Abstract
Objective
To develop a cell‐ELISA method to detect antineuronal antibodies (anti‐Ns) and evaluate the diagnostic value of anti‐Ns in central nervous system involvement in systemic lupus erythematosus (CNS‐SLE).
Method
Anti‐N was assessed in both serum and cerebrospinal fluid (CSF) samples from 38 patients with CNS‐SLE, 29 with SLE without CNS involvement (non‐CNS‐SLE), 36 with other rheumatic diseases and 59 with non‐rheumatic diseases with the CNS manifestations using a cell‐ELISA method with 1% paraformaldehyde‐fixed SK‐N‐MC neuroblastoma cells as substrate. Serum samples from 37 healthy donors were also included in this study. Patients with CNS‐SLE who were anti‐N positive in CSF were studied serially for CSF anti‐N levels at times of treatment‐associated improvement in CNS symptoms.
Results
Serum anti‐N levels were significantly increased in patients with SLE compared with other groups, with a sensitivity of 61.2% (41/67) and a specificity of 91.8% (p<0.001). CSF anti‐N levels were significantly increased in patients with CNS‐SLE, with a sensitivity of 47.4% (18/38) and a specificity of 89.7%, whereas only 10.3% (3/29) of patients with non‐CNS‐SLE had increased anti‐N in CSF (p<0.001). CSF anti‐N levels decreased significantly after effective treatment of CNS‐SLE (p<0.05).
Conclusion
Serum anti‐N is relatively specific to SLE. CSF anti‐N is a sensitive and relatively specific antibody in diagnosing CNS‐SLE and correlates with CNS‐SLE activity.
Central nervous system (CNS) involvement is a common and severe complication of systemic lupus erythematosus (SLE).1,2,3,4 Prompt diagnosis and treatment could considerably alleviate the disease and improve prognosis.
The most commonly applied complementary tests, such as CT and MRI, are static image techniques and are not sensitive in reflecting the pathophysiological changes in CNS‐SLE.3,5 It is imperative to develop more sensitive and specific tests to better diagnose the patients, especially those with atypical neuropsychiatric manifestations or those at an early stage. In the past two decades, the role of autoantibodies, including antiphospholipid antibody and antiribosomal P antibody, in the pathogenesis of CNS‐SLE has been increasingly recognised.2,4,6 A few reports deal with the role of antineuronal antibodies (anti‐Ns) in CNS‐SLE, and the results are inconsistent because of the different techniques used and the patients included.6,7,8,9,10,11
The purpose of this study is to develop a cell‐ELISA method to prevent the interference of antinuclear antibodies in detecting anti‐N, and by assessing both cerebrospinal fluid (CSF) and serum samples in CNS‐SLE, non‐CNS‐SLE before and after treatment as well as in other disease controls to evaluate systematically the diagnostic and prognostic value of anti‐Ns in CNS‐SLE.
Methods
Patients
In all, 38 consecutive inpatients with CNS‐SLE at the Peking Union Medical College Hospital, Beijing, China, were enrolled in this study, and 29 patients with non‐CNS‐SLE who were hospitalised at the same time were randomly selected as controls. All patients fulfilled four or more of the 1997 American College Rheumatology revised criteria for SLE.12 Patients were diagnosed as having CNS‐SLE by both a rheumatologist and a neurologist because of significant and unequivocal change in neurological or psychiatric function, identified by history, physical examination, laboratory or radiographic tests and further proved by clinical course and response to treatment, as required by the American College Rheumatology criteria for CNS‐SLE.13 Both serum and CSF samples were obtained from patients with CNS‐SLE and non‐CNS‐SLE, 36 patients with other rheumatic diseases (systemic vasculitis, myositis, antiphospholipid syndrome, systemic scleroderma, primary Sjögren syndrome, rheumatoid arthritis, etc) with or without CNS complications and 59 patients with non‐rheumatic diseases involving CNS (CNS infection, lymphoma, cerebral tumour, multiple sclerosis, etc). In addition, serum samples from 37 healthy donors were included as normal controls. Consent to participate in the study was obtained from all patients or their family. This research was approved by the hospital ethics committee.
Measurement of anti‐N activity
Anti‐N activity in both serum and CSF samples was determined by cell‐ELISA using the human neuroblastoma cell line SK‐N‐MC. Cells were first fixed with 1% paraformaldehyde and then incubated with diluted samples or standard sera. Bound IgG anti‐N reacted with peroxidase‐conjugated F (ab′)2 fragments of goat antihuman IgG. After incubation with substrate solution, OD492 was read with a two‐wavelength microplate photometer.
Determinations of OD492 were normalised to values for anti‐N positive control. The relative concentration of anti‐N was defined as ODr = ODsample/ODpositive control.
