Abstract
Objectives
To determine the predictive factors of clinical response to infliximab in patients with refractory psoriatic polyarthritis.
Methods
A multicentre open study which included 69 patients with psoriatic polyarthritis refractory to methotrexate (15 mg/week at least for 8 weeks). Patients were treated with infliximab 5 mg/kg every 8 weeks in addition to their stable doses of methotrexate. A major clinical response was defined by the ACR50 at week 38. Logistic regression analysis was performed to analyse which of the following measures at the start of treatment were associated with an ACR50 response: demographic and clinical characteristics, duration of disease, tender and swollen joint counts, involvement of large joints (knee or hip, or both), erythrocyte sedimentation rate, C reactive protein (CRP), Health Assessment Questionnaire disability index, axial involvement, and the presence of erosions at baseline.
Results
In an intention to treat analysis 30/69 (44%) patients achieved an ACR50 response. In the univariate analysis both the presence of large joint involvement and severe disability were associated with a poor clinical response. In a multivariate logistic regression analysis high CRP values were independently associated with a good therapeutic response (odds ratio (OR) = 18.7; 95% confidence interval (CI) 1.8 to 181.6; p = 0.011). In contrast, large joint involvement and severe disability were associated with a poor response, which reached significance for large joint involvement (OR = 29.3; 95% CI 3.2 to 266.3; p = 0.003).
Conclusion
A lower disability and, in particular, the absence of large joint involvement and higher CRP serum levels at the start of infliximab treatment are factors that seem to influence the probability of achieving a good therapeutic response in patients with psoriatic arthritis.
Keywords: psoriatic arthritis, infliximab, clinical response, predictive factors
Psoriatic arthritis (PsA) is a chronic inflammatory disease that occurs in association with skin psoriasis. The prevalence of psoriasis in the general population is about 1–3%, and 6–39% of these patients will develop PsA.1,2,3 Despite correct treatment, many patients with PsA have severe disease, with physical limitations, work related disability, and even increased mortality in comparison with controls.4,5,6 Methotrexate (MTX), sulfasalazine, and ciclosporin A are the most widely used disease modifying antirheumatic drugs (DMARDs), alone and in combination, in the treatment of PsA, although unfortunately a significant percentage of patients do not show any clinical significant response.7,8,9 High levels of tumour necrosis factor α (TNFα) are found in the joint fluid, synovium, and skin lesions of these patients.10,11,12 Several placebo controlled and open trials have shown that active PsA refractory to many DMARDs has an excellent response to TNFα blocking agents.13,14,15,16 However, given the high costs and the potential risks of treatment with TNFα blockers, the patients suitable for this kind of treatment should be carefully selected.17
We were interested to identify which variables might be potential predictive measures of good clinical response to infliximab in patients with PsA. To accomplish this, we analysed the data from patients with peripheral polyarthritis refractory to MTX included in a multicentre open study conducted in Spain.
Material and methods
Data were obtained from a 38 week, multicentre, prospective, open study evaluating the efficacy and tolerability of infliximab in patients with refractory PsA, conducted in Spain. The study included 69 patients who had (a) peripheral polyarthritis (⩾5 swollen and tender joints) and (b) one of the three following baseline criteria (morning stiffness >45 minutes and/or erythrocyte sedimentation rate >30 mm/1st h and/or C reactive protein (CRP) >15 mg/l), despite a minimum of 8 weeks of a stable dosage of 15 mg/week of MTX or a minimum of 7.5 mg/week in patients with severe intolerance to this drug. Patients testing positive for rheumatoid factor were excluded. Thus the primary goal of this study was to treat patients with psoriasis who presented a peripheral polyarthritis. A major clinical response was defined by the ACR50 (an improvement of at least 50% of the initial ACR composite index) at 38 weeks of active infliximab treatment.
