Abstract
Aim
Neuropsychiatric systemic lupus erythematosus (NPSLE) is a serious treatment‐resistant phenotype of systemic lupus erythematosus. A standard treatment for NPSLE is not available. This report describes the clinical and laboratory tests of 10 patients with NPSLE before and after rituximab treatment, including changes in lymphocyte phenotypes.
Methods
Rituximab was administered at different doses in 10 patients with refractory NPSLE, despite intensive treatment.
Results
Treatment with rituximab resulted in rapid improvement of central nervous system‐related manifestations, particularly acute confusional state. Rituximab also improved cognitive dysfunction, psychosis and seizure, and reduced the SLE Disease Activity Index Score at day 28 in all 10 patients. These effects lasted for >1 year in five patients. Flow cytometric analysis showed that rituximab down regulated CD40 and CD80 on B cells and CD40L, CD69 and inducible costimulator on CD4+ T cells.
Conclusions
Rituximab rapidly improved refractory NPSLE, as evident by resolution of various clinical signs and symptoms and improvement of radiographic findings. The down regulation of functional molecules on B and T cells suggests that rituximab modulates the interaction of activated B and T cells through costimulatory molecules. These results warrant further analysis of rituximab as treatment for NPSLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterised by multiple lesions induced by activation of autoreactive T cells and overproduction of autoantibodies by B cells. The involvement of the central nervous system (CNS) in SLE is often intractable, complicating the course of the disease in about 12–75% of patients with SLE. The involvement of the CNS has a negative clinical impact with a 5‐year survival of 55–85% and is associated with poor prognosis.1,2 Neuropsychiatric systemic lupus erythaematosus (NPSLE) exhibits a wide range of symptoms unrelated to SLE activation, which include organic and mental disorders, often associated with impairment of consciousness and/or convulsions. These organic disorders may become permanent, eventually leading to long‐term or irreversible decline in higher mental functions.
CNS immune abnormalities have an important role in such disease states. Therefore, a trial of intensive treatment, including the combination of potent immunosuppressive treatment and plasma exchange (PE), depending on the disease type and its severity, may be advisable in an effort to control autoreactive lymphocytes.3,4,5,6,7,8,9,10 Although the severity of NPSLE correlates with prognosis, there is no established treatment protocol and many cases are resistant to treatment making this condition difficult to control.
This study describes the results of treatment of patients with NPSLE who had previously failed to respond to various immunosuppressants. Our approach was based mainly on the use of anti‐CD20 antibody (rituximab), a chimeric antibody that directly targets B cells.11,12 Rituximab is a biological preparation that eliminates B cells through a variety of mechanisms such as antibody‐dependent cellular cytotoxicity, complement‐dependent cytotoxicity and apoptosis. Rituximab has recently been used for the treatment of a variety of SLE disease conditions and good therapeutic response has been reported.13,14,15,16 We investigated the short‐term and long‐term responses to rituximab treatment in 10 patients with NPSLE, and report that some showed marked improvement following rituximab treatment. Moreover, the results showed that rituximab modulated the functional molecules of activated lymphocytes, implying the efficacy of anti‐CD20 antibody treatment for CNS lesions in patients with SLE, otherwise resistant to other treatments.
Materials and methods
Patients
The study subjects were 10 patients who had been previously diagnosed with SLE based on the American College of Rheumatology criteria.17 The inclusion criteria were (1) the presence of a highly active disease and (2) CNS lesions resistant to conventional treatment. None of the patients showed improvement in CNS‐related symptoms in response to conventional immunosuppressive treatment such as intravenous cyclophosphamide pulse treatment (IV‐CY), cyclosporine A (CsA), PE and immunoadsorption therapy. All patients completed the course of anti‐CD20 antibody treatment described in this study. Patients 1–8, and patients 9 and 10 were treated at the University of Occupational and Environmental Health Hospital and Kyoto University Hospital, respectively, from 2000 to 2005. Informed consent was obtained from all patients in accordance with the regulations of the aforementioned two hospitals, and rituximab was administered in accordance with the study protocol approved by the ethics committee of each hospital.
Treatment protocol
Patients 1–5 and 10 were treated with 375 mg/m2 rituximab once a week for 2 weeks, and patient 9 received a single administration of the same dose. Patients 6 and 7 received 500 mg rituximab once a week for 4 weeks, while patient 8 was treated with 1000 mg once biweekly for 4 weeks. Blood pressure and ECG were monitored within the first 3.5 h of the administration to check for any reaction to the drug infusion.