To determine the specificity of our cell‐ELISA, the immunofluorescence staining types were compared between anti‐N positive control and eight serum samples that were antinuclear antibody (ANA) positive but anti‐N negative on cell‐ELISA.
Statistical analysis
Significant differences in the number of patients with positive baseline characteristics and laboratory findings between patients with CNS‐SLE and non‐CNS‐SLE were determined using χ2 tests. Significant differences in the positive rates and levels of anti‐N in CSF and serum between different study groups was determined using the unpaired t test and χ2 test or Fisher's exact test, depending on sample size (n>5 and n⩽5, respectively). Changes in CSF anti‐N after treatment were calculated using the paired t test. The SPSS, V.11 software was used to analyse the data. In all tests, the probability values were two‐sided and p<0.05 was considered significant.
Results
Table 1 lists the baseline characteristics and laboratory data of patients with CNS‐SLE and non‐CNS‐SLE.
Table 1 Baseline characteristics and laboratory data of patients with CNS‐SLE and non‐CNS‐SLE controls.
| Characteristic | CNS‐SLE (n = 38) | Non‐CNS‐SLE (n = 29) | p Value |
|---|---|---|---|
| Men/women (n) | 2/36 | 2/27 | – |
| Mean (SD) age (years) | 33 (18) | 35 (21) | – |
| Mean (SD) disease duration (years) | 3.1 (2.6) | 3.5 (2.8) | – |
| Nephritis | 31 (81.6) | 18 (62.1) | 0.074 |
| Leucopenia/thrombocytopenia | 18 (47.4) | 14 (48.3) | 0.94 |
| Hypocomplementia | 28 (73.7) | 17 (58.6) | 0.19 |
| Elevated anti‐dsDNA | 26 (68.4) | 16 (59.2) | 0.27 |
| Antiribosomal P | 11 (28.9) | 3 (10.3) | 0.064 |
| aCL | 14 (36.8) | 7 (24.1) | 0.27 |
aCL, anticardiolipid antibody; CNS‐SLE, central nervous system involvement in systemic lupus erythematosus; dsDNA, double‐stranded DNA.
Values are the number (percentage) of patients unless otherwise indicated.
The sex ratio, age and mean disease duration were similar in patients with CNS‐SLE and non‐CNS‐SLE controls. Although they did not reach significance, the positive rates of antiribosomal P and anticardiolipid antibody as well as active disease in the CNS‐SLE group were higher than in the control group.
Specificity of cell‐ELISA in detecting anti‐N
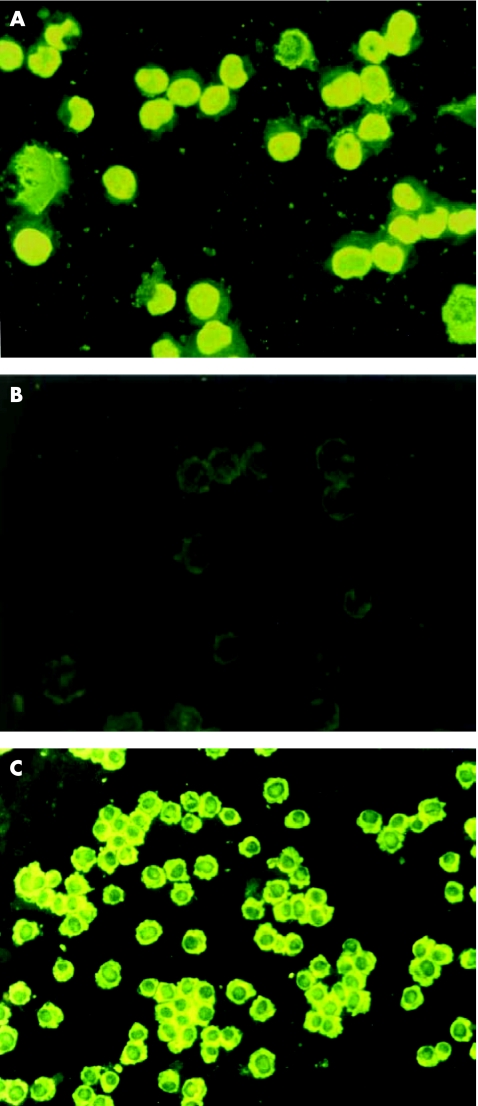
As shown in fig 1, while detecting eight serum samples that are ANA positive but anti‐N negative with SK‐N‐MC cells, we found that all eight samples were positive on acetone/methyl alcohol‐fixed substrate (fig 1A) and negative on paraformaldehyde‐fixed substrate (fig 1B). In contrast, indirect immunofluorescence staining showed that anti‐N positive control bound to paraformaldehyde‐fixed SK‐N‐MC cells, with homogeneous cytoplasmic fluorescence staining, and did not bind to the nucleus (fig 1C).
