Abstract
Objective
To study the effects of the antirheumatic drug sulphasalazine (SASP) on the immune system by analysing systemic and gut‐associated immune responses.
Methods
A total of 23 healthy volunteers were treated with either SASP or placebo for 5 weeks in a double‐blind fashion and immunised 2 weeks after the initiation of treatment. Specific immune responses were triggered by subcutaneous immunisation with tetanus toxoid and by peroral immunisation with inactivated influenza vaccine. The effects of treatment on specific immunity to tetanus and influenza were evaluated by enzyme‐linked immunospot assay quantifying the number of circulating specific and total antibody‐producing cells (spot‐forming cells (SFC)) at 6, 8 and 10 days after immunisation.
Results
An immunosuppressive effect of SASP on systemic immune response was observed with a decrease in the total number of IgG‐SFC, IgG anti‐tetanus SFC and IgG anti‐tetanus antibody levels in serum. SASP also exerted an immunosuppressive effect on the mucosa‐associated immune system as seen from its down‐regulating effect on the total number of circulating IgA SFC.
Conclusions
These data show firstly that SASP exerts an immunosuppressive effect on defined immune responses to immunisation in vivo, and secondly that both mucosa‐associated and systemic immunity are affected by SASP treatment.
Development of therapeutic strategies against inflammatory diseases such as rheumatoid arthritis today make use of several different options, among them combination therapies with new as well as older drugs. A rational use of these therapies requires more knowledge on the mode of action of all the drugs used, and also on their potential adverse effects, such as a reduced immune defence against various microbes. In many cases, however, even the effects of well‐accepted and commonly used antirheumatic drugs on immune responses are unknown. This is at least partly due to the lack of adequate methods to describe the effects of antirheumatic drugs on adaptive immune responses in vivo in humans.
In this study, we wanted to investigate whether a vaccination protocol followed by evaluation of the adaptive immune response by means of analysis of immunoglobulin‐producing cells could be used as a tool to study the effects of an antirheumatic drug on adaptive immune responses. Specifically, the effects of sulphasalazine (SASP) on host adaptive immune responses to the antiviral influenza vaccine and to the antibacterial tetanus toxoid vaccine were investigated.
SASP has been marketed for many years and is still commonly used both as monotherapy against arthritis and inflammatory bowel disease, and as a component in various combination therapies for rheumatoid arthritis.1,2,3,4,5 Nevertheless, surprisingly little is known about the effects of SASP on an immune response in vivo. We know that the treatment of patients with rheumatoid arthritis and other inflammatory diseases in vivo causes reduction of inflammatory parameters such as sedimentation rate and acute‐phase reactants, and may lead to a decrease in serum immunoglobulin levels.6,7,8,9,10 In vitro experiments have documented effects both on non‐specific inflammatory events such as granulocyte and mast cell activation, and on lymphocyte functions—that is, SASP can, in certain concentrations, inhibit both T and B cell proliferation, and immunoglobulin production.8,11,12,13,14,15,16 In addition, the inhibition of macrophage activation and NfkB‐dependent transcription has been described.17,18
The fact that the in vitro effects on lymphocyte function are seen for concentrations of SASP, which in vivo are only encountered within the gut, has supported the hypothesis that SASP preferentially exerts its action on the gut‐associated immune system.19,20,21 However, we still, we do not know to what extent SASP in vivo affects the adaptive immune response triggered from the gut or systemically.
One of the obstacles in studying immune responses triggered in the gut resides in the fact that mucosa‐derived immunity is only incompletely reflected by changes in the serum levels of IgA; instead, bone marrow cells are the main source of IgA in serum. A potential way to overcome this problem has been highlighted by data indicating that IgA production of B lymphocytes in the blood reflects a mucosa‐associated immune response much better than serum IgA levels.22,23,24
To study the mode and the site of action of SASP on defined immune response in vivo, we immunised healthy individuals in a double‐blind manner perorally and systemically after treatment with SASP or placebo for 2 weeks. Immune responses were evaluated by measuring both circulating Ig‐producing cells of different isotypes with the enzyme‐linked immunospot (ELISPOT) assay and serum immunoglobulin levels. In this way, we were able to evaluate the effects of SASP on both the systemic and the mucosa‐associated immune responses.
