughout all studies? Patients with rheumatoid arthritis may have problems when positioned in a whole‐body scanner owing to pain in the shoulder and neck area, and may therefore benefit from comfortable positioning outside the magnet. As a result, fewer motion artefacts occur, which is a possible reason for the higher detection rates of erosions and synovitis shown for lfMRI.
The OMERACT RAMRIS for erosions26 was not used in our study, for two reasons: this score is defined for MCP joints only, making a distinction between the metacarpal bone and the base of the proximal phalanx. Each of these two joint segments in the RAMRIS is evaluated separately on a scale of 0–10 (with 1 point corresponding to an erosion of 10% of the total bone volume). This scale allows a very detailed description of even minute changes in erosion size. However, modification of this scoring system for evaluation of the PIP joints in our study was considered unsuitable because of the small size of the PIP joints. Therefore, a modified Larsen Scale was used in the current study. Although this scoring system is systematic, it differs from the RAMRIS Scoring System for erosions and is not standardised. Evaluation of inter‐reader agreement showed excellent κ and intraclass correlation coefficient values, underlining the ease of use of our newly developed scoring system. Further studies for the evaluation of the differences between the two imaging systems investigated here using the OMERACT Scoring System for erosions are advisable.
An obvious limitation of our study is the small number of patients. Minimal slice thickness was different in both devices. However, this does not seem to influence the results as the device with thicker slices (lfMRI) overall detected more erosions. Another drawback is the coil selection. Although lfMRI was performed with a dedicated double‐phased hand coil, a comparable coil (eg, four‐channel hand coil) was not available for cMRI, and a standard flexible surface coil was used. On the other hand, our results reflect the true clinical situation, where only the standard equipment is available in many instances.
Bone marrow oedema, which has a high predictive value for the development of bone erosions,9 was not evaluated in our study owing to the rather low image quality of the 0.2 T imaging device in this regard. Different studies investigating this pathology on lfMRI showed only a low sensitivity in the detection of bone marrow oedema.19
Our study does not include a dedicated, age‐matched control group of healthy subjects. Such a group has been investigated by Ejbjerg et al.35 Here, only in a few individuals, mild changes consistent with synovitis were detected. It is important to exclude any possibility to score normal joints as mild synovitis. Although no control group exists, our data of inter‐reader agreement suggest a high level of confidence separating low‐grade inflammatory changes from normal joints and hence adds to the validity of our study results.
In summary, the results presented here confirm the clinical usefulness of dedicated low‐field magnetic resonance scanners for evaluating patients with rheumatoid arthritis. High agreement rates were found between lfMRI and cMRI not only in the detection of erosions and synovitis, as already shown by other studies, but also when scoring those lesions. The performance of the two devices in the detection and grading of tenosynovitis was investigated for the first time. Dedicated magnetic resonance scanners have a role wherever a universal whole‐body magnetic resonance scanner cannot be purchased for reasons of cost or for sparsely populated areas, but still a timely and sensitive diagnosis of rheumatoid arthritis and other inflammatory joint diseases is seeked.
Acknowledgements
The use of the MRI device was possible owing to a research contract between Charité medical school and Esaote Biomedica SA, Genoa, Italy.
Abbreviations
cMRI - conventional magnetic resonance imaging
Gd‐DTPA - gadolinium diethylenetriaminepentaacetic acid
lfMRI - low‐field magnetic resonance imaging
MCP - metacarpophalangeal
OMERACT - Outcome Measures in Rheumatology
PIP - proximal interphalangeal
RAMRIS - Rheumatoid Arthritis Magnetic Resonance Imaging Score
Footnotes
Competing interests: None.
