Abstract
Objectives
To study potential risk factors for the development of lung cancer in patients with scleroderma and explore the chronological relationship between onset of scleroderma symptoms and subtypes of lung cancer.
Method
Linkage of two population‐based registers to identify lung cancer cases and gender‐matched controls with scleroderma, followed by retrospective case note review for clinical details.
Results
Patients with scleroderma who smoke are seven times more likely to develop lung cancer than non‐smokers (p = 0.008). Smokers with scleroderma and cancer smoke more than smokers with scleroderma without cancer (p = 0.019). Pulmonary fibrosis and anti‐topoisomerase antibody do not increase the risk of lung cancer. Peripheral lung tumours occur earlier after the onset of scleroderma symptoms than bronchogenic tumours (p = 0.05).
Conclusions
Smokers with scleroderma should be monitored for the presence of lung cancer and counselled to quit smoking. The earlier development of peripheral lung tumours is not consistent, with pulmonary fibrosis being an aetiological factor.
Most, but not all, population‐based studies of cancer in scleroderma have shown an increased incidence of all cancers and, most consistently, an increased risk of lung cancer.1,2,3,4 The relationship between these two diseases remains unclear. It has been suggested that the common link between lung cancer and scleroderma is pulmonary fibrosis; however, this has not been formally tested in a cohort study.5
Methods
Scleroderma ascertainment
Patients with scleroderma were identified from the South Australian Scleroderma Registry (SASR), which has been described elsewhere.6 The registry was established in 1993 and aims to identify all residents with scleroderma in South Australia (SA).
Patients were ascertained from hospital discharge indices, state immunodiagnostic laboratories (all positive centromeres and Scl‐70 were pursued), referrals from SA rheumatologists and other specialist clinics. Scleroderma was validated according to the American Rheumatology Association criteria7 by examining referring clinical letters, case notes and autopsy reviews. Patients were subdivided into three clinical variants—namely limited, diffuse or overlap scleroderma—according to the extent of skin involvement and other criteria according to LeRoy et al.8 Patients with localised scleroderma were not included. Written consent was obtained from all patients before confidential enrolment, and the registry's use for analyses of this type had the approval of human ethical committees of all teaching hospitals in SA. Although complete ascertainment cannot be guaranteed, a high ascertainment rate in our population was inferred by the fact that scleroderma prevalence in SA, a statistic derived from the SASR, was high when compared with other regional world studies.6
Cancer ascertainment
There is compulsory notification of cancer in SA to the South Australian Cancer Registry (SACR)9 from pathology laboratories, hospital medical record departments, radiotherapy departments and oncologists, with 95% completeness verified by multiple electronic searches of SA laboratory and hospital records each year. In addition, the International Agency for Research on Cancer checks the quality of the SACR data every 5 years.
Using Automatch software (Matchware Technologies, Kenrebunk, Maine, USA), all subjects on the SASR were linked to the SACR to identify all cases of lung cancer among these patients prior to 1 January 2003. Case notes were then reviewed for confirmation of lung cancer histology, presence of pulmonary fibrosis and method of diagnosis, smoking history and year of onset of scleroderma symptoms including Raynaud's phenomenon. Gender‐matched controls were selected using computer‐generated random numbers from the remainder of the SASR and their case notes similarly reviewed. Odds ratios for risk factors for lung cancer in scleroderma were determined, and categorical variables were analysed using χ2, Fisher's exact and t tests.
Results
There were 632 patients on the SASR as of 1 January 2003 and 20 lung cancers were identified in 19 of them (table 1). One individual developed two histologically distinct tumours in different lobes. The average age at diagnosis of cancer was similar for all subtypes (squamous cell, mean 65.3 years; large cell, 64.3 years; adenocarcinoma, 60.7 years; small cell, 65.3 years; and bronchoalveolar cell (BAC), 72.5 years). Of those patients with lung cancer, 90% were smokers. Only two patients were non‐smokers, both of whom developed BAC. There were 5 (20%) cases of PF in the patients with lung cancer (2 squamous cell, 1 BAC, 1 large cell and 1 small cell lung cancer).