Methods
Study subjects
A total of 25 healthy volunteers, aged 17–48 (mean 32) years were recruited mainly from students and staff at the Uppsala University Hospital, Uppasala, Sweden by procedures approved by the local ethical committee. They were examined clinically, and those with a negative history of clinical disease and no previous salicyl or sulpha hypersensitivity reaction were included. The selected individuals were randomised and given either placebo or SASP (Pharmacia, presently Pfizer, Uppsala, Sweden) in a double‐blind manner. The active drug was given at a dose of 500–2000 mg (1000 mg twice daily), gradually increased from the initial dose over the first week, and with a constant dose of 2000 mg daily during the second week and throughout the study. Compliance was controlled during the study with personal communication with the study nurse at each time point the when blood samples were obtained. No major complications were encountered.
The study subjects were immunised with 0.5 ml of tetanus vaccine (Statens bakteriologiska laboratorium, SBL, Stockholm, Sweden, 7.5 Lf/ml detoxicated in formalin) subcutaneously at day 14. None had received a booster within 1 year, but all had received tetanus vaccination earlier in life. They were also perorally given 5 ml of influenza vaccine (Vaxigrip, Pasteur Mérieux, Lyon, France) solubilised in 150 ml of water with 500 mg of sodium bicarbonate to stabilise the vaccine (Tarkowski A, personal communication). The drug (SASP or placebo) was given immediately before the immunisation and before breakfast.
Blood samples were drawn before drug treatment (day 0), on the day of immunisation (day 14), and on days 20, 22 and 24 after initiation of drug treatment. These intervals of sampling were chosen on the basis of pilot experiments (data not shown) showing a peak of antibody‐producing cells (spot forming cells (SFC)) within this time interval post immunisation.
Preparation of mononuclear cells
Heparinised blood was separated on Ficoll‐Hypaque and the mononuclear cells were suspended in RPMI‐HPG (hepes, penicilline, glutamine) with 10% foetal calf serum. Cell concentration was adjusted to 105 or 106 cells/well for ELISPOT assay.
ELISPOT
For analysis of the total number of cells producing antibodies, 96‐well plastic plates (Immulon2; Dynatech, Chantilly, Virginia, USA) were coated with anti‐IgA (Dako, Denmark), anti‐IgG (Fab'2 fragment; Jackson, West Grove, Pennsylvania, USA) and anti‐IgM (Cappell, Organon Teknika, West Chester, Pennsylvania, USA), respectively. After washing, cells were added to the wells and incubated in 5% CO2 at 37°C for 3 h. Subsequently, the plates were washed in distilled water, and biotinylated secondary IgA, IgG and IgM antibodies were added (Tago, Burlingame, California, USA). After overnight incubation and washing avidin‐alkaline‐phosphatase (Dako) and subsequently the chromogen BCIP were added (Sigma, St Louis, Missouri, USA). For further details see Feltelius et al and Czerkinsky et al.21,25
For analysis of antigen‐specific Ig‐producing cells, the plastic plates were coated with influenza vaccine (Vaxigrip kindly provided by Pasteur‐Mérieux) and tetanus toxoid (SBL, conc. 400 Lf/ml), both diluted 1:20. Subsequent procedures were the same as described for total numbers of Ig‐producing cells.
The plates were evaluated under a reversed microscope at a magnification of ×40. All plates were counted by one observer. Three different cell concentrations and duplicate wells were set up for each sample to control for intra‐assay variability.
Elisa
Antigen‐specific antibodies in serum against tetanus and influenza were measured with ELISA at days 14 (day of vaccination) and 24 (10 days after vaccination).
96‐well plates (Costar, Cambridge, Massachusetts, USA) were coated with antigens diluted 1:20 in phosphate‐buffered saline at 4°C overnight. The plates were washed in phosphate‐buffered saline‐Tween, incubated with sera for 1 h, washed and incubated with secondary antibodies (peroxidase‐conjugated anti‐IgG and anti‐IgA antibodies, Dako) for 1 h, washed and incubated with substrate. The results are presented as arbitrary units.