Logistic regression analysis, Student's t test, χ2, and Fisher's exact tests using SPSS software were performed to investigate which of the following variables at the start of the treatment were associated with an ACR50 response: sex, age, disease duration, tender and swollen joint counts using the ACR66 articular index, axial involvement defined by the presence of radiological sacroiliitis (according to the New York modified criteria for ankylosing spondylitis), involvement of large joints (knee or hip, or both) by clinical assessment, erythrocyte sedimentation rate, CRP, clinical disability measured by the validated Health Assessment Questionnaire (HAQ) test, and presence of erosive arthritis (radiological erosions). Interpretation of the x ray findings was carried out by two expert rheumatologists. To determine the accuracy of our statistical model, we also calculated the receiver operating characteristics (ROC) curve, the sensitivity, specificity, and predictive values of the whole model, and goodness of fit with the Hosmen‐Lemeshow test.
An intention to treat analysis was carried out at 38 weeks after starting the treatment; however; we also analysed the results at 14 weeks. Moreover, we evaluated the prediction of response using the ACR20 and ACR70 (an improvement of at least 20% and 70%, respectively, in the initial ACR composite index) instead of the ACR50 to define the major treatment response.
Results
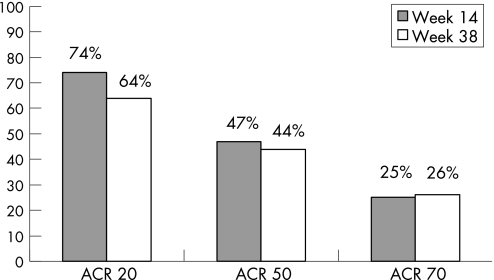
The study included 69 patients (42 women, 27 men). The mean (SD) disease duration was 8 (8), range 1–31 years. Among the patients included 49 (71%) had an erosive arthritis at entry, and 46 (68%) presented with arthritis in the knees or hips, or both. Table 1 shows the baseline demographic, clinical and biological characteristics of the patients at the start of infliximab treatment. Overall, a major clinical response (ACR50 at 38 weeks) was achieved by 30/69 (44%) patients, while an ACR20 and ACR70 was achieved by 44/69 (64%) and 18/69 (26%), respectively (fig 1).
Table 1 Demographic and clinical variables of patients included in the study at the start of treatment.
| Variable | Number of patients evaluated (%) | Mean | SD | Patients affected No (%) |
|---|---|---|---|---|
| Age (years) | 69 (100) | 42.5 | 12.8 | |
| Weight (kg) | 66 (96) | 72.7 | 14.7 | |
| SJC | 68 (99) | 13.5 | 10.7 | |
| TJC | 68 (99) | 20.7 | 12.4 | |
| VAS pain (cm) | 68 (99) | 5.9 | 2.0 | |
| VAS patient (cm) | 69 (100) | 6.2 | 1.6 | |
| VAS physician (cm) | 66 (96) | 5.8 | 1.4 | |
| HAQ | 68 (99) | 1.5 | 0.6 | |
| CRP (mg/l) | 58 (84) | 23.5 | 18.9 | |
| ESR (mm/1st h) | 67 (97) | 38.4 | 26.2 | |
| PASI | 15 (22) | 5.7 | 6.5 | |
| Arthritis of large joints | ||||
| Hip | 68 (99) | 10 (15) | ||
| Knee | 68 (99) | 40 (59) | ||
| Hip and/or knee | 68 (99) | 46 (68) | ||
| Axial involvement* | 64 (93) | 20 (31) | ||
| Erosive arthritis | 69 (100) | 49 (71) |
*Defined by radiological sacroiliitis.
SJC, swollen joint count; TJC, tender joint count; VAS pain, visual analogue scale for pain (0–10 cm); VAS patient, patient's assessment of global disease on a visual analogue scale (0–10 cm); VAS physician, physician's assessment of global disease on a visual analogue scale (0–10 cm); HAQ, Health Assessment Questionnaire; CRP, C reactive protein; ESR, erythrocyte sedimentation ratio; PASI, Psoriasis Area and Severity Index.
