Abstract
Backgrounds and aims
To evaluate the prognosis of primary biliary cirrhosis (PBC) together with systemic sclerosis (SSc), as this is unknown.
Methods and results
A PBC database of 580 patients identified 43 with PBC and SSc: two patients with PBC alone were matched to each PBC‐SSc patient for serum bilirubin concentration at the initial visit. Forty (93%) patients had limited cutaneous SSc. At diagnosis of PBC, median values were: 49.7 years, bilirubin 17 μmol/l, and albumin 40.5 g/l. Liver diagnosis occurred a median 4.9 years after SSc in 24 (56%) patients. In matched patients, median values at diagnosis were: 53.2 years, bilirubin 12 μmol/l, and albumin 41 g/l. Median follow up was similar: 3.16 years (PBC‐SSc) and 4.8 years (PBC alone). The risk of transplantation or death from diagnosis, adjusting for sex, age, log bilirubin, and alkaline phosphatase was significantly lower in PBC‐SSc (hazard ratio 0.116, p = 0.01) due to less transplantation (hazard ratio 0.068, p = 0.006). The rate of bilirubin increase was less in PBC‐SSc (p = 0.04). Overall survival was similar (hazard ratio 1.11, p = 0.948); there were nine deaths (21%) in PBC‐SSc (six SSc related and two liver related) and nine (11%) in PBC alone (six liver related).
Conclusions
Liver disease has a slower progression in PBC‐SSc compared with matched patients with PBC alone.
Keywords: liver transplantation, survival, antimitochondrial antibody, anticentromere antibody, autoimmune disease
Primary biliary cirrhosis (PBC) often occurs in association with other autoimmune conditions. Systemic sclerosis (SSc) is a chronic systemic connective tissue disease whose cardinal feature is sclerosis of the skin with potential involvement of other organs but usually not the liver. There are two main subsets, diffuse cutaneous (dcSSc) and limited cutaneous (lcSSc) disease, associated with varying morbidity and mortality.1 lcSSc is associated with little organ involvement except isolated pulmonary hypertension.2,3,4,5 Case reports6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 and some series report that 3–50% of PBC patients have SSc, mostly lcSSc.9,11,19,21,22,23,24 A positive anticentromere antibody (ACA), a hallmark antibody of SSc, is reported in 9–30% of PBC patients25,26,27,28 and 22–25% of all SSc patients, almost exclusively lcSSc. Conversely, 25% of SSc patients are antimitochondrial antibody (AMA) positive,29,30 the pathognomic antibody of PBC. ACA positivity is greater in PBC‐SSc than either disease on its own, but there is no cross reactivity between mitochondrial and centromere antigens.31 The autoimmune mechanisms behind the PBC‐SSc association are not fully understood. This patient group has clonally expanded T cells expressing TCRBV3, which may be involved in disease pathogenesis.32 Both SSc and PBC are associated with increased morbidity and mortality compared with the general population.33,34,35,36,37,38
Some case reports20,39 suggest that PBC‐SSc is associated with a more favourable prognosis of PBC whereas others report increased mortality due to SSc.40 However, there are no data regarding the prognosis of PBC‐SSc compared with a matched population with PBC alone.
Our aim was to compare survival and time to liver transplantation and to occurrence of complications in patients with both PBC and SSc, versus PBC alone, using Cox modelling, Mayo41 and Royal Free42 prognostic models.
Patients and methods
The PBC database
The database comprised 580 consecutive PBC patients referred to the Royal Free Hospital from 1 January 1977 to 1 January 2004. The diagnosis of PBC was established by positive AMA, alkaline phosphatase level >1.5 times the upper normal limit, and/or liver histology compatible with PBC: definite PBC fulfilled all of the above three criteria and probable PBC two of them. The following were prospectively recorded: baseline clinical and laboratory features at diagnosis, at referral, and during follow up at routine 3–6 month intervals or whenever the patient re‐attended hospital, as well as associated medical and surgical conditions, and time to occurrence of complications and comorbidity. Hepatic complications (at diagnosis, referral, or during follow up) were: ankle oedema, ascites, oesophageal varices (endoscopically or radiologically proven), variceal and non‐variceal gastrointestinal bleeding, spontaneous bacterial peritonitis (SBP), other infections, portosystemic encephalopathy (PSE), hepatocellular carcinoma (HCC), cancers, and surgical interventions.
Cause of death was liver related (resulting from a complication of liver disease) or non‐liver related. Patients who had liver transplantation were censored at the date of surgery whereas all patients alive without transplantation at the end of the study were censored at the date of the last follow up, updated to 15 April 2004. None was lost to follow up in either group.
Current study
Patients with SSc in the PBC database were cross referenced with the SSc database of 1700 consecutive patients referred to the Royal Free Hospital Centre for Rheumatology from 1980 onwards (SSc was classified using defined criteria).43 In addition, case notes of PBC patients with positive ACA antibody and SSc patients with positive AMA were also evaluated. In total, 43 patients with clinically evident PBC and SSc were identified.
Thirty eight of these 43 PBC‐SSc patients were already present in the PBC database, 15 of these referred to us for PBC, and SSc was followed up elsewhere. The remaining five of the 43 PBC‐SSc patients were referred to us for SSc, and PBC was followed up elsewhere. Those not previously followed for both diseases at the Royal Free Hospital all underwent subsequent evaluation with us. Therefore, all patients with suspicion of PBC in the Rheumatology Clinic and of SSc in the Hepatology Clinic were identified.