Table 1 Characteristics of the patients with scleroderma and lung cancer.
| Lung cancer histology | Age at onset of scleroderma (years) | Age at diagnosis of cancer (years) | Interval (years) | Scleroderma subtype | Gender | Pulmonary fibrosis (method of diagnosis) | Smoking (pack years) |
|---|---|---|---|---|---|---|---|
| Squamous cell | 58 | 59 | >1 | Unknown | M | Yes (CT) | 100 |
| Squamous cell | 36 | 61 | >25 | L | M | Yes (CT) | 30 |
| Squamous cell | 47 | 76 | >29 | L | F | no (CT) | 50 |
| Large cell | 54 | 56 | >2 | L | M | Yes (biopsy) | 40 |
| Large cell | Unknown | 72 | Unknown | M | No (CT) | 12 cig/day | |
| Large cell* | 60 | 65 | >5 | L | M | No (CT) | 60 |
| Adenocarcinoma* | 60 | 65 | >5 | L | M | No (CT) | 60 |
| Adenocarcinoma | 58 | 62 | >4 | L | F | No (CT) | 58 |
| Adenocarcinoma | 28 | 46 | >18 | D | F | No (PFT) | 20 cig/day |
| Adenocarcinoma | 57 | 58 | >1 | O | F | No (CT ) | 25 cig/day |
| Adenocarcinoma | 48 | 56 | >8 | L | F | No (CXR) | 15 |
| Adenocarcinoma | 72 | 76 | >4 | O | M | No (CT) | 50 |
| Adenocarcinoma | 29 | 62 | >33 | L | F | No (CT) | 40 |
| Small cell | 51 | 57 | >5 | D | M | Yes (CT) | 15 |
| Small cell | 26 | 65 | >39 | L | F | No (CT) | 70 |
| Small cell | 50 | 72 | >22 | L | F | No (CXR) | 40 |
| Small cell | 40 | 67 | >27 | L | M | No (CXR) | 58 |
| BAC | 51 | 69 | >18 | L | F | Yes (CT) | 0 |
| BAC | 60 | 76 | >16 | L | F | No (CT) | 0 |
| unspecified | 62 | 81 | >19 | D | F | Unknown | 14 |
BAC, bronchoalveolar carcinoma; CXR, chest x ray; D, diffuse; F, female; M, male; L, limited; O, overlap; PFT, pulmonary function testpack years (1 pack year = 20 cigarettes per day for 1 year).
cig/day = number of cigarettes smoked per day, duration of smoking (years) unknown.
*Patient with two histologically distinct lung tumours.
Cigarette smoking was a risk factor for development of lung cancer (table 2), with smokers being seven times more likely to develop lung cancer than non‐smokers. Having pulmonary fibrosis, diffuse disease or being anti‐topoisomerase antibody positive did not increase the risk of lung cancer. Smokers with scleroderma and lung cancer were more likely to smoke larger quantities than controls with scleroderma who smoked. The number of pack years (1 pack year = 20 cigarettes per day for 1 year) smoked was known for 15 of the 18 (83%) cases of lung cancer in smokers, and in 18 of the 23 (78%) controls who smoked. The average number of pack years in the smokers who developed cancer (46.7 years) was significantly greater than in controls who smoked (29.6 years; p = 0.019).