Figure 1 ACR response at 14 and 38 weeks of treatment with infliximab (intention to treat analysis).
Table 2 summarises the main data on efficacy obtained in our study. When an ACR50 at 38 weeks in an intention to treat analysis was assumed as the main outcome, univariate analysis disclosed that the involvement of large joints (hip or knee, or both) (30% v 78%, p<0.001) and a high disability expressed by an HAQ ⩾2 (27% v 53%, p = 0.05) were both predictors of a smaller response to infliximab than in patients with no involvement of the large joints and an HAQ <2 (table 3). None of the other variables analysed predicted response to treatment.
Table 2 Summary of the main data and therapeutic response to infliximab at 38 weeks of treatment.
| Data | Baseline (mean) | 38 Weeks (mean) | Reduction in values (%)* | p Value |
|---|---|---|---|---|
| TJC | 20.7 | 6.0 | 73.1 | <0.001 |
| SJC | 13.5 | 1.7 | 77.7 | <0.001 |
| CRP (mg/l) | 23.5 | 5.8 | 59.4 | <0.001 |
| ESR (mm/1st h) | 38.5 | 17.2 | 37.2 | <0.001 |
| VAS pain (cm) | 6.2 | 2.7 | 50.2 | <0.001 |
| VAS physician (cm) | 5.8 | 2.2 | 61.6 | <0.001 |
| VAS patient (cm) | 5.8 | 2.7 | 58.4 | <0.001 |
| HAQ | 1.5 | 0.8 | 54.3 | <0.001 |
| PASI | 5.7 | 0.1 | 47.8 | <0.001 |
*Intention to treat analysis.
TJC, tender joint count; SJC, swollen joint count; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; VAS pain, visual analogue scale for pain (0–10 cm); VAS physician, physician's assessment of global disease on a visual analogue scale (0–10 cm); VAS patient, patient's assessment of global disease on a visual analogue scale (0–10 cm); HAQ, Health Assessment Questionnaire; PASI, Psoriasis Area and Severity Index.
Table 3 Factors influencing the ACR50 response: results are shown for univariate analysis.
| Variables | ACR50 response No (%) | Univariate analysis OR (95% CI) | p Value |
|---|---|---|---|
| Age (years) | – | 0.99 (0.99 to 1.04) | NS |
| Sex (female/male) | 19 (43)/13 (48) | 0.87 (0.34 to 2.3) | NS |
| Disease duration (years) | – | 1.8 (0.8 to 4.1) | NS |
| SJC | – | 1.01 (0.9 to 1.03) | NS |
| Arthritis in: | |||
| Main large joints (knee and/or hip) | 14 (30) v 18 (78)† | 8.23 (2.54 to 26.6) | <0.001 |
| Knee joints | 12 (30) v 20 (61)† | 3.58 (1.36 to 9.49) | 0.009 |
| Hip joints | 4 (40) v 28 (48)† | 0.73 (0.19 to 2.89) | NS |
| CRP (>10 mg/l) | 23 (52) v 7 (39)† | 1.72 (0.56 to 5.26) | NS |
| ESR (⩾30 mm/1st h) | 19 (46) v 12 (48)† | 0.89 (0.39 to 2.90) | NS |
| Axial involvement* | 12 (60) v 19 (43)† | 0.51 (0.17 to 1.50) | NS |
| Erosive arthritis | 26 (53) v 6 (30)† | 2.6 (0.9 to 7.9) | NS |
| HAQ (⩾2) | 4 (27) v 28 (53)† | 3.1 (0.87 to 10.9) | 0.05 |
*Determined by radiological sacroiliitis; †results shown for response of patients with and without the variable listed.
SJC, swollen joint count; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire.
When the univariate analysis was performed at 14 weeks of treatment, the results were the same, except for CRP and age. The presence of a CRP ⩾10 mg/l at the start of treatment was associated with a significantly high rate of response (62% v 28%, p = 0.025). Moreover, patients who achieved an ACR50 were younger than others (mean (SD) 39 (12) v 45 (13) years, p = 0.05).