To increase comparability between groups, PBC‐SSc patients were matched to PBC without SSc, based on serum bilirubin concentration at the time of Royal Free Hospital referral (grouped in bands of 5 μmol/l), using an SAS software algorithm (SAS version 8.2, Cary, North Carolina, USA). Matching was one PBC‐SSc patient to two PBC alone patients. Two PBC‐SSc patients could not be matched, leaving a matched cohort of 41. There were also 13 PBC patients with positive ACA but without any clinical features of SSc. These were not matched but the same clinical end points were evaluated.
The main end point was occurrence of liver transplantation or death. As the Royal Free Hospital is a transplant centre with specific referral for liver transplantation, time to death or transplantation was taken from the time of diagnosis of PBC to minimise selection bias. Indications for liver transplantation were refractory or resistant ascites, repeated variceal bleeding, SBP, intractable PSE, HCC, and/or increase in serum bilirubin >170 μmol/l, or control of symptoms. Other end points were de novo fatigue, pruritus, ankle oedema, ascites, oesophageal varices, variceal bleeding, SBP, PSE, HCC, septicaemia, and non‐variceal gastrointestinal bleeding.
Statistical analysis
SAS package version 8.2 was used. Clinical characteristics were compared using χ2 tests, the Mann‐Whitney U test, and two sample t tests (continuous variables). Differences in Kaplan‐Meier survival plots were tested by log rank testing. Cox proportional hazards regression was used to adjust for differences in baseline characteristics associated with death or liver transplantation. Observed survival was compared with that predicted by Mayo Clinic41 and Royal Free prognostic scores.42
Results
In the study cohort of 43, 40 (93%) were female and 40 (93%) had limited cutaneous SSc, with 27 (63%) having definite PBC and 16 probable PBC (three AMA negative, with histology compatible with PBC). There was one overlap with Sjögren's syndrome and one with systemic lupus erythematosus. At diagnosis of PBC, median bilirubin was 17 μmol/l (range 4–109), median albumin 40.5 g/l (29–49 g/L), and only one (2.3%) presented with fluid retention (table 1). Median age was 49.7 years (range 22.1–70.1), with the PBC diagnosis made after SSc in 24 (56%), at a median of 4.9 years (range 0.1–26.7) (table 2). Twenty nine patients with PBC and lcSSc and one with dcSSc were positive for ACA. Among ACA negative patients with lcSSc, one had antinuclear ribonucleoprotein (nRNP) antibody, two anti‐double strand DNA antibody, and one had an unspecified antinuclear antibody. Of the two ACA negative patients with dcSSc, one had anti‐topoisomerase I (Scl70) and one anti‐nRNP antibody.
Table 1 Primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc) patients matched with 82 with PBC alone by serum bilirubin, at referral to the Royal Free Hospital.
| PBC‐SSc | PBC | |
|---|---|---|
| No of patients | 43 | 82 |
| Females | 40 (93%) | 76 (93%) |
| Age at diagnosis of PBC (y) | 49.7 (22.1–70.1)* | 53.2 (25.9–82.1)* |
| AMA positive | 40 (93%) | 77 (94%) |
| ACA positive | 30 (70%) | 0 (0%) |
| Bilirubin at diagnosis (μmol/l) | 17 (4–109)† | 12 (2–120)† |
| Albumin at diagnosis (g/l) | 40.5 (29–49)† | 41 (31–48)† |
| Alkaline phosphatase at diagnosis (U/l) | 334 (74–1038)† | 403 (89–2300)† |
| UDCA treatment | 22 (51%) | 43 (52%) |
| UDCA treatment at dosage ⩾13 mg/kg/day | 12 (28%) | 35 (43%) |
| Start of UDCA (y after diagnosis of PBC) | 2.1 (0–10.2) | 0.8 (0–10.3) |
Values are number (%) or median (range).
AMA, antimitochondrial antibody; ACA, anticentromere antibody; UDCA, ursodeoxycholic acid
*Mann‐Whitney U test was done for comparison of the two cohorts. No significant differences were observed.
†Two sample t test with equal variances was done using log transformed values for comparison of the two cohorts. No significant differences were observed.
Table 2 Features of 43 patients with primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc).
| PBC‐SSc | |
|---|---|
| Limited cutaneous SSc (lcSSc) | 40 (93%) |
| Diffuse cutaneous SSc (dcSSc) | 3 (7%) |
| SSc diagnosed before PBC | 24 (56%) |
| Time interval between diagnosis of SSc and PBC (y) | 4.87 (0.06–26.7) |
| PBC diagnosed before SSc | 19 (44%) |
| Time interval between diagnosis of PBC and SSc (y) | 4.08 (0.30–13.7) |
Values are number (%) or median (range).
In the 82 matched patients, 76 (93%) were female, 53 (65%) had definite PBC and 29 probable PBC (five AMA negative with histology consistent with PBC). Median values at diagnosis were: 53.2 years (range 25.9–82.1), bilirubin 12 μmol/l (range 2–130), albumin 41 g/l (range 31–48) and two (2.4%) had fluid retention (table 1).
At diagnosis, pruritus and fatigue were significantly more frequent in the PBC alone group (p = 0.03 and p = 0.05, respectively) whereas only diarrhoea was significantly more frequent in patients with PBC and SSc (p = 0.03) (table 3).