Assessment
Clinical symptoms and treatment‐induced adverse reactions were assessed before treatment, every week during treatment, every week within 1 month after treatment and once monthly thereafter. Laboratory tests included blood count, erythrocyte sedimentation rate, liver and renal function tests, urinary protein, serum complement titre and autoantibody level (such as anti‐ds‐DNA antibody). To evaluate the impact of rituximab on CNS lesions, we measured the immunoglobulin (Ig)G index and interleukin (IL)6 level in the cerebrospinal fluid, MRI, cerebral flood flow scintillator (single‐photon‐emission computed tomography (SPECT), and 18FTG‐positron emission tomography. To assess SLE activity, the SLE Disease Activity Index (SLEDAI) was determined before and after treatment. The level of expression of functional molecules on the lymphocyte cell surface was assessed by flow cytometry.
Flow cytometry
Mononuclear cells were isolated from peripheral blood using lymphocyte separation medium (ICN/Cappel Pharmaceuticals, Aurora, Ohio, USA). After washing twice with phosphate‐buffered saline (PBS), the cells were incubated in blocking buffer (0.25% human globulin, 0.5% human albumin (Yoshitomi, Osaka, Japan), and 0.1% NaN3 (Sigma Aldrich, St Louis, Missouri, USA) in PBS) and left to stand in a 96‐well plate at 4°C for 15 min. In the next step, the cells were incubated in 100 μl of fluorescence‐activated cell sorter (FACS) solution (0.5% human albumin and 0.1% NaN3 in PBS) and then treated with fluorescein isothiocyanate‐labelled mouse IgG1 and antihuman CD40, CD69, inducible costimulator (ICOS), CD19, CD4 (Pharmingen, San Diego, California, USA), CD80 (Chemicon Europe, Chandlers Ford, UK), or CD40L (Ancell, Bayport, USA) antibody, and left to react for 30 min at 4°C. The cells were washed three times with FACS solution and analysed using FACScalibar (Becton–Dickinson, San Jose, California, USA).
Statistical analysis
All data were expressed as mean (SD). Differences between data collected before and after treatment were examined for statistical significance using the Student's t test. p<0.05 denoted the presence of a significant difference.
Results
Characteristics of patients
Table 1 summarises the NPSLE classification and laboratory data of the 10 patients. All patients were females with a mean (range) age of 31 (20–55) years. The mean (range) duration of illness from the onset of SLE to administration of rituximab was 9.6 years (3 months to 25 years). Immunosuppressants used for treatment before enrollment in the rituximab protocol included CsA, cyclophosphamide, mizoribine, and azathioprine. In addition, five patients with intractable disease did not respond to the combination treatment, and thus received PE as well.
Table 1 Characteristics of 10 female patients with neuropsychiatric systemic lupus erythaematosus at study entry.
| Patient | Age (years) | Duration of disease | Previous treatment | NP classification | MRI/SPECT | IgG index /IL6 (pg/ml) | Clinical manifestations | SLEDAI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 19 years | CS (40 mg, pulse 14), IV‐CY (22), VCR (10 mg), CsA (300 mg, 3 years), AZ (100 mg, 2 months), MTX (8 mg/w, 4 months), PE (11), IA (15) | Acute confusional state, seizure, psychosis | Normal/abnormal | Not done/not done | Fever, fatigue, nephritic syndrome, leukopenia, low Hb, high ESR, CH50, anti‐ds DNA↑ | 49 | |||
| 2 | 55 | 25 years | CS (40 mg, pulse 3), IV‐CY (7), PE (2) | Acute confusional state | II, III/abnormal | 0.73↑/ 1.8 | Paresthesia of fingers, severe AIHA, anti‐ds DNA↑ | 2 | |||
| 3 | 46 | 3 months | CS (50 mg), IV‐CY (1), PE (2), IA (3) | Acute confusional state, seizure | II, III/abnormal | 0.46/33.8↑ | Leukopenia, low Hb, thrombocytopenia, proteinuria, AIH, anti‐ds DNA↑ | 37 | |||
| 4 | 20 | 1 year | CS (50 mg), CsA (175 mg, 1 m) | Headache | Normal/not done | 1.05↑/3.1 | Fever, fatigue, skin rash, alopecia, cardiomyopathy, polyneuropathy, leukopenia, C4↓, anti‐ds DNA↑ | 16 | |||
| 5 | 34 | 3 years | CS (60 mg), IV‐CY (8), MZ (150 mg, 25 months) | Demyelinating syndrome | II, III/normal | 0.85↑/0.9 | Sensory deficit, photosensitivity, mouth ulcer, lymphocytopenia, C4↓ | 16 | |||
| 6 | 30 | 22 years | CS (40 mg), MZ (150 mg, 22 years) | Mood disorder | Normal/abnormal | 0.54/1.5 | Polyneuropathy, muscular pain, skin rash, leukopenia, anti‐ds DNA↑ | 17 | |||
| 7 | 21 | 7 years | CS (60 mg, pulse 3), IV‐CY (14), MTX (intrathecal 30 mg), MZ (300 mg, 2 years) | Myelopathy, mood disorder, anxiety disorder | II, III/abnormal | 0.80↑/4.7 | Periungual erythaema, leukopenia | 3 | |||
| 8 | 20 | 9 months | CS (45 mg), IV‐CY (6), AZ (50 mg, 1 month) | Psychosis, cognitive dysfunction | III/abnormal | 0.56/1.0 | Lymphadenopathy, alopecia, malar rash, lymphocytopenia | 18 | |||
| 9 | 20 | 8 months | CS (60 mg, pulse 3), IV‐CY, DFPP (4) | Acute confusional state, psychosis | III/abnormal | 0.98↑/4.2 | Fever, lymphadenopathy, low Hb, lymphocytopenia, high ESR, anti‐Sm↑ | 28 | |||
| 10 | 29 | 17 years | CS (40 mg, pulse 2), AZ (100 mg, ly), CsA (300 mg, 1 month), IV‐CY (2), PE (4) | Acute confusional state, psychosis | Normal/abnormal | 0.60/2.4 | Severe AIHA, CH50↓ | 18 |
The disease activity was high in all patients and none had responded to conventional immunosuppressants.