Instead of the ACR50 as an indicator of a major treatment response, we also performed a prediction analysis for ACR20 and ACR70 clinical response at 38 weeks. However, the univariate analysis applied did not show any advantage over the previous analysis performed using ACR50 as the main outcome.
Multiple adjustment for the different variables included in the study showed that a good model built by a stepwise regression method contained the following co‐variables: age, sex, arthritis in the large joints, CRP, and HAQ. When this model was used only CRP (odds ratio (OR) = 18.7; 95% confidence interval (CI) 1.8 to 181.6; p = 0.011) and the absence of arthritis in the hip or knee, or both (OR = 29.3; 95% CI 3.2 to 266.3; p = 0.003) were independent predictors of major clinical response (table 4). Moreover a trend towards an association was also seen for the HAQ (OR = 6.4; 95% CI 0.91 to 45.17; p = 0.06). Table 4 summarises the main data obtained from the regression analysis.
Table 4 Factors influencing the ACR50 response: results are shown for multivariate logistic regression analysis.
| Variable | Multivariate analysis OR (95% CI) | p Value |
|---|---|---|
| Age (years) | 1.02 (0.97 to 1.08) | NS |
| Sex (female) | 1.5 (0.4 to 5.5) | NS |
| No arthritis in large joints | 29.3 (3.2 to 266.3) | 0.003 |
| CRP (>10 mg/l) | 18.7 (1.8 to 181.6) | 0.011 |
| HAQ (<2) | 6.4 (0.9 to 45.2) | 0.06 |
CRP, C reactive protein; HAQ, Health Assessment Questionnaire.
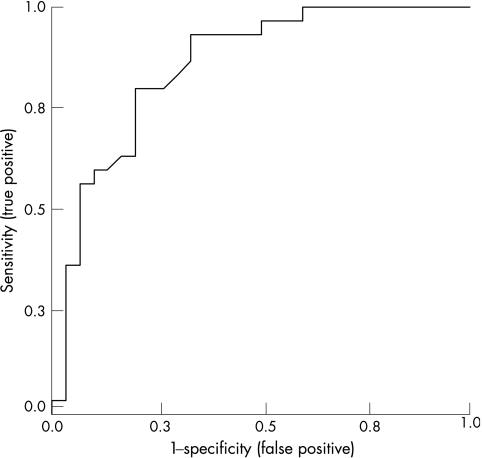
The statistical value and the accuracy of the model were expressed by the area under the ROC curve (0.855, p<0.001; fig 2). This model included the variables age, sex, arthritis in large joints, CRP, and HAQ. The sensitivity and the specificity of the model, using a cut off point of 0.5, were 76% (75–78) and 80% (78–81), respectively, with a positive predictive value of 79% (77–81), and a negative predictive value of 77% (75–79).
Figure 2 Receiver operating characteristics (ROC) curve for the multivariate model assessing infliximab therapeutic response (ACR50). This model included the variables: age, sex, arthritis in large joints, CRP, and HAQ. Area under curve = 0.855.
Discussion
To our knowledge this is the first analysis focusing on variables predicting a major clinical response to infliximab in refractory psoriatic polyarthritis. A similar attempt was reported in a study published by Rudwaleit et al18 in active ankylosing spondylitis. In our study, the likelihood of achieving a major clinical response to infliximab in PsA refractory to MTX was significantly higher in patients with better functional status, absence of arthritis in the hips and/or knees, and raised CRP serum levels.
We found a clear relationship between raised CRP serum levels at the start of treatment and good clinical response to infliximab (OR = 18.7 95% CI 1.8 to 181.6, p = 0.011). These data were in accordance with the data previously reported in recent studies of TNFα blockers in different inflammatory rheumatic diseases.18,19 Thus, serum CRP levels seems to be an important factor that should be considered in the decision to give infliximab treatment to a patient with PsA; a raised CRP level at the start of treatment may contribute to a high response rate.