Table 3 Clinical features at diagnosis of primary biliary cirrhosis (PBC) in 43 patients with PBC‐systemic sclerosis (PBC‐SSc) and 82 with PBC alone matched by serum bilirubin at referral to the Royal Free Hospital, and in 13 patients with PBC who were anticentromere antibody positive but had no features of SSc†.
| PBC‐SSc (n = 43) | PBC (n = 82) | PBC‐ACA positive (n = 13) | |
|---|---|---|---|
| Asymptomatic (no liver symptoms) | 17 (39%) | 22 (27%) | 3 (23%) |
| Symptoms at diagnosis | |||
| Pruritus | 14 (33%)* | 43 (52%)* | 4 (31%) |
| Jaundice | 4 (9%) | 16 (19.%) | 2 (15%) |
| Fatigue | 7 (16%)‡ | 27 (33%)‡ | 2 (15%) |
| Abdominal pain | 6 (14%) | 13 (16%) | 4 (31%) |
| Diarrhoea | 5 (12%)§ | 2 (2%)§ | 1 (8%) |
| Oesophageal varices (at endoscopy) | 2 (5%) | 4 (5%) | 1 (8%) |
| Bleeding varices | 2 (5%) | 2 (2%) | 1 (8%) |
| Haematemesis or maelena | 2 (5%) | 3 (4%) | 1 (8%) |
| Bone pain | 6 (14%) | 15 (18%) | 1 (8%) |
| Fever | 0 (0%) | 2 (2%) | 0 (0%) |
| Weight loss | 2 (5%) | 10 (12%) | 0 (0%) |
| Amenorrhoea | 4 (10%) | 6 (7%) | 2 (15%) |
| Dry eyes and/or dry mouth | 8 (20%) | 9 (11%) | 3 (23%) |
| Ankle swelling | 1 (2%) | 1 (1%) | 0 (0%) |
| Abdominal swelling | 0 (0%) | 1 (1%) | 0 (0%) |
| Signs at diagnosis | |||
| Jaundice | 5 (12%) | 17 (21%) | 1 (8%) |
| Hepatomegaly | 10 (27%) | 27 (33%) | 5 (38%) |
| Splenomegaly | 7 (20%) | 11 (13%) | 3 (23%) |
| Hyperpigmentation | 2 (5%) | 9 (11%) | 2 (15%) |
| Xanthomata/xantelasma | 0 (0%) | 4 (5%) | 0 (0%) |
| Spider naevi | 2 (5%) | 6 (7%) | 1 (8%) |
| Ankle swelling | 1 (2%) | 1 (1%) | 0 (0%) |
| Ascites | 0 (0%) | 1 (1%) | 0 (0%) |
| Portosystemic encephalopathy | 0 (0%) | 0 (0%) | 0 (0%) |
| Muscular wasting | 0 (0%) | 2 (2%) | 0 (0%) |
| Finger clubbing | 0 (0%) | 1 (1%) | 0 (0%) |
| Pyrexia | 0 (0%) | 2 (2%) | 0 (0%) |
Values are number (%).
†Comparison was done between patients with PBC‐SSc and patients with PBC alone (that is, first two columns).
*p = 0.03 (Pearson χ2 test), 95% 95% confidence interval (CI) 0.34–0.98.
‡p = 0.047 (Pearson χ2 test), 95% CI 0.26–1.06.
§p = 0.03 (Pearson χ2 test), 95% CI 1.29–3.79.
Matching (at the time of referral to the Royal Free Hospital) resulted in no significant differences in pruritus (57% PBC alone, 49% of PBC‐SSc) or fatigue (44% PBC alone, 33% of PBC‐SSc), in the proportion without liver symptoms (that is, pruritus, fatigue, abdominal pain, jaundice, varices, variceal bleeding, ankle swelling, ascites, portosystemic encephalopathy; 22% PBC alone and 30% PBC‐SSc), or in serum albumin or alkaline phosphatase (table 4). Serum bilirubin, alkaline phosphatase, and albumin level at diagnosis, at referral to the Royal Free Hospital, and at the last follow up are shown in table 4. Comparison between the two cohorts, using log transformed values, did not show any significant difference.
Table 4 Biochemical data at diagnosis of primary biliary cirrhosis (PBC), at referral to the Royal Free Hospital, and at the last follow up, in 43 patients with PBC‐systemic sclerosis (PBC‐SSc), and 82 with PBC alone matched by serum bilirubin at referral to the Royal Free Hospital.
| At diagnosis | At referral to Royal Free Hospital | At last follow up* | |
|---|---|---|---|
| Bilirubin (μmol/l)‡ | |||
| PBC‐SSc | 17 (4–109) | 13 (4–174)† | 15.5 (5–590) |
| PBC | 12 (2–120) | 13 (3–112) | 28 (5–438) |
| Albumin (g/l)‡ | |||
| PBC‐SSc | 40.5 (29–49) | 41.5 (27–47) | 38.5 (27–47) |
| PBC | 41 (31–48) | 42 (27–49) | 40 (20–49) |
| Alkaline phosphatase (U/l)‡ | |||
| PBC‐SSc | 334 (74–1038) | 291 (94–2090) | 247 (83–1694) |
| PBC | 403 (89–2300) | 282 (82–1039) | 249 (66–1009) |
Values are median (range).
*Values at the last follow up for patients alive, last values before transplantation for transplanted patients, and last values before death for patients who died.
†Bilirubin values used for matching the cohort of patients with PBC alone.
‡Two sample t test with equal variances was done using log transformed values for comparison of the two cohorts. No significant differences were observed.
Median index of bilirubin change over time, calculated for each group from diagnosis and from referral to the Royal Free Hospital, was higher in PBC patients with respect to PBC‐SSc patients: 1.39 μmol/l/year versus 0.25 μmol/l/year from diagnosis (p = 0.04), 2.39 μmol/l/year versus 0.54 μmol/l/year from referral to the Royal Free Hospital (p = 0.15), respectively. At referral, 66% with PBC and SSc and 63% with PBC alone had bilirubin level ⩽17 μmol/l whereas at the last follow up 58% versus 39%, respectively, were still ⩽17 μmol/l (table 5).