AIHA, autoimmune haemolytic anaemia; AZ, azathioprine; CS, corticosteroid; CsA, cyclosporine; CY, cyclophosphamide; DFPP, double filtration plasmapheresis; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; IA, immunoadsorption; MTX, methotrexate; MZ, mizoribine; PE, plasma exchange; SLE‐DAI, Systemic Lupus Erythaematosus Disease Activity Index; VCR, vincristine.
For IV‐CY, PE and IA, numbers in parentheses represent the number of treatments. For CS, CsA, AZ and MZ, the doses in parentheses represent maximum dosage. For VCR in patient 1 and MTX in patient 7, the dose in parentheses expresses total dosage. MRI finding: II, small areas of increased signal intensity secondary to microinfarctions; III, focal areas of signal intensity in grey matter (Am J Roentgenol 1985;144:1027–31).
With regard to CNS‐related symptoms, acute confusional state was noted in 5, psychosis in 4, seizures in 2, mood disorders in 2, and one patient each had headache, demyelinating syndrome, myelopathy, anxiety disorder and cognitive dysfunction, based on the NPSLE classification of the American College of Rheumatology.18,19 MRI findings included abnormal signals in the cerebral white matter in six patients. SPECT showed reduced cerebral blood flow in eight patients. Although a high IgG index20 was noted in five patients (>0.66), an increase in IL6 was confirmed in only one patient.
Serious haemolytic anaemia, cardiomyopathy‐associated decreased cardiac function, muscle pain, mucocutaneous disorders, peripheral neural deficits such as abnormal sensation and neurogenic bladder were also seen in these patients, in addition to the CNS‐related changes (tables 1 and 2). In all participants, conventional immunosuppressive therapy produced either no improvement of symptoms or only a poor response. The SLEDAI values (range, 2–49) reflected the presence or absence of organ system‐specific activity, with large scores representing involvement of CNS and low scores reflecting haematological activity. In the present study, involvement of organs was limited to those that could be confirmed objectively, while subjective signs such as fatigue and paresthesia were not recorded. Thus, using this approach, the SLEDAI scores of patients with objective signs reflecting multiple involvement of CNS were high whereas those of patients with subjective symptoms only were low. In our study, patients 1 and 3 had multiple CNS signs, patients 1 (49 points) and 3 (37 points) had seizures, psychosis and organic brain syndrome. On the other hand, patient 2 had MRI abnormality in the medulla oblongata but had only paresthesia as a subjective symptom (2 points), and patient 7 had MRI abnormality in the dorsal medulla spinalis and paralysis of the lower extremities, mood and anxiety disorders. However, the SLEDAI scores of both patients were based on subjective symptoms, and thus the scores were low (2 and 3, respectively).