We might argue that patients who had high HAQ values had more structural damage and that, therefore, the pain reported for these patients could not be attributed entirely to acute inflammation of the joints but might rather be related to permanent mechanical joint changes. If we assume that infliximab acts mainly at sites of acute inflammation, it is not surprising that the response rate of infliximab treatment was significantly less in patients with severe disability (27% v 53%, p = 0.05), although it did not achieve statistical significance with multivariate analysis, probably owing to the small sample size. These results are also in accordance with previous studies published in patients with active AS.18
The number of painful or swollen joints at the start of treatment did not predict the rate of clinical response. Instead, the absence of arthritis in the hips or knees, or both, in our study, had the highest predictive value for a good response to infliximab (OR = 29.3; 95% CI 3.2 to 266.3, p = 0.003). A recent study reported that hip arthritis is one of the main prognostic factors among patients with PsA.20 In our study, although knee arthritis is the major predictive factor of a poor response to anti‐TNF treatment (30% v 61%; p = 0.009), the presence of hip arthritis is also a predictive factor of poor response to treatment (40% v 50%), although it did not achieve statistical significance. However we should not rule out definitively hip arthritis as a predictive factor of poor response to infliximab owing to the power of our study. Indeed, addition of hip arthritis to the analysis increases the likelihood of no response to infliximab, compared with knee arthritis alone (table 3).
As far as we know, the influence of inflammation of the large joints on the probability of achieving a good therapeutic response to infliximab has not been previously reported in any inflammatory rheumatic disease. We decided to determine the importance of this clinical feature as a predictive factor, as a result of our clinical experience of treatment with TNFα blockers. The rate of good response to infliximab in those patients with arthritis in the main large joints (30%), does not exclude these patients as suitable candidates for anti‐TNFα treatment. Moreover, some of these patients, even those not achieving an ACR50, may receive some satisfaction from a minor response of the joints, or may benefit significantly from some improvement in the skin or their quality of life.
Rudwaleit et al found that age and disease duration were predictive factors of anti‐TNF response in patients with active AS.18 As for patients with AS, it might be assumed for patients with PsA that both factors are associated with longstanding disease, and increased structural damage. So, a smaller response rate in older patients and patients with longstanding disease might be expected. However, neither of these factors was significantly associated with the response rate, suggesting that the influence of both factors in the treatment response, through structural damage, might be better explained by other variables such as the HAQ values. However, the possibility that the small sample size of our study affected these results cannot be excluded. In the multivariate analysis, age was one of the co‐variables definitively included in the best model built to calculate the response rate to infliximab in PsA.
We chose the ACR50 as the measure to define a good clinical response because of the similarity of our patients with peripheral PsA and patients with rheumatoid arthritis (RA). Thus the ACR50 is widely used to measure the minimum significant clinical response in therapeutic trials of patients with RA. We also applied the ACR20, widely accepted in patients with RA as the minimum statistical change, instead of ACR50 to define a major clinical response and found no relevant differences in the measures predicting such a response.
We recognise the limitations of our study: (a) the small sample size may limit the ability to detect other predictive factors of therapeutic response, especially those with a low predictive value, and may influence the precision of estimates, with in some cases a wide range of confidence intervals. However, the variables we found that significantly influence the probability of a good clinical response to infliximab in patients with PsA refractory to MTX are among the strongest predictive factors. Additionally, the accuracy of the statistical model is demonstrated by the area under the ROC curve (0.855). (b) The patients included in this study are those with the most severe and refractory PsA usually seen in clinical practice (71% erosive disease). Thus extrapolation of the data to the general population of patients with PsA must be made with caution. Nevertheless, these patients included those often considered to be suitable candidates for anti‐TNF treatment in clinical practice.
Finally, (c) the predictive values we found for our model, did not permit its use in clinical practice as a definitive rule for deciding which patients should or should not be given anti‐TNF treatment, because 20–25% of patients theoretically classified as non‐responders may have a good response.