Table 5 Proportion of patients with serum bilirubin concentration within the normal range (⩽17 μmol/l) at diagnosis, at referral to the Royal Free Hospital, and at the last follow up, for 43 patients with primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc) and 82 with PBC alone matched by serum bilirubin at referral to the Royal Free Hospital.
| Bilirubin ⩽17 μmol/l | Proportion of patients on UDCA treatment | |||
|---|---|---|---|---|
| At diagnosis | At referral to Royal Free Hospital† | At last follow up* | ||
| PBC‐SSc | 52% | 66% | 58% | 19% |
| PBC | 64% | 63% | 39% | 16% |
*Values at last follow up for patients alive, last values before transplantation for transplanted patients, and last value before death for patients who died.
†Serum bilirubin values at this time were used for matching the cohort of patients with PBC alone.
There were no significant differences for histological stage at diagnosis (p = 0.34), with 35% with PBC‐SSc versus 33% with PBC alone having histological stage I or II disease.44
Twenty two (51%) PBC‐SSc patients were treated with ursodeoxycholic acid (UDCA) started a median of 2.1 years (range 0.3–10.2) after diagnosis of PBC whereas among patients with PBC alone 43 (52%) received UDCA started a median time of 0.8 years (range 0–10.3) after diagnosis of PBC. Median dose of UDCA was 600 mg/day for both groups (range 150–750 for PBC‐SSc, 300–1500 for PBC alone); 12 patients (28%) with PBC‐SSc and 35 (43%) with PBC alone received UDCA at dosages ⩾13 mg/kg/day.
Ten (23%) PBC‐SSc patients received corticosteroid treatment for a maximum of six months; in three corticosteroids were commenced before PBC diagnosis.
Median time from PBC diagnosis to referral to the Royal Free Hospital was similar in the two cohorts (1.81 years (range 0–23.4) for PBC‐SSc; 1.19 years (range 0–16.3) for PBC alone). Median follow up from Royal Free Hospital referral was also similar (3.2 years (range 0.1–19.4) for PBC‐SSc; 4.8 years (range 0.1–13.6) for PBC alone).
There were no differences in number or in time to occurrence of hepatic complications (from either diagnosis of PBC or Royal Free Hospital referral) between the two cohorts, except for first occurrence of SBP and septicaemia, which were more frequent in PBC‐SSc patients (tables 6, 7).
Table 6 Rate of advent of complications from diagnosis of primary biliary cirrhosis (PBC) to the last follow up in 43 patients with PBC‐systemic sclerosis (PBC‐SSc) and 82 with PBC alone, matched by serum bilirubin at referral to Royal Free Hospital.
| Complication‡ | PBC‐SSc (n = 43) | PBC (n = 82) |
|---|---|---|
| New pruritus | 7 (16%) | 7 (9%) |
| New fatigue | 13 (30%) | 23 (28%) |
| Ankle oedema | 15 (35%) | 22 (27%) |
| Ascites | 11 (26%) | 21 (26%) |
| Oesophageal varices | 13 (30%) | 28 (34%) |
| Variceal bleeding | 8 (19%) | 13 (16%) |
| Upper gastrointestinal non‐variceal bleeding | 5 (12%) | 4 (5%) |
| Spontaneous bacterial peritonitis | 4 (9%)* | 1 (1%)* |
| Portosystemic encephalopathy | 5 (12%) | 7 (9%) |
| Hepatocellular carcinoma | 0 (0%) | 2 (2%) |
| Septicaemia | 4 (9%)† | 0 (0%)† |
Values are number (%).
*p = 0.03 (Pearson χ2 test), 95% confidence interval (CI) 1.48–4.09.
†p = 0.005 (Pearson χ2 test), 95% CI 2.39–4.02.
‡Log rank test was done for comparison of time to occurrence of complications. No significant differences were observed.
Table 7 Rate of advent of complications from referral through last follow up in 43 patients with primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc) and 82 with PBC alone matched by serum bilirubin at referral to Royal Free Hospital.
| Complication‡ | PBC‐SSc (n = 43) | PBC (n = 82) |
|---|---|---|
| New pruritus | 0 (0%) | 3 (4%) |
| New fatigue | 6 (14%) | 14 (17%) |
| Ankle oedema | 8 (19%) | 18 (22%) |
| Ascites | 5 (12%) | 15 (18%) |
| Oesophageal varices | 4 (9%) | 18 (22%) |
| Variceal bleeding | 4 (9%) | 6 (7%) |
| Upper gastrointestinal non‐variceal bleeding | 3 (7%) | 4 (5%) |
| Spontaneous bacterial peritonitis | 4 (9%)* | 1 (1%)* |
| Portosystemic encephalopathy | 3 (7%) | 7 (9%) |
| Hepatocellular carcinoma | 0 (0%) | 2 (2%) |
| Septicaemia | 4 (9%)† | 0 (0%)† |
Values are number (%).
*p = 0.03 (Pearson χ2 test).
†p = 0.005 (Pearson χ2 test).
‡Log rank test was done for comparison of time to occurrence of complications. No significant differences were observed.