Table 2 Characteristics of lung cancer cases and gender‐matched controls.
| Cancer cases | Controls | ||
|---|---|---|---|
| Number | 20 | 41 | |
| Male | 9 (45%) | 19 (46.3%) | |
| Mean age (years) | 65 | 69 | p>0.05, unpaired t test |
| Smokers | 18 (90%) | 23 (56%) | OR 7.04, p = 0.008, χ2square |
| Average pack years in smokers for whom value is known (table 1; years) | 46.7 | 29.6 | p = 0.019, unpaired t test |
| Pulmonary fibrosis | 5 (25%) | 11 (27%) | OR 1.19, p = 1, Fisher's |
| Diffuse scleroderma | 3 (15%) | 7 (17%) | OR 0.86, p = 1, Fisher's |
| Anti‐topoisomerase antibody | 0 (0%) | 6 (14.6%) | |
| Anti‐centromere antibody | 3 (15%) | 17(41.5%) | p<0.001, χ2square |
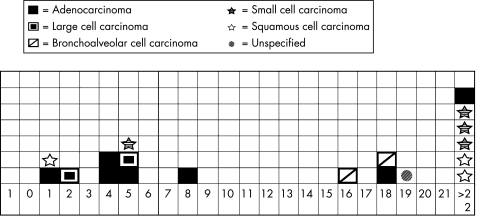
All lung cancers occurred after onset of scleroderma symptoms (fig 1). The median time to cancer after onset of scleroderma symptoms was 25 years (range 1–39 years) for the bronchogenic tumours (small and squamous cell) and 5 years (range 1–33 years) for the peripheral tumours (large cell, BAC and adenocarcinoma) (p = 0.05). Only two of the seven bronchogenic tumours occurred early and both these patients had pulmonary fibrosis, whereas none of those with adenocarcinoma did.
Figure 1 Chronological relationship between onset of scleroderma symptoms and subtypes of lung cancer. 0, year of onset of scleroderma symptoms, subsequent number = years after onset of scleroderma that lung cancer was diagnosed.
Discussion
We have demonstrated a strong association between lung cancer and heavier smoking in patients with scleroderma, which is similar to that seen in the UK general population.10 We found no association between lung cancer and pulmonary fibrosis, scleroderma subtype or anti‐topoisomerase status. Furthermore, we have shown that peripheral lung tumours occur earlier than bronchogenic tumours in the course of scleroderma.
Three previous studies in the English literature have considered potential risk factors for lung cancer in scleroderma.11,12,13 Similar but separate studies from Baltimore and Ontario that prospectively followed a total of 319 scleroderma outpatients found 10 patients who developed lung cancer, all of whom had evidence of pulmonary fibrosis and three of whom were smokers.11,12 In a single‐centre study from the Pittsburgh standard metropolitan statistical area,13 four cases of lung cancer in 262 patients with scleroderma were identified. All had evidence of pulmonary fibrosis and one was a smoker. Conclusions regarding the lung cancer, smoking and pulmonary fibrosis relationship in scleroderma are difficult to draw from these studies owing to the lack of control groups and use of outpatient‐based rather than population‐based registers. The latter point is likely to lead to a higher proportion of patients with PF and smaller numbers of those with cancer. Patients on the SASR with diffuse scleroderma, the variant associated with pulmonary fibrosis, make up 22%. This is a low figure compared with many cohort studies, but reflects higher detection rates of limited and mild disease in our population.6
Although our pulmonary fibrosis ascertainment methods are similar to large cryptogenic fibrosing alveolitis cohorts14 and the aforementioned scleroderma studies in which all patients with lung cancer had pulmonary fibrosis, the investigation used to demonstrate pulmonary fibrosis varies between patients in all these cohorts. A smaller prospective study would eliminate this variable, but lose the strength of complete cancer ascertainment. CT and biopsy results were available in 75% of our cancer cases, and although the prevalence of pulmonary fibrosis in our control group may be underestimated, this would not diminish, but would increase the significance of our findings. Data on immunosuppressant therapy in this cohort were incomplete and so not included, but this factor could potentially affect the incidence of cancer.