Immunoglobulin measurements
Serum immunoglobulin levels were analysed with a nephelometer using standards of The World Health Organization for calibration and expressed as grams/litre.
Statistical analyses
The results of ELISPOT at day 6 were compared between the placebo and SASP groups by the Mann Whitney U test. Evaluation of the effect of immunisation on SFC at different time points was performed by means of the paired Student's t test.
Results
Effects of immunisation in the placebo‐treated group
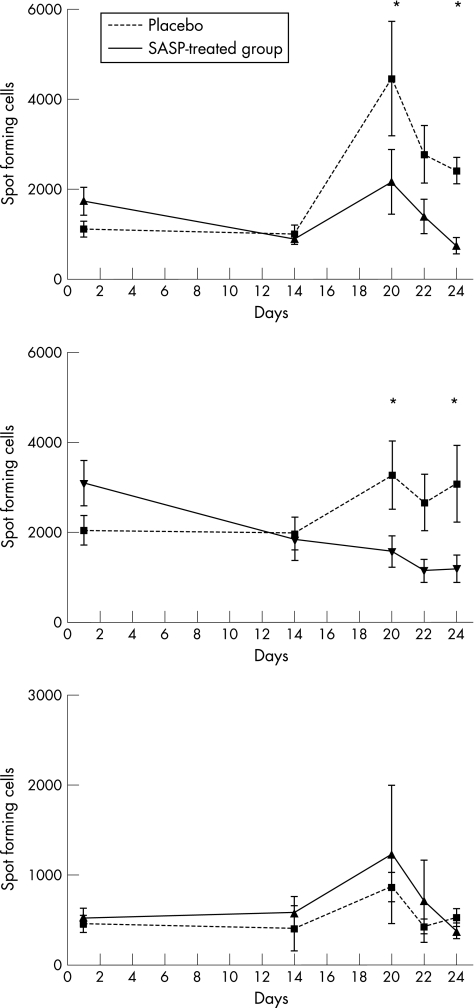
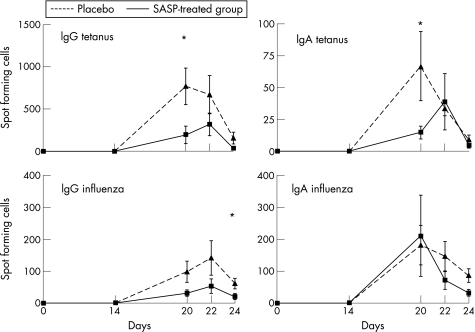
Figure 1 shows the effects of the immunisations on mucosa‐associated and systemic immune systems; it shows the change in absolute numbers of immunoglobulin‐producing cells (SFC) per microliter of blood. The total numbers of IgG‐SFC and IgA‐SFC increased markedly after immunisation in the placebo‐group, whereas no changes were observed in the levels of total IgG or IgA antibodies in serum after immunsation (data not shown). A transient appearance of anti‐tetanus toxoid IgG‐SFC was seen after immunisation26 (fig 2), together with a minor number of IgA‐anti‐tetanus toxoid SFC. IgA‐anti‐influenza SFC appeared with dynamics similar to IgG SFC, although the IgG SFC were fewer in number. In addition, serum levels of IgG‐anti‐tetanus antibodies increased strongly after vaccination (table 1). A small increase in serum IgA‐anti‐tetanus antibody levels was also evident. No measurable increase was seen in the serum levels of IgA or IgG anti‐influenza antibodies.
Figure 1 Collected data for total spots (isotype specific) are indicated in three different graphs, illustrating the mean (standard error of the mean, SEM) values for spot‐forming cells (SFC) at each time point expressed as SFC/ml of blood. For isotypes IgG and IgA, the number of SFC was significantly lower for the SASP‐treated group, as compared with the placebo group, indicated by an asterisk (*) (‐‐‐placebo, ___SASP‐treated group).