Death and liver transplantation
During follow up, nine patients (21%) with PBC‐SSc (all with lcSSc) died, six due to SSc related causes (two pulmonary hypertension, three interstitial lung disease, and one sepsis secondary to central venous cannulation for pulmonary hypertension) and two liver related causes (both oesophageal variceal bleeding) whereas nine (11%) with PBC alone died, six due to liver related causes (five liver failure, one HCC) (table 8) and two due to extrahepatic malignancy. Cause of death was unknown in two (one in each cohort). Four of nine with PBC‐SSc who died (including the two liver related deaths) and seven of nine deceased with PBC alone (including four deaths from liver failure) had been treated with UDCA.
Table 8 Death or liver transplantation in 43 patients with primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc) and 82 patients with PBC alone matched by serum bilirubin at referral to Royal Free Hospital.
| PBC‐SSc (n = 43) | PBC (n = 82) | |
|---|---|---|
| Death | 9 (21%) | 9 (11%) |
| Liver related deaths | 2 (5%) | 6 (7%) |
| SSc related deaths | 6 (14%) | – |
| Non‐liver and non SSc related deaths | – | 2 (2%) |
| Cause of death unknown | 1 (2%) | 1 (1%) |
| Liver transplantation | 7 (16%) | 21 (26%) |
| Liver transplantation for liver failure | 6 (14%) | 20 (24%) |
| Liver transplantation for symptoms | 1 (2%) | 1 (1%) |
Values are number (%).
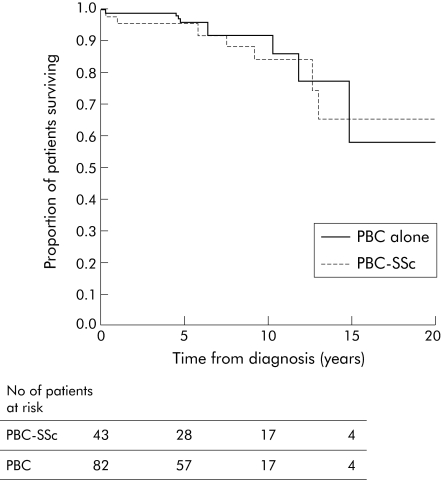
Risk of transplantation or death from time of diagnosis, adjusting for sex, age, log transformed bilirubin, and alkaline phosphatase, was significantly lower in PBC‐SSc patients (hazard ratio 0.116, p = 0.01). This was due to a higher liver transplantation rate of 21 (26%) in the group with PBC alone versus seven (16%) with PBC‐SSc. Thus risk of liver transplantation alone from diagnosis (adjusting as above) was significantly lower for the PBC‐SSc group (hazard ratio 0.068, p = 0.006) compared with the matched PBC alone. This difference was maintained even when adjusting for other baseline characteristics. However, for overall survival, there was no difference between the two groups (hazard ratio 1.11, p = 0.948) (fig 1).
Figure 1 Kaplan‐Meier curve of mortality in 43 patients with primary biliary cirrhosis‐systemic sclerosis (PBC‐SSc) and 82 patients with PBC alone matched by serum bilirubin at referral to the Royal Free Hospital (time of transplant censored). Number of patients at risk is shown for 0, 5, 10, and 15 years of follow up.
The lower rate of liver transplantation in the PBC‐SSc cohort reflects a real reduced need for transplantation, as all patients considered for liver transplantation went on to have one. Among transplanted patients, five of seven with PBC‐SSc and 11 of 21 with PBC alone had received UDCA.
The Mayo and Royal Free prognostic scores predicted average survival fairly well in the two cohorts: Mayo predicted 96% at two years and 84% at five years in PBC‐SSc (observed survival 95% and 92% at two and five years, respectively) and 98% at two years and 96% at five years in PBC (observed survival 99% and 96% at two and five years, respectively). Royal Free prognostic score predicted 97% (PBC‐SSc) and 98% (PBC) survival at two years.
Patients with PBC and ACA positivity
Clinical characteristics for the 13 patients with PBC and positive ACA but without clinical features of SSc are shown in table 3. These were similar to our study cohort. No patient died at two years and there was 89% survival at five years. Three (23%) died during follow up (two liver failure, one unknown) and five (38%) underwent liver transplantation (four complications of liver disease and one pruritus). Median follow up was 5.3 years (range 0.3–15.4). The advent of de novo complications was: three (23%) pruritus, four (31%) fatigue, two (15%) ankle oedema, three (23%) ascites, three (23%) oesophageal varices, two (15%) variceal bleeding, two (15%) non‐variceal gastrointestinal bleeding, and one (8%) septicaemia; none developed SBP, PSE, or HCC.
Discussion
This is the first study that has specifically addressed the prognosis of PBC associated with SSc compared with PBC alone, using serum bilirubin, the most important prognostic marker, to match patients.
In our PBC database 7.4% had SSc, similar to the 8% observed in a study reporting on the prevalence of autoimmune conditions in unselected PBC patients,21 suggesting we did not have selection bias, despite being a tertiary referral centre for both diseases. Furthermore, most of our patients (93%) suffered from the lcSSc subtype, again as previously reported.12,19,21 The diagnosis of PBC occurred after SSc in 56% of cases, similar to the only other study where this was evaluated.12
In the PBC‐SSc group, the diagnosis of PBC occurred at a slightly lower age than in patients with PBC alone: this difference was probably due to the effect of lead time bias (that is, screening for PBC in SSc patients and thus early diagnosis of asymptomatic PBC—56% presented with SSc alone). Median age at diagnosis of PBC, made after SSc diagnosis, was lower (46.1 years) than PBC diagnosed before SSc (51.1 years) and lower than diagnosis in PBC alone (53.2 years). The difference in age is taken into account by both Cox modelling and the comparison with the Mayo Clinic41 and Royal Free prognostic42 scores, both of which have age as an independent prognostic variable.