We have previously seen no difference in proportions of lung cancer subtypes in our cohort compared with the general SA population.2 Peripheral lung cancers, however, occur earlier after the onset of scleroderma symptoms than traditionally smoking‐related bronchogenic tumours. This contradicts the hypothesis of scleroderma‐induced pulmonary fibrosis leading to tumour development in the damaged lung as the time frame is inconsistent and none of the patients with adenocarcinoma had pulmonary fibrosis. A multitude of factors in a lung with scleroderma, including cytokines and disturbance of cellular immunity, may predispose an individual to peripheral tumours as well as pulmonary fibrosis, the former being a cause of death before the development of pulmonary fibrosis. Alternatively, the dominance of smoking as a risk factor in our study suggests that its effect may over‐ride or synergise with that of scleroderma, making the smoking pattern, including year of cessation, important information to plot chronologically with diagnosis of lung cancer and of scleroderma symptoms onset. More detailed smoking histories are required and this information should be collected prospectively in scleroderma registries, followed by counselling to quit smoking. Different histological types of cancer may represent a heterogeneous end point and could also be a point for further study.
Using two population‐based registries, we demonstrated a strong association between lung cancer and heavier smoking in patients with scleroderma. By contrast, we found no association with pulmonary fibrosis, scleroderma subtype and anti‐topoisomerase status. Scleroderma registers should prospectively collect detailed smoking histories, and patients with scleroderma who smoke should be monitored for the presence of lung cancer, and strongly encouraged to quit.
Acknowledgements
We thank Mr Kevin Priest and Anh‐Minh Nguyen, Department of Health, South Australia; Dr David Roder, Cancer Council of South Australia; and Professor Oliver FitzGerald, Department of Rheumatology, St Vincent's University Hospital, Dublin, Ireland for their assistance.
Abbreviations
SASR - South Australian Scleroderma Registry
SACR - South Australian Cancer Registry
Footnotes
Competing interests: None.
The use of the SASR for studies such as this has the approval of ethics committees of all teaching hospitals in South Australia, and all patients with scleroderma are mailed a request to enrol on the confidential register.
References
- 1.Pearson J E, Silman A J. Risk of cancer in patients with scleroderma. Ann Rheum Dis 200362697–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill C L, Nguyen A ‐ M, Roder D, Roberts‐Thomson P. Risk of cancer in patients with scleroderma: a population based cohort study. Ann Rheum Dis 200362728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal A K, McLaughlin J K, Gridley G, Nyren O. Incidence of cancer among patients with systemic sclerosis. Cancer 199576910–914. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Dombi G W, Severson R K, Mayes M D. Risk of malignancy in scleroderma. A population‐based cohort study. Arthritis Rheum 2005522415–2424. [DOI] [PubMed] [Google Scholar]
- 5.Daniels C E, Jett J R. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med 200511431–437. [DOI] [PubMed] [Google Scholar]
- 6.Roberts‐Thomson P J, Jones M, Hakendorf P, Kencana Dharmapatni A A S S, Walker J G, MacFarlane J G.et al Scleroderma in South Australia: epidemiological observations of possible pathogenic significance. Intern Med J 200131220–229. [DOI] [PubMed] [Google Scholar]
- 7.Subcommittee for scleroderma criteria of the American Rheumatology Association Diagnostic and Therapeutic Criteric Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 198323581–590. [DOI] [PubMed] [Google Scholar]
- 8.LeRoy E C, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger T A., Jret al Scleroderma: classification, subset and pathogenesis. J Rheumatol 198825688–694. [PubMed] [Google Scholar]
- 9.Bonett A, Roder D, Milliken L. The South Australian Cancer Registry: a means of assessing cancer incidence, mortality and case survival. Eur J Cancer 1992281923–1926. [DOI] [PubMed] [Google Scholar]
- 10.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case‐control studies. BMJ 2000321323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu‐Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis Rheum 199336460–464. [DOI] [PubMed] [Google Scholar]
- 12.Peters‐Golden M, Wise R A, Hochberg M, Stevens M B, Wigley F M. Incidence of lung cancer in systemic sclerosis. J Rheumatol 1985121136–1139. [PubMed] [Google Scholar]
- 13.Roumm A D, Medsger T A., Jr Cancer and systemic sclerosis. Arthritis Rheum 1985281336–1340. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population‐based cohort study. Am J Respir Crit Care Med 20001615–8. [DOI] [PubMed] [Google Scholar]