Figure 2 Collected data for total spots (IgG and IgA isotypes) are indicated in four different graphs, illustrating the mean (SEM) values for spot‐forming cells at each time point expressed as spot‐forming cells (SFC)/ml of blood. For the tetanus SFC the difference between the placebo and the SASP groups was statistically significant (Mann–Whitney U test), indicated by an asterisk (*) (‐‐‐‐placebo, ___SASP‐treated group).
Table 1 Antibodies to tetanus toxoid and influenza serum levels of anti‐tetanus and influenza antibodies were measured by ELISA.
| SASP | Placebo | SASP vs placebo p value | |
|---|---|---|---|
| IgG‐ anti‐TT | |||
| Day 14 | 376 (87) | 309 (69) | |
| Day 24 | 921 (210) | 1745 (243)* | 0.019 |
| IgA‐anti‐TT | |||
| Day 14 | 34 (8) | 53 (22) | |
| Day 24 | 152 (49) | 487 (126)* | 0.027 |
| IgG‐anti‐influenza | |||
| Day 14 | 915 (186) | 716 (1020 | |
| Day 24 | 912 (188) | 745 (1050 | NS |
| IgA‐anti‐influenza | |||
| Day 14 | 618 (209) | 662 (184) | |
| Day 24 | 605 (201) | 845 (170) | NS |
TT, tetanus toxoid; SASP, sulphasalazine.
All values are arbitrary units, defined separately for each antigen and isotype (mean (SEM)).
*Statistically significant (paired t test) between days 14 and 24.
Effects of SASP treatment
Preimmunisation
There was a relatively constant number of total Ig‐SFC of all subclasses between days 0 and 14 in the placebo group. By contrast, in the SASP‐treated group, during this preimmunisation period a significant reduction in the numbers of IgA‐SFC was observed when compared with the placebo‐treated group (p = 0.036). Furthermore, the number of IgG‐SFC decreased slightly in the SASP group. No effects of SASP treatment were seen on the numbers of IgM‐SFC.
Post‐immunisation
Figures 1 and 2 show comparisons between SASP and placebo‐treated groups concerning ELISPOT data. The largest difference between the two groups was seen for total IgA‐SFC, where the statistically significant increase after immunisation in the placebo group was replaced by a steady decrease in the numbers of antibody‐producing cells in the SASP‐treated group. In addition total numbers of IgG‐SFC were clearly reduced in response to immunisation in the SASP‐treated group when compared with the placebo group. No difference between the SASP and placebo group was seen for IgM‐SFC.
Analysis of the antigen‐specific SFC (fig 2) showed significant differences between SASP and placebo‐treated groups for IgG‐anti‐tetanus SFC and for IgA‐anti‐tetanus SFC, despite low numbers for IgA SFC in both groups. No differences however, were, seen for IgA‐anti‐influenza SFC.
The clear increase of the IgG‐anti‐tetanus antibody level (table 1) in the placebo group was replaced by a blunted response in the SASP group. A clear but less dramatic effect of SASP on IgA‐anti‐tetanus antibody levels was seen. The lack of measurable serum antibody response to the peroral influenza vaccine precluded further analysis of anti‐influenza serum antibodies.
Finally, no differences between SASP and placebo‐treated groups as to serum IgM levels or antigen‐specific IgM‐SFC were observed (data not shown).
Discussion
This study presents two sets of data with direct clinical implications. Firstly, a method is presented where the ELISPOT assay for enumeration of single antibody‐producing cells in the blood is used to quantify the effects of drugs on both systemic IgG and mucosa‐associated IgA responses. Secondly, parallel usage of subcutaneous and peroral immunisations shows that SASP affects both systemic and gut‐associated immunity, observed as changes in IgA‐SFC and IgG‐SFC in peripheral blood after immunisations. The effects of SASP on systemic immunity were further confirmed by changes in the numbers of IgG‐anti‐tetanus toxoid SFC and serum levels of IgG anti‐tetanus antibodies after immunisation.