Despite a less rapid increase in serum bilirubin concentration, PBC‐SSc patients had a higher incidence of first episode of SBP and septicaemia during follow up. This could be related to an increased risk of infections due to immune abnormalities and organ system manifestations associated with SSc.45
Similar proportions of patients in both groups commenced UDCA treatment, with a similar median dose and median interval from diagnosis. Although UDCA significantly reduced serum bilirubin concentration, whether or not it affects the prognosis of PBC is controversial.46,47 The proportion of patients with serum bilirubin concentrations ⩽17 μmol/l at last follow up was similar in both cohorts taking UDCA.
Patients with PBC alone had a higher rate of liver transplantation, reflecting the more rapid worsening of bilirubin. Median index of bilirubin change was at least fivefold higher in PBC alone, whether calculated from time of diagnosis or from referral to Royal Free Hospital. This more rapid progression of PBC is paralleled by the greater liver related mortality in PBC alone (67% deaths due to liver disease) whereas in the PBC‐SSc group 67% of deaths were related to SSc (22% of deaths due to liver causes). However, the improvement in liver related survival in the PBC‐SSc cohort was outweighed by an increase in non‐liver deaths, due to SSc, and thus overall survival was not different. These data emphasize the importance of comorbidity in PBC. Prince and colleagues38 observed an increase in non‐hepatic deaths in asymptomatic PBC, even with a reduced liver related mortality, in comparison with symptomatic PBC. Mayo and Royal Free Hospital scores resulted in good prediction for average survival at two years in both the PBC‐SSc and PBC alone groups. However, in PBC‐SSc patients the causes of death were mainly due to SSc and not liver disease (compared with nearly 70% for PBC alone), so that if SSc related deaths were excluded, the models overestimated mortality. Thus patients with PBC and associated autoimmune diseases may need different prognostic models in order to better predict their liver related survival.
With SSc, a possible interaction between fibrosing alveolitis associated with SSc, with a reduced mortality of fibrosing alveolitis compared with idiopathic fibrosing alveolitis alone, has been reported.48,49,50 Patients with polymyositis‐SSc overlap may also have a less severe course than polymyositis alone, responding to smaller doses of steroids and requiring less intensive therapy.51,52
Although lead time bias may explain the differences found, in our cohorts matching was designed to minimise this. Moreover, the proportion of patients without liver related symptoms was neither significantly different at diagnosis of PBC nor at referral in both groups. This similarity, and the similarity in serum bilirubin at both referral (matching time point) and diagnosis, gives internal validity to our matched comparison. Moreover, there were no significant differences at either diagnosis or referral for both serum albumin, a well documented independent prognostic variable in PBC,41,42 and alkaline phosphatase, also an independent prognostic variable, but in early PBC.38 Thus protective effects from treatment of SSc or alternative pathogenetic mechanisms may be responsible for the differences found between the two groups. However, only the latter could be a more likely hypothesis as only 10 PBC‐SSc patients were treated with corticosteroid therapy and there were no clear differences between patients receiving or not receiving immunosuppression.
SSc related mortality in our PBC‐SSc patients was not surprising, as SSc has an increased mortality, up to fourfold with respect to the general population.35,37,53 In a UK study, 79% of these excess deaths were related to SSc (for example, multiorgan involvement) and half of these secondary to lung disease.36 Other increased mortality is due to excess infection, malignancy, and cardiovascular disease.37
The group with PBC and ACA positivity but without clinical features of SSc at the last follow up had the same rate (67%) of liver related deaths as those with PBC alone but ACA negativity, behaving as if PBC was the major determinant of outcome. Although ACA positivity is not pathognomic of SSc, it is associated with an increased risk of developing connective tissue disease.54 A review article55 reported a sensitivity of 32% (17–56%) for SSc, 57% (32–96%) for lcSSc, and specificity of at least 93% while ACA positivity was present in 5% with other connective tissue disease and fewer than 1% of disease free controls. Therefore, we suggest assessment of PBC patients should always include screening for SSc related symptoms, such as Raynaud's phenomenon. The use of nailfold capillaroscopy in patients suspected of having connective tissue disease may be a useful indicator.56
In conclusion, patients with PBC and SSc had a lower rate of liver transplantation and a lower rate of liver related deaths, with a slower rate of bilirubin increase with respect to patients with PBC alone. However, these differences were not due to earlier SSc related deaths. The reasons for the different progression are not known but one could argue that some immunological interactions in the presence of an additional autoimmune disorder may influence the clinical picture and favour a better outcome of the liver disease.31 It would be useful if our data are confirmed in a larger group of patients in a multicentre study.
Prognostic models for PBC alone may not be applicable for PBC associated with SSc or for other associated autoimmune diseases, to assess the risk of liver related mortality and the need for liver transplantation. New models may need to be developed for these groups of patients.
Abbreviations
ACA - anticentromere antibody
AMA - antimitochondrial antibody
ANA - antinuclear antibody
CREST - calcinosis, Raynaud's phenomenon, oesophageal dysfunction, sclerodactyly, telangiectasia
dcSSc - diffuse cutaneous systemic sclerosis
HCC - hepatocellular carcinoma
lcSSc - limited cutaneous systemic sclerosis
nRNP - nuclear ribonucleoprotein
PBC - primary biliary cirrhosis
PSE - portosystemic encephalopathy
SBP - spontaneous bacterial peritonitis
Scl70 - antitopoisomerase I
SSc - systemic sclerosis
UDCA - ursodeoxycholic acid
Footnotes
Conflict of interest: None declared.