Table 2 Clinical outcomes of neuropsychiatric systemic lupus erythaematosus after anti‐CD20 antibody treatment.
| Patient | Dose of rituximab | Other treatments at study entry (mg) | CNS manifestations | Objective NPSLE findings after treatment | Duration of remission (m) | |
|---|---|---|---|---|---|---|
| before | after | |||||
| 1 | 375 mg/m2 day 1, 8 | Bet 1.0 | Consciousness disorder, seizure, psychosis | Complete recovery (GCS 7–11→15/5 days) | Improvement of SPECT | 22 |
| 2 | 375 mg/m2 day 1, 15 | Bet 1.5 | Consciousness disorder | Improved consciousness | No follow‐up data | 18 |
| 3 | 375 mg/m2 day 1, 8 | Bet 1.0 | Consciousness disorder, seizure | Complete recovery (GCS 3→14/2 days) | No improvement in MRI and SPECT | 23 |
| 4 | 375 mg/m2 day 1, 8 | m‐PSL 20 | Headache | Resolution of headache | Improved IgG index (1.05 →0.84/4 w) | 29 |
| 5 | 375 mg/m2 day 1, 8 | Bet 1.25 | Paresthesia of fingers, toes and left precordial‐back | Resolution of paresthesia | Improvement of neck MRI | 7 |
| 6 | 500 mg day 1, 8, 15, 22 | Bet 2.5 | Depressive state, insomnia | Improvement of depressive state | Improvement of SPECT | 7 |
| 7 | 500 mg day 1, 8, 15, 22 | Bet 1.25 | Paresis of both lower limbs, muscle weakness, depressive state | Reduction of paresis, improvement of depressive state (SDS 58→50/2 w) | Improvement of SPECT, improvement of IgG index (0.80 →0.72/3 m) | 14 |
| 8 | 1000 mg day 1, 15 | Bet 1.25, AZ 50 | Psychosis, cognitive dysfunction | Improvement of psychosis (BPRS 26→7/8 w) | Improvement of SPECT | 11 |
| 9 | 375 mg/m2 day 1 | PSL 45 | Consciousness disorder, psychosis, paresis of both lower limbs, neurological bladder | Complete recovery | Improvement of PET and MRI, improved IgG index (0.98 →0.61/2 w) | 10 |
| 10 | 375 mg/m2 day 1, 8 | Bet 3 | Consciousness disorder, hallucination, cataplexy | Complete recovery | No significant improvement in objective findings | 4 |
Bet, betamethasone; BPRS, brief psychiatric rating scale; CNS, central nervous system; GCS, Glasgow Coma Scale; m‐PSL, methylprednisolone; MRI, magnetic resonance imaging; NPSLE, neuropsychiatric systemic lupus erythaematosus; PET, 18FTG‐positron emission tomography; PSL, prednisolone; SDS, self‐rating depression scale; SPECT, single photon emission computed tomography. For other abbreviations, see table 1.
Clinical outcome
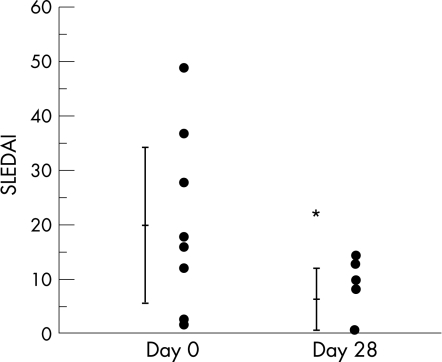
At the start of rituximab treatment, patients were treated with low to moderate doses of corticosteroids (15–40 mg of prednisolone, 1–3 mg betamethasone), and continued to use this treatment during the rituximab arm of the study. However, immunosuppressants were stopped at entry to the study in all patients except for patient 8 who continued her treatment of 50 mg azathioprine. The postrituximab follow‐up period was 7–45 months. Table 2 provides details of the clinical symptoms and laboratory tests before and 28 days after rituximab treatment (unless otherwise indicated in the table). Improvement in the skin and mucocutaneous lesions was fast, and the ejection fraction recovered from 44% to 72.1% in patient 4. All patients showed improvement in haematopenia and complement titre and marked falls in PE‐resistant autoantibodies after treatment. Analysis of SLE activity before and after the treatment showed a significant decrease in SLEDAI from 19.9 (range, 49–2) before treatment to 6.2 (range, 15–0) after treatment (p = 0.013, fig 1). Moreover, SLEDAI decreased to 0 in 9 of the 10 patients at 1–6 months after rituximab treatment.
Figure 1 Systemic lupus erythaematosus disease activity index (SLEDAI) score before and 28 days after rituximab treatment. A decrease in SLEDAI score was detected in 9 of the 10 patients. Data are mean (SD). *p<0.05.
Rituximab treatment was also effective against CNS lesions in all patients. In particular, the consciousness state of all the five patients who were in acute confusional state before treatment, improved rapidly after the treatment. For example, the GCS score of patient 1 improved from 7–11 to 15 after 5 days of treatment, and that of patient 2 from 3 to 14 after 2 days of treatment. This rapid recovery was clinically significant. In addition, even in three patients who were in a dazed state and needed to be woken up before rituximab treatment, became alert the next day (patient 2) or after a few days of treatment (patients 9 and 10). Furthermore, rituximab also improved neuropsychiatric symptoms such as psychosis and mood disorder within a few weeks to a few months after treatment. For example, the Brief Psychiatric Rating Scale, which is used for the assessment of schizophrenia, markedly decreased in patient 8 from 26 to 7 points within 2 months, together with recovery of communication skills. In addition, patients 1 and 9 showed rehabilitation into society after rituximab treatment although they had serious neuropsychiatric symptoms before treatment. In addition to the improvement in SLE activity and clinical symptoms, rituximab also improved the quality of life of the patients.