The starting point of this study was our ambition to investigate the effects of SASP on defined immune responses originating either in the mucosa or systemically. This met, however, with methodological difficulties mainly concerning feasible means to monitor and quantify a defined mucosa‐associated immune reaction. These local reactions lead mainly to IgA secretion from mucosal surfaces into the exterior lumen, and result to a lesser extent in increased levels of serum IgA. Production of IgA locally in the gut, on the other hand, is difficult to measure repeatedly in drug trials. A potential solution to this problem was provided by the demonstration that a local mucosal immunologic stimulus22,27,28 rapidly causes local B cells to recirculate in the body, thus being transiently present in the peripheral circulation before subsequently homing to their site of destination.29 Quantification of the numbers of circulating IgA‐SFC thus represents a way of monitoring events in the gut through analysis of peripheral blood.22 Using the ELISPOT assay to obtain qualitative characteristics of IgA production in terms of subclass distribution and the relationship between polymeric and monomeric Ig has indeed shown circulating IgA‐producing cells to be migrating progenitors of IgA‐precursor B cells, previously activated in secretory tissues.24,27,28
Concerning systemic immunity, similar studies applying the ELISPOT assay for quantitation of IgG‐producing cells after subcutaneous immunisation with antigens such as tetanus toxoid26 have shown a rapid and distinct increase in total IgG‐SFC as well as antigen‐specific IgG‐SFC in the circulation after immunisation.
On this basis, we designed a dual immunisation protocol using two immunogens, influenza vaccine for peroral and tetanus toxoid for systemic immunisation, to quantify the potential immunomodulatory effects of SASP. This protocol yielded an efficient activation of both systemic and mucosa‐associated immune responses as shown by the clear increase in the numbers of both IgG‐SFC and IgA‐SFC in peripheral blood, as well as circulating antigen‐specific B cells (influenza and tetanus) measured by the ELISPOT assay at 6–8 days after immunisation. Measurements of antigen‐specific IgG and IgA antibody levels showed that the systemic immunisation was also adequately mirrored by levels of tetanus‐specific antibodies. The fact that no effect of immunisation was observed when measuring total serum levels of IgA or influenza‐specific IgA antibodies confirms the difficulties in measuring mucosal immune responses through serum antibody analysis, in particular when serum titres were measured relatively early after immunisation. The high reproducibility of the ELISPOT assay, including intra‐assay and inter‐assay variability, allows this method to be used for these types of studies.
The data obtained from the analysis of the placebo‐treated group and from previous pilot experiments indicated that the total numbers of IgA‐SFC and IgG‐SFC as well as the numbers of antigen‐specific IgA‐SFC and IgG‐SFC are useful parameters for estimating SASP effects on mucosal and systemic immunity, respectively. Measurements of total Ig‐SFC have an extra advantage in yielding a “baseline” value, permitting calculations of proportional changes in Ig‐SFC for each individual. This is an obvious advantage in a situation such as the present one, where inter‐individual variations in magnitude of responses are large, both concerning total Ig‐SFC as well as antigen‐specific responses.
The effects of SASP treatment on systemic immunity were clear cut—that is, a statistically significant decrease in IgG‐responses was seen in all measured parameters: total numbers of Ig‐producing cells, number of anti‐tetanus IgG‐SFC and serum levels of IgG—anti‐tetanus toxoid. The situation is obviously more complex with respect to mucosal immunity. The fact that the statistically significant increase in the total numbers of IgA‐SFC in the placebo group was replaced by a gradual decrease in the numbers of IgA‐SFC in the SASP‐treated group provides an indication in favour of a suppressive effect of SASP on the mucosa‐associated immune response to vaccination. On the other hand, no significant decrease in the numbers of IgA anti‐influenza SFC was observed, whereas a decrease was seen in IgA‐anti‐tetanus‐SFC. We have at present no satisfactory explanation for this discrepancy, but on the basis of the effect on the parameter that we consider most reliable, that is, total numbers of IgA‐SFC, where proportional increases and decreases could be measured to compensate for inter‐individual variations in responses, we have chosen to interpret the data in favour of an effect of SASP on the mucosal immune response.