References
- 1.LeRoy E C, Black C, Fleischmajer R.et al Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 198815202–205. [PubMed] [Google Scholar]
- 2.McCarty G A, Rice J R, Bembe M L.et al Anticentromere antibody. Clinical correlations and association with favorable prognosis in patients with scleroderma variants. Arthritis Rheum 1983261–7. [DOI] [PubMed] [Google Scholar]
- 3.Steen V D, Ziegler G L, Rodnan G P.et al Clinical and laboratory associations of anticentromere antibody in patients with progressive systemic sclerosis. Arthritis Rheum 198427125–131. [DOI] [PubMed] [Google Scholar]
- 4.Steen V D, Powell D L, Medsger T A., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum 198831196–203. [DOI] [PubMed] [Google Scholar]
- 5.Bunn C C, Denton C P, Shi‐Wen X.et al Anti‐RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol 19983715–20. [DOI] [PubMed] [Google Scholar]
- 6.Murray‐Lyon I M, Thompson R P, Ansell I D.et al Scleroderma and primary biliary cirrhosis. BMJ 19701258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds T B, Denison E K, Frankl H D.et al Primary biliary cirrhosis with scleroderma, Raynaud's phenomenon and telangiectasia. New syndrome. Am J Med 197150302–312. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien S T, Eddy W M, Krawitt E L. Primary biliary cirrhosis associated with scleroderma. Gastroenterology 197262118–121. [PubMed] [Google Scholar]
- 9.Sherlock S, Scheuer P J. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med 1973289674–678. [DOI] [PubMed] [Google Scholar]
- 10.Geffroy Y, Colin R, Hemet J.et al Primary biliary cirrhosis and scleroderma. Med Chir Dig 19732281–286. [PubMed] [Google Scholar]
- 11.Clarke A K, Galbraith R M, Hamilton E B.et al Rheumatic disorders in primary biliary cirrhosis. Ann Rheum Dis 19783742–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell F C, Schroeter A L, Dickson E R. Primary biliary cirrhosis and the CREST syndrome: a report of 22 cases. Q J Med 19876275–82. [PubMed] [Google Scholar]
- 13.Maekawa S, Yano E, Shintani S. A case of rheumatoid arthritis associated with progressive systemic sclerosis and primary biliary cirrhosis in the presence of various autoantibodies. Ryumachi 199232515–521. [PubMed] [Google Scholar]
- 14.Ueno Y, Shibata M, Onozuka Y. Association of extrahepatic autoimmune diseases in primary biliary cirrhosis—clinical statistics and analyses of Japanese and non‐Japanese cases. Nippon Rinsho 1998562687–2698. [PubMed] [Google Scholar]
- 15.Akimoto S, Ishikawa O, Takagi H.et al Immunological features of patients with primary biliary cirrhosis (PBC) overlapping systemic sclerosis: a comparison with patients with PBC alone. J Gastroenterol Hepatol 199813897–901. [DOI] [PubMed] [Google Scholar]
- 16.Goring H D, Panzner M, Lakotta W.et al Coincidence of scleroderma and primary biliary cirrhosis. Results of a systematic study of a dermatologic patient sample. Hautarzt 199849361–366. [DOI] [PubMed] [Google Scholar]
- 17.Akimoto S, Ishikawa O, Muro Y.et al Clinical and immunological characterization of patients with systemic sclerosis overlapping primary biliary cirrhosis: a comparison with patients with systemic sclerosis alone. J Dermatol 19992618–22. [DOI] [PubMed] [Google Scholar]
- 18.Brzezinska‐Kolarz B, Undas A, Dyczek A.et al Reynolds syndrome: the combination of scleroderma and primary biliary cirrhosis. Pol Arch Med Wewn 2001105231–234. [PubMed] [Google Scholar]
- 19.Marasini B, Gagetta M, Rossi V.et al Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis 2001601046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadie V, Wohlrab J, Marsch W C. Reynolds syndrome—a rare combination of 2 autoimmune diseases. Med Klin (Munich) 20029740–43. [DOI] [PubMed] [Google Scholar]
- 21.Watt F E, James O F, Jones D E. Patterns of autoimmunity in primary biliary cirrhosis patients and their families: a population‐based cohort study. Q J Med 200497397–406. [DOI] [PubMed] [Google Scholar]
- 22.Culp K S, Fleming C R, Duffy J.et al Autoimmune associations in primary biliary cirrhosis. Mayo Clin Proc 198257365–370. [PubMed] [Google Scholar]
- 23.Modena V, Marengo C, Amoroso A.et al Primary biliary cirrhosis and rheumatic diseases: a clinical, immunological and immunogenetical study. Clin Exp Rheumatol 19864129–134. [PubMed] [Google Scholar]
- 24.Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann Rheum Dis 200463123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein R M, Callender M E, Neuberger J M.et al Anticentromere antibody in primary biliary cirrhosis. Ann Rheum Dis 198241612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell F C, Winkelmann R K, Venencie‐Lemarchand F.et al The anticentromere antibody: disease specificity and clinical significance. Mayo Clin Proc 198459700–706. [DOI] [PubMed] [Google Scholar]
- 27.Hansen B U, Eriksson S, Lindgren S. High prevalence of autoimmune liver disease in patients with multiple nuclear dot, anti‐centromere, and mitotic spindle antibodies. Scand J Gastroenterol 199126707–713. [DOI] [PubMed] [Google Scholar]