References
- 1.Lee D M, Weinblatt M E. Rheumatoid arthritis. Lancet 2001358903–911. [DOI] [PubMed] [Google Scholar]
- 2.McGonagle D, Conaghan P G, Wakefield R, Emery P. Imaging the joints in early rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol 20011591–104. [DOI] [PubMed] [Google Scholar]
- 3.Kainberger F, Peloschek P, Langs G, Boegl K, Bischof H. Differential diagnosis of rheumatic diseases using conventional radiography. Best Pract Res Clin Rheumatol 200418783–811. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D. Quantification of radiological damage in inflammatory arthritis: rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Best Pract Res Clin Rheumatol 200418847–860. [DOI] [PubMed] [Google Scholar]
- 5.Taylor P C. The value of sensitive imaging modalities in rheumatoid arthritis. Arthritis Res Ther 20035210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostergaard M, Szkudlarek M. Imaging in rheumatoid arthritis—why MRI and ultrasonography can no longer be ignored. Scand J Rheumatol 20033263–73. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard M, Ejbjerg B. Magnetic resonance imaging of the synovium in rheumatoid arthritis. Semin Musculoskelet Radiol 20048287–299. [DOI] [PubMed] [Google Scholar]
- 8.Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf K J.et al Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast‐enhanced magnetic resonance imaging. Arthritis Rheum 1999421232–1245. [DOI] [PubMed] [Google Scholar]
- 9.McQueen F M, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S.et al Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum 2003481814–1827. [DOI] [PubMed] [Google Scholar]
- 10.Guermazi A, Taouli B, Lynch J A, Peterfy C G. Imaging of bone erosion in rheumatoid arthritis. Semin Musculoskelet Radiol 20048269–285. [DOI] [PubMed] [Google Scholar]
- 11.McQueen F M, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan P L.et al Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis 199857350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostergaard M, Hansen M, Stoltenberg M, Jensen K E, Szkudlarek M, Pedersen‐Zbinden B.et al New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum 2003482128–2131. [DOI] [PubMed] [Google Scholar]
- 13.Benton N, Stewart N, Crabbe J, Robinson E, Yeoman S, McQueen F M. MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann Rheum Dis 200463555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterfy C G, van Dijke C F, Lu Y, Nguyen A, Connick T J, Kneeland J B.et al Quantification of the volume of articular cartilage in the metacarpophalangeal joints of the hand: accuracy and precision of three‐dimensional MR imaging. Am J Roentgenol 1995165371–375. [DOI] [PubMed] [Google Scholar]
- 15.Gandy S J, Brett A D, Dieppe P A, Keen M C, Maciewicz R A, Taylor C J.et al Measurement of cartilage volumes in rheumatoid arthritis using MRI. Br J Radiol 20057839–45. [DOI] [PubMed] [Google Scholar]
- 16.Savnik A, Malmskov H, Thomsen H S, Graff L B, Nielsen H, Danneskiold‐Samsoe B.et al Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol 2001282193–2200. [PubMed] [Google Scholar]
- 17.Savnik A, Malmskov H, Thomsen H S, Bretlau T, Graff L B, Nielsen H.et al MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high‐field MRI (1.5 T). Eur Radiol 2001111030–1038. [DOI] [PubMed] [Google Scholar]
- 18.Taouli B, Zaim S, Peterfy C G, Lynch J A, Stork A, Guermazi A.et al Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. Am J Roentgenol 2004182937–943. [DOI] [PubMed] [Google Scholar]
- 19.Ejbjerg B J, Narvestad E, Jacobsen S, Thomsen H S, Ostergaard M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis 2005641280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostergaard M, Ejbjerg B, Szkudlarek M. Imaging in early rheumatoid arthritis: roles of magnetic resonance imaging, ultrasonography, conventional radiography and computed tomography. Best Pract Res Clin Rheumatol 20051991–116. [DOI] [PubMed] [Google Scholar]
- 21.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 22.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 23.Eshed I, Althoff C E, Schink T, Scheel A K, Schirmer C, Backhaus M.et al Low‐field MRI for assessing synovitis in patients with rheumatoid arthritis. Impact of Gd‐DTPA dose on synovitis scoring. Scand J Rheumatol 200635277–282. [DOI] [PubMed] [Google Scholar]
- 24.Ejbjerg B, McQueen F, Lassere M, Haavardsholm E, Conaghan P, O'Connor P.et al The EULAR‐OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Ann Rheum Dis 200564(Suppl 1)i23–i47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conaghan P, Bird P, Ejbjerg B, O'Connor P, Peterfy C, McQueen F.et al The EULAR‐OMERACT rheumatoid arthritis MRI reference image atlas: the metacarpophalangeal joints. Ann Rheum Dis 200564(Suppl 1)i11–i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B.et al OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA‐MRI scoring system. J Rheumatol 2003301385–1386. [PubMed] [Google Scholar]
- 27.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 197718481–491. [DOI] [PubMed] [Google Scholar]
- 28.Cicchetti D V, Feinstein A R. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol 199043551–558. [DOI] [PubMed] [Google Scholar]
- 29.Ostergaard M, Szkudlarek M. Magnetic resonance imaging of soft tissue changes in rheumatoid arthritis wrist joints. Semin Musculoskelet Radiol 20015257–274. [DOI] [PubMed] [Google Scholar]
- 30.Klarlund M, Ostergaard M, Jensen K E, Madsen J L, Skjodt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. The TIRA Group. Ann Rheum Dis 200059521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swen W A, Jacobs J W, Hubach P C, Klasens J H, Algra P R, Bijlsma J W. Comparison of sonography and magnetic resonance imaging for the diagnosis of partial tears of finger extensor tendons in rheumatoid arthritis. Rheumatology (Oxford) 20003955–62. [DOI] [PubMed] [Google Scholar]
- 32.Backhaus M, Burmester G R, Sandrock D, Loreck D, Hess D, Scholz A.et al Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis 200261895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostergaard M, McQueen F M, Bird P, Ejbjerg B, Lassere M N, Peterfy C G.et al Magnetic resonance imaging in rheumatoid arthritis advances and research priorities. J Rheumatol 2005322462–2464. [PubMed] [Google Scholar]
- 34.Peterfy C G. Magnetic resonance imaging in rheumatoid arthritis: current status and future directions. J Rheumatol 2001281134–1142. [PubMed] [Google Scholar]
- 35.Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen H S.et al Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum 2004501097–1106. [DOI] [PubMed] [Google Scholar]