We also assessed the effects of rituximab treatment by comparing the findings of MRI and SPECT before and after treatment. In four patients (patients 1, 6, 7 and 8), rituximab treatment improved cerebral blood flow as determined by SPECT; in patient 1, such improvement was noted at the early stage of treatment and paralleled the improvement in clinical symptoms. For patient 5, rituximab treatment resulted in improvement in the abnormal findings in T2‐weighted images of the cervical cord on MRI, along with the improvement in sensory deficits due to inflammation at the same site. For patient 9, rituximab treatment resulted in reduction of the high‐intensity lesion in the head MRI T2‐weighted image.
Four of our patients had peripheral neuropathies in addition to CNS lesions. Treatment with rituximab resulted in remission or marked improvement of paresthesia in patient 2, radiculopathy in patient 4, ulnar neuropathy in patient 6, and neurological bladder in patient 9. Rituximab also improved quality of life based on improvement of peripheral neuropathy‐related symptoms although such symptoms tended to persist after treatment.
While the overall therapeutic effect of rituximab was excellent, some patients developed relapse after long‐term remission. Six of the 10 patients showed reactivation of SLE including reappearance of CNS‐related symptoms. For patient 1, remission was maintained with low‐dose steroid for 22 months after rituximab treatment. However, the patient showed recurrence associated with an increase in autoantibodies and proteinuria. Recurrence was also noted 18 months after treatment in patient 2, associated with haemolysis. Both patients 1 and 2 required retreatment with rituximab. At 23 months after completion of rituximab treatment, patient 3 showed worsening of the head MRI findings and cerebrospinal fluid abnormalities and developed witnessed seizure attacks. In patient 5, a reduction in the steroid dose was followed by recurrence of CNS‐related symptoms after 7 months. Generalised skin rashes appeared in patient 9 after 10 months and patient 10 reported worsening of lupus headache after 4 months. Patients 3 and 5 received IV‐CY treatment, and patient 9 and 10 required an increase in the steroid dose. However, four patients (patients 4, 6, 7 and 8) maintain a remission state at the time of writing this report (at 35 months in patient 4, at 7 months in patient 6, at 19 months in patient 7 and 16 months in patient 8) after the completion of rituximab treatment.
Adverse effects
Of the 10 patients, two developed pneumonia, one had herpes zoster, one developed chickenpox and one had intractable infection of decubitus ulceration. These infections were successfully controlled with antibiotics.
Phenotypic analysis of SLE lymphocytes
T cells and B cells are activated by antigen stimulation via T cell receptors and signals from costimulatory molecules. The responsible costimulatory molecules, such as CD40/40L, CD80, CD86/CD28 and ICOS/B7h, are known to be expressed in patients with active SLE.21,22,23,24,25,26
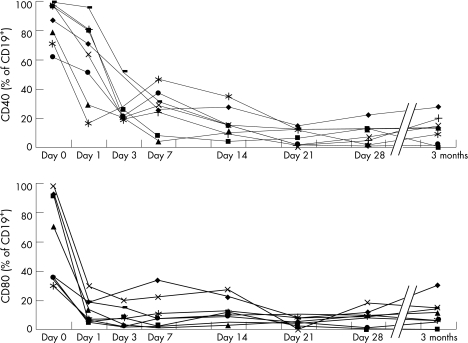
We performed serial analysis of the expression of functional molecules in eight patients with SLE before and after rituximab treatment by flow cytometry. Rituximab treatment resulted in rapid disappearance of CD20, a specific antigen to B cells, marked decrease in CD19‐positive cells, within several days to 2 weeks after treatment. Rituximab also resulted in rapid falls in the percentages of CD40‐expressing and CD80‐expressing CD19 cells within 1 day and both were hardly detected after the second day (fig 2). The expression levels of these molecules were still low at 3 months after completion of rituximab treatment.
Figure 2 Serial changes in CD40 and CD80 expression on CD19‐positive cells after rituximab treatment in eight patients with systemic lupus erythaematosus. CD40 and CD80 expression was measured before and 28 days after rituximab treatment.