In summary, this study has, for the first time, shown that some variables may significantly influence the therapeutic response to infliximab in patients with PsA. Because anti‐TNF treatment is expensive and has possible severe side effects, the opportunity to preselect patients with a high probability of achieving a good clinical response (ACR50) may be important in clinical practice. Unfortunately, the data reported here cannot be used as a definitive guide for deciding which patients should be given anti‐TNF treatment. Thus we consider that the decision to start infliximab treatment in these patients should be made on the basis of anti‐TNF consensus treatment in patients with PsA,21,22 and for the individual patient, aspects related to work incapacity, or quality of life owing to the disease, rather than merely the variables analysed herein, should be considered. Finally, large studies supporting our data will be needed in order to prove our statistical model and to establish more accurately the predictive factors for clinical response to infliximab in patients with PsA.
Acknowledgements
We would like to thank Dra. Ma Carmen Garcia for her help in the monitoria of this study.
Participating investigators in the MIPRA Study (MIPRA Study Group)
B Alvarez Lario (Hospital General Yagüe, Burgos), M Álvarez de Mon (Hospital Universitario Príncipe de Asturias, Alcalá de Henares), E Brito (Hospital Ramón y Cajal, Madrid), J Cañete (Hospital Clínic i Provincial, Barcelona), E Chamizo (Hospital del Insálud, Mérida), E Collantes (Hospital Universitario Reina Sofía, Córdoba), M Crespo (Hospital Severo Ochoa, Leganés), F Galdo (Hospital Juan Canalejo, A Coruña), MA González‐Gay (Hospital Xeral, Lugo), T González‐García (Hospital Universitario de Canarias, La Laguna), M Guzman (Hospital Universitario Virgen de las Nieves, Granada), A Larrea (Hospital Puerta de Hierro, Madrid), M Larrosa (Hospital Parc Taulí, Sabadell), JMa Llobet (Hospital Sant Pau, Barcelona), J Manero (Hospital Miguel Servet, Zaragoza), JL Marenco (Hospital Nuestra Señora de Valme, Sevilla), S Marsal (Hospital Vall d'Hebrón, Barcelona), JM Martín (Hospital Río Hortega, Valladolid), I Mateo (Hospital 12 de Octubre, Madrid), J Maymó (Hospital del IMAS, Barcelona), I Monteagudo (Hospital Gregorio Marañón, Madrid), F Navarro (Hospital Universitario Virgen de la Macarena, Sevilla), R Ortega (Hospital Infanta Cristina, Badajoz), J Pérez (Hospital SAS, Jerez), M Pérez (Hospital Universitario Carlos Haya, Málaga), C Rodríguez‐Lozano (Hospital Doctor Negr'n, Las Palmas de Gran Canaria), J Rodríguez‐Moreno (Hospital de Bellvitge, Hospitalet de Llobregat), C Rubio (Hospital Nuestra Señora del Carmen, Ciudad Real), J Sampedro (Hospital Virgen de la Salud, Toledo), X Tena (Hospital Germans Trias i Pujol, Badalona), J Tornero (Hospital de Guadalajara, Guadalajara), JC Torre (Hospital Monte Naranco, Oviedo), JC Vesga (Hospital Txagorritxy, Vitoria), P Zarco (Fundación Hospital de Alcorcón, Alcorcón)
Abbreviations
CI - confidence interval
CRP - C reactive protein
DMARDs - disease modifying antirheumatic drugs
HAQ - Health Assessment Questionnaire
MTX - methotrexate
OR - odds ratio
PsA - psoriatic arthritis
RA - rheumatoid arthritis
ROC - receiver operating characteristics
TNFα - tumour necrosis factor α
Footnotes
Disclosure: Study sponsored by Schering‐Plough SA.