The effects of SASP on the numbers of total IgA‐SFC—that is, the supposed effect on mucosal immunity in vivo, was interesting, but not unexpected, considering that SASP has been reported to inhibit both T and B cell activation in vitro in concentrations only present locally in the gut after therapeutic doses of SASP.8,14,20,30,31 Less expected was the clear effect on systemic IgG‐mediated immunity as concentrations of SASP attained systemically are considered to be lower than required for in vitro effects on lymphocyte activation.20,30
Our results can obviously not determine at which level of the immune system the immunosuppressive effects are exerted. However, because the changes are seen not only in the number of circulating Ig‐SFC but also in the serum levels of IgG—anti‐tetanus antibodies, the effects are indeed on the level of lymphocyte activation and not merely a result of cell migration. The lack of effects on IgM responses is moreover an indirect argument in favour of an effect of SASP mainly on T –cell‐dependent mechanisms.
Two main conclusions concerning the clinical use of SASP can be drawn from this study. Regarding its beneficial use as an immunosuppressive drug, it is possible that the effects of SASP on both systemic and mucosa‐associated immunity may help us to design improved protocols for combination therapy with drugs that do not exert such effects. In the case of unwanted effects of immunosuppression, it is probable, however, that SASP has negative effects on any type of vaccination.
In a more general sense, this study also shows the potential of the current immunisation and monitoring protocols for elucidating the mode of action not only of SASP but also of other drugs, where a better understanding of their in vivo action may assist in establishing combined therapy regimens with new or presently established disease‐modifying antirheumatic drugs acting in different sites of the body and at different levels of the immune system.
Acknowledgements
Ingrid Martling and Märta Ryde provided invaluable help in recruiting and monitoring the volunteers involved in this study. Pasteur‐Mérieux generously provided us with the Vaxigrip influenza vaccine. Professor Andrej Tarkowski provided essential information and ideas concerning the procedures for peroral immunization and ELISPOT assays. The study was supported by grants from the Swedish Medical Research Council, from King Gustaf V:s 80‐year foundation and from the Swedish Association against Rheumatism.
Abbreviations
ELISPOT - enzyme‐linked immunospot, SASP, sulphasalazine
SFC - spot forming cells
Footnotes
Competing interests: None.
Ethics approval: This study was approved by the local ethical committee at Uppsala University Hospital, Uppsala, Sweden.
References
- 1.Cannella A C, O'Dell J R. Is there still a role for traditional disease‐modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis? Curr Opin Rheumatol 200315185. [DOI] [PubMed] [Google Scholar]
- 2.Scott D L, Smolen J S, Kalden J R, van de Putte L B, Larsen A, Kvien T K.et al Treatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann Rheum Dis 200160913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Dell J R, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff P J.et al Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two‐year, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2002461164. [DOI] [PubMed] [Google Scholar]
- 4.Haagsma C J, van Riel P L, de Jong A J, van de Putte L B. Combination of sulphasalazine and methotrexate versus the single components in early rheumatoid arthritis: a randomized, controlled, double‐blind, 52 week clinical trial. Br J Rheumatol 1997361082. [DOI] [PubMed] [Google Scholar]
- 5.Aletaha D T, Stamm T, Kapral G, Eberl J, Grisar K, Machold P.et al Survival and effectiveness of leflunomide compared with methotrexate and sulfasalazine in rheumatoid arthritis: a matched observational study. Ann Rheum Dis 200362944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr M, Bacon P A, Coppock J, Scott D L. Long term experience of salazopyrin EN in rheumatoid arthritis (RA). Scand J Rheumatol Suppl 19876437. [DOI] [PubMed] [Google Scholar]
- 7.Feltelius N, Hallgren R. Sulphasalazine in ankylosing spondylitis. Ann Rheum Dis 198645396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symmons D P, Salmon M, Farr M, Bacon P A. Sulfasalazine treatment and lymphocyte function in patients with rheumatoid arthritis. J Rheumatol 198815575. [PubMed] [Google Scholar]