Figure 1 The staining patterns of antineuronal antibodies (anti‐N) and antinuclear antibody (ANA) were different on 1% paraformaldehyde‐fixed SK‐N‐MC cells (indirect fluorescence staining,×100). An ANA‐positive sample showed homogeneous nucleus type on SK‐N‐MC cells fixed with acetone/methyl alcohol (A) and was negative on 1% paraformaldehyde‐fixed SK‐N‐MC cells (B) in contrast, anti‐N IgG appeared as homogeneous cytoplasmic type on 1% paraformaldehyde‐fixed SK‐N‐MC cells (C).
Anti‐N in sera and CSF by cell‐ELISA
CSF ODr was 0.10 (0.07) in the non‐rheumatic disease group (n = 59); an ODr of >0.31 (mean+3SD) was considered anti‐N positive in CSF. The serum ODr was 0.18 (0.06) in the healthy donor group (n = 37); an ODr >0.36 (mean+3SD) was considered anti‐N positive in serum.
As shown in table 2, the positive rates and levels of serum anti‐N in both CNS‐SLE and non‐CNS‐SLE groups were significantly higher than in the rheumatic disease group and the healthy donor group (p<0.001). Although the level was higher in the CNS‐SLE group than in the non‐CNS‐SLE group, the difference did not reach significance (p>0.05). The sensitivity and specificity of serum anti‐N in diagnosing SLE was 61.2% and 91.8%, respectively, and the positive and negative predicting values were 85.4% and 71.7%, respectively.
Table 2 Antineuronal antibodies in serum and cerebrospinal fluid by cell‐ELISA.
| Groups (n) | Serum ODr positivity ODr level (%) | CSF ODr positivity ODr level (%) | ||
|---|---|---|---|---|
| CNS‐SLE (38) | 23 (60.5) | 0.59 (0.36)* | 18 (47.4) | 0.33 (0.19)* |
| Non‐CNS‐SLE (29) | 18 (62.1) | 0.44 (0.25)* | 3 (10.3) | 0.19 (0.10)* |
| Other rheumatic diseases (36) | 6 (16.2) | 0.25 (0.18)* | 2 (5.6) | 0.12 (0.07)* |
| Non‐rheumatic diseases (59) | ND | ND | 2 (3.4) | 0.10 (0.07)* |
| Healthy donors (37) | 1 (2.7) | 0.18 (0.06)* | ND | ND |
CNS, central nervous system; CSF, cerebrospinal fluid; ND, not done; ODr, relative concentration of antineuronal antibodies; SLE, systemic lupus erythematosus.
Values are number (percentage) of cases.
*Values are mean (SD).
The CSF anti‐N level in the CNS‐SLE group was significantly higher than in the other three groups, including the non‐CNS‐SLE group (p<0.001). The positive rates of CSF anti‐N in the CNS‐SLE and non‐CNS‐SLE groups were 47.4% and 10.3%, respectively (p<0.001). The sensitivity and specificity of CSF anti‐N in diagnosing CNS‐SLE were 47.4% and 89.7%, respectively, and the positive and negative predicting values were 85.7% and 56.5%, respectively.
Among 21 patients who were anti‐N positive in both serum and CSF, 18 were consistent with the diagnosis of CNS‐SLE. However, in 20 patients who were positive in serum but not in CSF, only 5 were clinically diagnosed with CNS‐SLE. None of these 67 patients were positive in CSF but negative in serum.
Of the 18 CNS‐SLE patients who were anti‐N positive in CSF, 17 were studied serially for CSF anti‐N levels at times of treatment‐associated improvement in CNS symptoms. The CSF anti‐N levels before and after treatment were 0.47 (0.22) and 0.22 (0.09), respectively (p<0.05).
Discussion
The pathogenesis of CNS‐SLE remains unclear. Small‐vessel vasculopathy, mediated by immune complexes, antiphospholipid antibody, antiribosomal P antibody as well as other autoantibodies, and vasculitis were suggested in the pathogenesis of CNS‐SLE.2,14
In 1978, Bluestein6 showed that 90% of patients with diffuse‐type CNS‐SLE had increased IgG anti‐N in the CSF. Their result was further supported by Isshi and Hirohata's report,7 whereas Kelly8 reported a much less positive rate of anti‐N in the CSF of CNS‐SLE. Thus, it remains to be clarified whether anti‐N is a sensitive and, more importantly, specific antibody in diagnosing CNS‐SLE. In our study, we used cell‐ELISA, with formaldehyde‐fixed SK‐N‐MC neuroblastoma cells as substrate, and found a marked increase in serum IgG anti‐N level in patients with SLE compared with other diseases and normal controls; the sensitivity and specificity were 61.2% and 91.8%, respectively, suggesting that serum anti‐N is relatively sensitive and specific in the diagnosis of SLE. We also noted, although the difference was not significant, the higher number of patients with antiribosomal P antibodies and anticardiolipid antibody in patients with CNS‐SLE. Interestingly, the positive rate and level of anti‐N in the CSF samples of CNS‐SLE were significantly higher than in non‐CNS‐SLE as well as in other groups, and were rather specific (89.7%); thus, the occurrence of anti‐N in CSF samples suggests the diagnosis of CNS‐SLE. We also conducted a serial study in 17 patients with CNS‐SLE who were anti‐N positive in CSF before treatment, and found that CSF anti‐N levels correlated with CNS‐SLE activity and decreased dramatically after successful treatment. We proved that this cell‐ELISA method is relatively specific in detecting anti‐N. Using paraformaldehyde to fix the SK‐N‐MC cell line, we could block the reaction of autoantibodies targeting nuclear antigens with SK‐N‐MC cells, as shown by indirect immunofluorescence staining, and prevent the interference of ANA in detecting anti‐N.