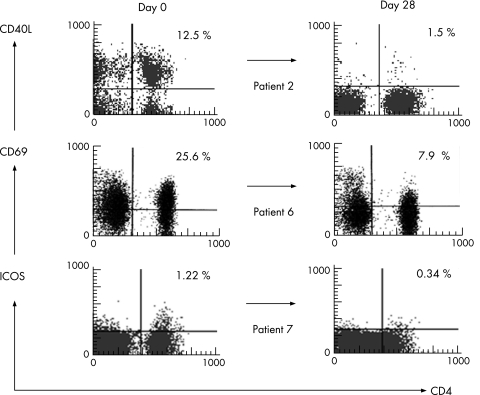
We also assessed the effects of treatment on the expression levels of CD40L (a costimulatory molecule on CD4‐positive cells), ICOS and CD69 (an early activation antigen). While only three patients showed high expression of these molecules before treatment, rituximab treatment reduced the expression levels of these molecules in all three patients (fig 3), suggesting that rituximab does not only affect B cells but also T cells in patients with SLE.
Figure 3 Changes in expression of functional molecules on CD4‐positive cells induced by rituximab treatment. The expression of CD40L (patient 2), CD69 (patient 6) and ICOS (patient 7) on CD4‐positive cells was measured before (day 0) and 28 days after rituximab treatment. Percentages represent the percentage of CD4‐positive cells expressing the functional molecules.
Discussion
To date, reports on rituximab treatment for autoimmune diseases have covered various conditions, including RA, SLE, dermatomyositis, Sjögren's syndrome and vasculitis.27,28,29,30 Rituximab treatment resulted in improvement, manifested by a decrease in the British Disease Activity score and SLE DAI score, of arthropathy, nephropathy, thrombocytopenia and haemolytic anaemia.11,12,13,14,15,16
Although few reports described the efficacy of rituximab treatment in patients with SLE with CNS lesions,11,14,31 to our knowledge, there are no published reports that provide detailed analysis of the effects of such treatment in a large group of patients. Rituximab has a large molecular weight of 146 kDa, and hence cannot readily cross the blood–brain barrier; therefore, it is unlikely to reach the cerebrospinal fluid following systemic administration. We measured rituximab concentration in the cerebrospinal fluid of patient 8 at 24 h after treatment. The value (0.3 μg/ml) was slightly higher than the lower detection limit of the assay, whereas the serum concentration was 279 μg/ml. Based on this finding, we assume that the central effects of rituximab are mediated through another mechanism, not through antibody‐dependent cellular cytotoxicity and/or complement‐dependent cytotoxicity.32
To assess autoreactive lymphocyte activity, we determined the expression of various functional molecules on the surface of peripheral blood lymphocytes before and after rituximab treatment by using flow cytometry. We previously proposed that rituximab could regulate SLE disease activity and correct autoimmune abnormalities.12 The present results showed a rapid decrease in the expression of functional surface molecules and maintenance of long‐term control following rituximab treatment (fig 2). Specifically, a marked decrease in the proportion of CD40‐expressing and CD80‐expressing cells was detected on the day after initiation of rituximab treatment. In this regard, Leng et al33 found CD40 overexpression in CD19 cells in patients with rheumatoid arthritis compared with healthy controls. Others also reported that the percentage of CD80‐positive cells among activated B cell subset was higher in SLE than the controls.34 These results suggest that the target of rituximab treatment is activated B cells. Anolik et al35 examined B cell phenotypes after rituximab treatment and reported that the proportion of autoreactive memory B cells was decreased after rituximab treatment. Considered together, the above results and those of the present study suggest that T cell activation is negatively influenced by a rapid decrease in B cell to T cell stimulation in parallel with the loss of B cells. Our results also showed that rituximab down regulated CD40L, ICOS and CD69 on CD4‐positive cells in patients with active SLE (fig 3). Sfikakis et al36 also reported that rituximab treatment decreased CD40L and CD69 expression in patients with SLE. These results imply that rituximab could eliminate B cells bearing functional molecules and inhibit the interaction between these B cells and activated T cells by down regulating costimulatory molecules, and also possibly by reducing the production of certain cytokines and complement activation, which could lead to rapid improvement of CNS manifestations of the disease.
At present, there is no treatment strategy for patients with NPSLE who fail to respond to conventional therapies. In such patients, large doses of steroids are provided on long‐term basis, and IV‐CY is administered continuously. Our study showed that rituximab is useful as a new treatment for such cases. However, recurrence after rituximab treatment was noted in our patients, as has been reported previously in patients with rheumatoid diseases.28 Two of our patients who experienced recurrence received rituximab re‐treatment. However, these patients experienced recurrence at 18 and 22 months after rituximab treatment, suggesting that remission could be maintained for a comparatively long period of time with rituximab treatment. Further studies are needed to develop strategies for the prevention of recurrence and counter measures for inhibiting the production of antichimeric antibodies.37,38 There is also a need to investigate the long‐term effects of rituximab treatment and its organ specificity.