- 9.Bird H A, Dixon J S, Pickup M E, Rhind V M, Lowe J R, Lee M R.et al A biochemical assessment of sulphasalazine in rheumatoid arthritis. J Rheumatol 1982936. [PubMed] [Google Scholar]
- 10.Kang B Y, Chung S W, Im S Y, Choe Y K, Kim T S. Sulfasalazine prevents T‐helper 1 immune response by suppressing interleukin‐12 production in macrophages. Immunology 19999898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klareskog L, Holmdahl R, Goldschmidt T, Bjork J. Immunoregulation in arthritis. A review on synovial immune reactions in RA and in some experimental animal models for arthritis. Scand J Rheumatol Suppl 1987647. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara M, Mitsui K, Ishida J, Yamamoto I. The effect of salazosulfapyridine on the in vitro antibody production in murine spleen cells. Immunopharmacology 19901915. [DOI] [PubMed] [Google Scholar]
- 13.Imai F, Suzuki T, Ishibashi T, Dohi Y. Effect of sulfasalazine on B cells. Clin Exp Rheumatol 19919259. [PubMed] [Google Scholar]
- 14.Comer S S, H E. Jasin. In vitro immunomodulatory effects of sulfasalazine and its metabolites. J Rheumatol 198815580. [PubMed] [Google Scholar]
- 15.MacDermott R P, Nash G S, Bertovich M J, Mohrman R F, Kodnerv D L. Delacroix 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology 198691379. [DOI] [PubMed] [Google Scholar]
- 16.Bissonnette E Y, Enciso J A, Befus A D. Inhibitory effects of sulfasalazine and its metabolites on histamine release and TNF‐alpha production by mast cells. J Immunol 1996156218. [PubMed] [Google Scholar]
- 17.Hasko G, Szabo C, Nemeth Z H, Deitch E A. Sulphasalazine inhibits macrophage activation: inhibitory effects on inducible nitric oxide synthase expression, interleukin‐12 production and major histocompatibility complex II expression. Immunology 2001103473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahl C, Liptay S, Adler G, Schmid R M. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 19981011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullar T, Hunter J A, Capell H A. Sulphasalazine in the treatment of rheumatoid arthritis: relationship of dose and serum levels to efficacy. Br J Rheumatol 198524269. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon P J, Webb C, Grindulis K A. Sulphasalazine in rheumatoid arthritis: pointers to a gut‐mediated immune effect. Br J Rheumatol 198726318. [DOI] [PubMed] [Google Scholar]
- 21.Feltelius N, Gudmundsson S, Wennersten L, Sjoberg O, Hallgren R, Klareskog L. Enumeration of IgA producing cells by the enzyme linked immunospot (ELISPOT) technique to evaluate sulphasalazine effects in inflammatory arthritides. Ann Rheum Dis 199150369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czerkinsky C, Svennerholm A M, Quiding M, Jonsson R, Holmgren J. Antibody‐producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun 199159996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol 19877265. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A M. Mucosal immunity: implications for vaccine development. Immunobiology 1992184157. [DOI] [PubMed] [Google Scholar]
- 25.Czerkinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarkowski A. A solid‐phase enzyme‐linked immunospot (ELISPOT) assay for enumeration of specific antibody‐secreting cells. J Immunol Methods 198365109. [DOI] [PubMed] [Google Scholar]
- 26.Tarkowski A, Czerkinsky C, Nilsson L A. Simultaneous induction of rheumatoid factor‐ and antigen‐specific antibody‐secreting cells during the secondary immune response in man. Clin Exp Immunol 198561379. [PMC free article] [PubMed] [Google Scholar]
- 27.Czerkinsky C, Prince S J, Michalek S M, Jackson S, Russell M W, Moldoveanu Z.et al IgA antibody‐producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A 1987842449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol 198740153. [DOI] [PubMed] [Google Scholar]
- 29.Picker L J, Butcher E C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol 199210561. [DOI] [PubMed] [Google Scholar]
- 30.McConkey B, Amos R S, Durham S, Forster P J, Hubball S, Walsh L. Sulphasalazine in rheumatoid arthritis. Br Med J 1980280442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDermott R P, Schloemann S R, Bertovich M J, Nash G S, Peters M, Stenson W F. Inhibition of antibody secretion by 5‐aminosalicylic acid. Gastroenterology 198996442. [DOI] [PubMed] [Google Scholar]