- 28.Chan H L, Lee Y S, Hong H S.et al Anticentromere antibodies (ACA): clinical distribution and disease specificity. Clin Exp Dermatol 199419298–302. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R C, Seibold J R, Krishnan M R.et al Precipitating autoantibodies to mitochondrial proteins in progressive systemic sclerosis. Clin Exp Immunol 19845868–76. [PMC free article] [PubMed] [Google Scholar]
- 30.Chou M J, Lai M Y, Lee S L.et al Reactivity of anti‐mitochondrial antibodies in primary biliary cirrhosis and systemic sclerosis. J Formos Med Assoc 1992911075–1080. [PubMed] [Google Scholar]
- 31.Whyte J, Hough D, Maddison P J.et al The association of primary biliary cirrhosis and systemic sclerosis is not accounted for by cross reactivity between mitochondrial and centromere antigens. J Autoimmun 19947413–424. [DOI] [PubMed] [Google Scholar]
- 32.Mayo M J, Jenkins R N, Combes B.et al Association of clonally expanded T cells with the syndrome of primary biliary cirrhosis and limited scleroderma. Hepatology 1999291635–1642. [DOI] [PubMed] [Google Scholar]
- 33.Mitchison H C, Lucey M R, Kelly P J.et al Symptom development and prognosis in primary biliary cirrhosis: a study in two centers. Gastroenterology 199099778–784. [DOI] [PubMed] [Google Scholar]
- 34.Mahl T C, Shockcor W, Boyer J L. Primary biliary cirrhosis: survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol 199420707–713. [DOI] [PubMed] [Google Scholar]
- 35.Abu‐Shakra M, Lee P. Mortality in systemic sclerosis: a comparison with the general population. J Rheumatol 1995222100–2102. [PubMed] [Google Scholar]
- 36.Bryan C, Howard Y, Brennan P.et al Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. Br J Rheumatol 1996351122–1126. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen S, Halberg P, Ullman S. Mortality and causes of death of 344 Danish patients with systemic sclerosis (scleroderma). Br J Rheumatol 199837750–755. [DOI] [PubMed] [Google Scholar]
- 38.Prince M I, Chetwynd A, Craig W L.et al Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 200453865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollak C, Minar E, Dragosics B.et al Primary biliary cirrhosis and scleroderma: long‐term benign course of a complex autoimmune disease. Leber Magen Darm 19851585–89. [PubMed] [Google Scholar]
- 40.Beswick D R, Klatskin G, Boyer J L. Asymptomatic primary biliary cirrhosis. A progress report on long‐term follow‐up and natural history. Gastroenterology 198589267–271. [PubMed] [Google Scholar]
- 41.Dickson E R, Grambsch P M, Fleming T R.et al Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989101–7. [DOI] [PubMed] [Google Scholar]
- 42.Hughes M D, Raskino C L, Pocock S J.et al Prediction of short‐term survival with an application in primary biliary cirrhosis. Stat Med 1992111731–1745. [DOI] [PubMed] [Google Scholar]
- 43. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 198023581–590. [DOI] [PubMed] [Google Scholar]
- 44.Scheuer P. Primary biliary cirrhosis. Proc R Soc Med 1967601257–1260. [PMC free article] [PubMed] [Google Scholar]
- 45.Juarez M, Misischia R, Alarcon G S. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Rheum Dis Clin North Am 200329163–184. [DOI] [PubMed] [Google Scholar]
- 46.Goulis J, Leandro G, Burroughs A K. Randomised controlled trials of ursodeoxycholic‐acid therapy for primary biliary cirrhosis: a meta‐analysis. Lancet 19993541053–1060. [DOI] [PubMed] [Google Scholar]
- 47.Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. Oxford: Update Software, 2002;CD000551, [DOI] [PubMed]
- 48.Wells A U, Cullinan P, Hansell D M.et al Fibrosing alveolitis associated with systemic sclerosis has a better prognosis than lone cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 19941491583–1590. [DOI] [PubMed] [Google Scholar]
- 49.Daniil Z D, Gilchrist F C, Nicholson A G.et al A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999160899–905. [DOI] [PubMed] [Google Scholar]
- 50.Bouros D, Wells A U, Nicholson A G.et al Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 20021651581–1586. [DOI] [PubMed] [Google Scholar]
- 51.Jablonska S, Chorzelski T P, Blaszczyk M.et al Scleroderma/polymyositis overlap syndromes and their immunologic markers. Clin Dermatol 199210457–472. [DOI] [PubMed] [Google Scholar]
- 52.Marguerie C, Bunn C C, Copier J.et al The clinical and immunogenetic features of patients with autoantibodies to the nucleolar antigen PM‐Scl. Medicine (Baltimore) 199271327–336. [DOI] [PubMed] [Google Scholar]
- 53.Geirsson A J, Wollheim F A, Akesson A. Disease severity of 100 patients with systemic sclerosis over a period of 14 years: using a modified Medsger scale. Ann Rheum Dis 2001601117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caramaschi P, Biasi D, Manzo T.et al Anticentromere antibody—clinical associations. A study of 44 patients. Rheumatol Int 199514253–255. [DOI] [PubMed] [Google Scholar]
- 55.Spencer‐Green G, Alter D, Welch H G. Test performance in systemic sclerosis: anti‐centromere and anti‐Scl‐70 antibodies. Am J Med 1997103242–248. [DOI] [PubMed] [Google Scholar]
- 56.Fonollosa V, Simeon C P, Castells L.et al Morphologic capillary changes and manifestations of connective tissue diseases in patients with primary biliary cirrhosis. Lupus 200110628–631. [DOI] [PubMed] [Google Scholar]