Acknowledgements
This work was supported in part by a Research Grant‐In‐Aid for Scientific Research by the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan and University of Occupational and Environmental Health, Japan.
Abbreviations
CNS - central nervous system
FACS - fluorescence‐activated cell sorter
NPSLE - neuropsychiatric systemic lupus erythematosus
PBS - phosphate‐buffered saline
PE - plasma exchange
SLE - systemic lupus erythematosus
SLEDAI - SLE Disease Activity Index
SPECT - single‐photon‐emission computed tomography
Footnotes
Competing interests: None declared.
References
- 1.Blanco F J, Gomez‐Reino J J, de la Mata J, Corrales A, Rodriguez‐Valverde V, Rosas J C.et al Survival analysis of 306 European Spanish patients with systemic lupus erythematosus. Lupus 19987159–163. [DOI] [PubMed] [Google Scholar]
- 2.Kasitanon N, Louthrenoo W, Sukitawut W, Vichainun R. Causes of death and prognostic factors in Thai patients with systemic lupus erythematosus. Asian Pac J Allergy Immunol 20022085–91. [PubMed] [Google Scholar]
- 3.Hahn B H. Systemic lupus erythematosus. In: Kasper DL, ed. Origins of Harrison's principles of internal medicine. 16th edn. Columbus: McGraw‐Hill, 20051960–1967.
- 4.Ad Hoc Working Group on Steroid‐Sparing Criteria in Lupus Criteria for steroid‐sparing ability of interventions in systemic lupus erythematosus: report of a consensus meeting. Arthritis Rheum 2004503427–3431. [DOI] [PubMed] [Google Scholar]
- 5.Goldblatt F, Isenberg D A. New therapies for systemic lupus erythematosus. Clin Exp Immunol 2005140205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petri M. Cyclophosphamide: new approaches for systemic lupus erythematosus. Lupus 200413366–371. [DOI] [PubMed] [Google Scholar]
- 7.Boumpas D T, Yamada H, Patronas N J, Scott D, Klippel J H, Balow J E. Pulse cyclophosphamide for severe neuropsychiatric lupus. Q J Med 199181975–984. [DOI] [PubMed] [Google Scholar]
- 8.Barile‐Fabris L, Ariza‐Andraca R, Olguin‐Ortega L, Jara L J, Fraga‐Mouret A, Miranda‐Limon J M.et al Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis 200564620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherer Y, Levy Y, Longevitz P, Lorber M, Fobrizzi F, Shoenfeld Y. Successful treatment of systemic lupus erythematosus cerebritis with intravenous immunoglobulin. Clin Rheumatol 199918170–173. [DOI] [PubMed] [Google Scholar]
- 10.Valesini G, Priori R, Francia A, Balestrieri G, Tincani A, Airo P.et al Central nervous system involvement in systemic lupus erythematosus: a new therapeutic approach with intrathecal dexamethasone and methotrexate. Springer Semin Immunopathol 199416313–321. [DOI] [PubMed] [Google Scholar]
- 11.Saito K, Nawata M, Nakayamada S, Tokunaga M, Tsukada J, Tanaka Y. Successful treatment with anti‐CD20 monoclonal antibody (rituximab) of life‐threatening refractory systemic lupus erythematosus with renal and central nervous system involvement. Lupus 200312798–800. [DOI] [PubMed] [Google Scholar]
- 12.Tokunaga M, Fujii K, Saito K, Nakayamada S, Tsujimura S, Nawata M.et al Down‐regulation of CD40 and CD80 on B cells in patients with life‐threatening systemic lupus erythematosus after successful treatment with rituximab. Rheumatology 200544176–182. [DOI] [PubMed] [Google Scholar]
- 13.Perrotta S, Locatelli F, La Manna A, Cennamo L, De Stefano P, Nobili B. Anti‐CD20 monoclonal antibody (Rituximab) for life‐threatening autoimmune haemolytic anaemia in a patient with systemic lupus erythematosus. Br J Haematol 2002116465–467. [PubMed] [Google Scholar]
- 14.Leandro M J, Edwards J C, Cambridge G, Ehrenstein M R, Isenberg D A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 2002462673–2677. [DOI] [PubMed] [Google Scholar]
- 15.Looney R J, Anolik J H, Campbell D, Felgar R E, Young F, Arend L J.et al B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose‐escalation trial of rituximab. Arthritis Rheum 2004502580–2589. [DOI] [PubMed] [Google Scholar]
- 16.Leandro M J, Cambridge G, Edwards J C, Ehrenstein M R, Isenberg D A. B‐cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology 2005441542–1545. [DOI] [PubMed] [Google Scholar]
- 17.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 18.ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature The American College of Rheumatology nomenclature and classification and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 199942599–608. [DOI] [PubMed] [Google Scholar]