References
- 1.Koo J. Population based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 199614485–496. [DOI] [PubMed] [Google Scholar]
- 2.Shbeeb M, Uramoto K M, Gibson L E, O'Fallon W M, Gabriel S E. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol 2000271247–1250. [PubMed] [Google Scholar]
- 3.Leonard D G, O'Duffy J D, Rogers R S. Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc 197853511–518. [PubMed] [Google Scholar]
- 4.Torre Alonso J C, Rodriguez Perez A, Arribas Castrillo J M, Ballina Garcia J, Riestra Noriega J L, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol 199130245–250. [DOI] [PubMed] [Google Scholar]
- 5.Husted J A, Gladman D D, Farewell V T, Cook R J. Health‐related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 200145151–158. [DOI] [PubMed] [Google Scholar]
- 6.Wong K, Gladman D D, Husted J, Long J A, Farewell V T. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum 1997401868–1872. [DOI] [PubMed] [Google Scholar]
- 7.Gladman D D, Hing E N, Schentag C T, Cook R J. Remission in psoriatic arthritis. J Rheumatol 2001281045–1048. [PubMed] [Google Scholar]
- 8.Pipitone N, Kingsley G H, Manzo A, Scott D L, Pitzalis C. Current concepts and new developments in the treatment of psoriatic arthritis. Rheumatology (Oxford) 2003421138–1148. [DOI] [PubMed] [Google Scholar]
- 9.Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003421460–1468. [DOI] [PubMed] [Google Scholar]
- 10.Partsch G, Steiner G, Leeb B F, Dunky A, Broll H, Smolen J S. Highly increased levels of tumor necrosis factor‐alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol 199724518–523. [PubMed] [Google Scholar]
- 11.Ritchlin C, Haas‐Smith S A, Hicks D, Cappuccio J, Osterland C K, Looney R J. Patterns of cytokine production in psoriatic synovium. J Rheumatol 1998251544–1552. [PubMed] [Google Scholar]
- 12.Ettehadi P, Greaves M W, Wallach D, Aderka D, Camp R D. Elevated tumour necrosis factor‐alpha (TNF‐alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol 199496146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mease P J, Kivitz A J, Burch F X, Siegel E L, Cohen S B, Ory P.et al Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004502264–2272. [DOI] [PubMed] [Google Scholar]
- 14.Antoni C E, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester G R, Schneider U.et al Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005521227–1236. [DOI] [PubMed] [Google Scholar]
- 15.Antoni C, Krueger G G, de Vlam K, Birbara C, Beutler A, Guzzo C.et al Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005641150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mease P J, Gladman D D, Ritchlin C T, Ruderman E M, Steinfeld S D, Choy E H.et al Adalimumab for the treatment of patients with moderatly to severely active psoriatic arthritis: results of a double blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005523270–3289. [DOI] [PubMed] [Google Scholar]
- 17.Bresnihan B, Cunnane G. Infection complications associated with the use of biologic agents. Rheum Dis Clin North Am 200329185–202. [DOI] [PubMed] [Google Scholar]
- 18.Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 200463665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busch M H, Seto Y, Bingham S J, Bejarano V, Bryer D, White J.et al C‐reactive portein as a predictor of infliximab treatment outcome in patients with rheumatoid arthritis: defining subtypes to non responders an subsequent respondres to etenercept. Arthritis Rheum 20055242–48. [DOI] [PubMed] [Google Scholar]
- 20.Michet C J, Mason T G, Mazlumzadeh M. Hip joint disease in psoriatic arthritis: risk factors and natural history. Ann Rheum Dis 2005641068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyle S, Chandler D, Griffiths C E, Helliwell P, Lewis J, McInnes I.et al Guideline for anti‐TNF‐alpha therapy in psoriatic arthritis. Rheumatology (Oxford) 200544390–397. [DOI] [PubMed] [Google Scholar]
- 22.Maksymowych W P, Inman R D, Gladman D, Thomson G, Stone M, Karsh J.et al Canadian Rheumatology Association Consensus on the use of anti‐tumor necrosis factor‐alpha directed therapy in the treatment of spondylarthritis. J Rheumatol 2003301356–1363. [PubMed] [Google Scholar]