Anti‐N was tested in both serum and CSF samples of these patients with SLE. Among 21 patients who had anti‐N in both serum and CSF, 18 were with CNS‐SLE. However, in 20 patients who were positive in serum but not in CSF, only 5 were with CNS‐SLE. None of these 67 patients were positive in CSF but negative in serum. These results suggest that the presence of anti‐N in the CSF of patients with CNS‐SLE is a consequence of increased vascular permeability or disrupted blood–brain barrier.
Acknowledgements
We thank Dr Shunei Hirohata from Teikyo University School of Medicine, Tokyo, Japan for kindly providing anti‐N positive control.
Abbreviations
ANA - antinuclear antibody
Anti‐N - antineuronal antibody
CNS - central nervous system
CSF - cerebrospinal fluid
ODr - relative concentration of antineuronal antibodies
SLE - systemic lupus erythematosus
Footnotes
Funding: This study was supported by the National Natural Sciences Foundation of China (30400410), New Century Excellent Talent, Ministry of Education of China (NCET‐04‐0191) and Beijing Natural Sciences Foundation (7052052).
Competing interests: None.
References
- 1.Zhang X, Dong Y, Tang F, Li H, Zhang F. Central nervous system involvement in systemic lupus erythematosus. Zhonghua Nei Ke Za Zhi 199938681–684. [PubMed] [Google Scholar]
- 2.Diamond B, Volpe B. On the track of neuropsychiatric lupus. Arthritis Rheum 2003482710–2712. [DOI] [PubMed] [Google Scholar]
- 3.Sibbitt W J, Sibbitt R, Brooks W. Neuroimaging in neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 1999422026–2038. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood D, Gitlits V, Alderuccio F, Sentry J, Toh B. Autoantibodies in neuropsychiatric lupus. Autoimmunity 20023579–86. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zhu Z, Zhang F, Shu H, Li F, Dong Y. Diagnostic value of single‐photon‐emission computed tomography in severe central nervous system involvement of systemic lupus erythematosus: a case‐control study. Arthritis Rheum 200553845–849. [DOI] [PubMed] [Google Scholar]
- 6.Bluestein H. Neurocytotoxic antibodies in serum of patients with systemic lupus erythematosus. Proc Natl Acad Sci USA 1978753965–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isshi K Hirohata S. Differential roles of the anti‐ribosomal P antibody and antineuronal antibody in the pathogenesis of central nervous system involvement in systemic lupus erythematosus. Arthritis Rheum 1998411819–1827. [DOI] [PubMed] [Google Scholar]
- 8.Kelly M C, Denburg J. Cerebral spinal fluid immunoglobulins and neuronal antibodies in neuropsychiatric systemic lupus erythematosus and related conditions. J Rheumatol 198714740–747. [PubMed] [Google Scholar]
- 9.Bresnihan B, Hohmeister R, Cutting J. The neuropsychiatric disorder in systemic lupus erythematosus: evidence for both vascular and immune mechanisms. Ann Rheum Dis 197938301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tishler M, Alosachie I, Chapman Y, Korcyn A, Lorber M, Mevorach D.et al Anti‐neuronal antibodies in antiphospholipid syndrome with central nervous system involvement: the difference from systemic lupus erythematosus. Lupus 19954145–147. [DOI] [PubMed] [Google Scholar]
- 11.Toh B, Mackay I R. Autoantibody to a novel neuronal antigen in systemic lupus erythematosus and in normal human sera. Clin Exp Immunol 198144555–559. [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997401725. [DOI] [PubMed] [Google Scholar]
- 13.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R.et al Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population‐based evaluation. Arthritis Rheum 200145419–423. [DOI] [PubMed] [Google Scholar]
- 14.Trysberg E, Tarkowski A. Cerebral inflammation and degeneration in systemic lupus erythematosus. Curr Opin Rheumatol 200416527–533. [DOI] [PubMed] [Google Scholar]