- 19.Borchers A T, Aoki C A, Naguwa S M, Keen C L, Shoenfeld Y, Gershwin M E. Neuropsychiatric features of systemic lupus erythematosus. Autoimmun Rev 20054329–344. [DOI] [PubMed] [Google Scholar]
- 20.French C A, Tracy R P, Rudick R A, Kraemer A M, Arvan D A. Simultaneous determination of cerebrospinal fluid oligoclonal bands and the “gamma‐protein index” by agarose electrophoresis and densitometry. Clin Chem 19863284–87. [PubMed] [Google Scholar]
- 21.Datta S K, Kalled S L. CD40‐CD40 ligand interaction in autoimmune disease. Arthritis Rheum 1997401735–1745. [DOI] [PubMed] [Google Scholar]
- 22.Koshy M, Berger D, Crow M K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest 199698826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yellin M J, Thienel U. T cells in the pathogenesis of systemic lupus erythematosus: potential roles of CD154‐CD40 interactions and costimulatory molecules. Curr Rheumatol Rep 2000224–31. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs B, Thomas D E, Tsokos G C. Elevated in vivo expression of the costimulatory molecule B7‐BB1 (CD80) on antigen presenting cells from a patient with SLE. Clin Exp Rheumatol 199614695–697. [PubMed] [Google Scholar]
- 25.Bijl M, Horst G, Limburg P C, Kallenberg C G. Expression of costimulatory molecules on peripheral blood lymphocytes of patients with systemic lupus erythematosus. Ann Rheum Dis 200160523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreas H, Kerstin B, Karin R, Hans J B, Marcus O, Annett J.et al Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum 2004503211–3220. [DOI] [PubMed] [Google Scholar]
- 27.Leandro M J, Edwards JC W, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis 200261883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keogh K A, Ytterberg S R, Fervenza F C, Carlson K A, Schroeder D R, Specks U. Rituximab for refractory Wegener's granulomatosis: report of a prospective, open‐label pilot trial. Am J Respir Crit Care Med 2006173180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine T D. Rituximab in the treatment of dermatomyositis: an open‐label pilot study. Arthritis Rheum 200552601–607. [DOI] [PubMed] [Google Scholar]
- 30.Pijpe J, van Imhoff G W, Spijkervet F K, Roodenburg J L, Wolbink G J, Mansour K.et al Rituximab treatment in patients with primary Sjogren's syndrome: an open‐label phase II study. Arthritis Rheum 2005522740–2750. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong D J, McCarron M T, Wright G D. SLE‐associated transverse myelitis successfully treated with Rituximab (anti‐CD20 monoclonal antibody). Rheumatol Int 2005181–2. [DOI] [PubMed] [Google Scholar]
- 32.Reff M E, Carner K, Chambers K S, Leonard J E, Raab R, Newman R A.et al Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 199483435–445. [PubMed] [Google Scholar]
- 33.Leng J H, Hu Z Y, Zhuo G C, Wang K Y, Wang Z W, Peng L.et al The expression and significance of costimulatory molecule in peripheral blood B lymphocytes in rheumatoid arthritis. Zhonghua Nei Ke Za Zhi 200443519–521. [PubMed] [Google Scholar]
- 34.Folzenlogen D, Hofer M F, Leung D Y, Freed J H, Newell M K. Analysis of CD80 and CD86 expression on peripheral blood B lymphocytes reveals increased expression of CD86 in lupus patients. Clin Immunol Immunopathol 199783199–204. [DOI] [PubMed] [Google Scholar]
- 35.Anolik J H, Barnard J, Cappione A, Pugh‐Bernard A E, Pugh‐Bernard A E, Felgar R E.et al Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 2004503580–3590. [DOI] [PubMed] [Google Scholar]
- 36.Sfikakis P P, Boletis J N, Lionaki S, Vigklis V, Fragiadaki K G, Iniotaki A.et al Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down‐regulation of the T cell costimulatory molecule CD40 ligand: an open‐label trial. Arthritis Rheum 200552501–513. [DOI] [PubMed] [Google Scholar]
- 37.Weide R, Heymanns J, Pandorf A, Koppler H. Successful long‐term treatment of systemic lupus erythematosus with rituximab maintenance therapy. Lupus 200312779–782. [DOI] [PubMed] [Google Scholar]
- 38.Edelbauer M, Jungraithmayr T, Zimmerhackl L B. Rituximab in childhood systemic lupus erythematosus refractory to conventional immunosuppression: case report. Pediatr Nephrol 200520811–813. [DOI] [PubMed] [Google Scholar]