Abstract
Background and aims
Total enteral nutrition (TEN) with a liquid formula can suppress gut inflammation and induce remission in active Crohn's disease. The mechanism is obscure. Studies have suggested that long term nutritional supplementation with a liquid formula (partial enteral nutrition (PEN)) may also suppress inflammation and prevent relapse. The aim of this study was to compare PEN with conventional TEN in active Crohn's disease.
Patients and methods
Fifty children with a paediatric Crohn's disease activity index (PCDAI) >20 were randomly assigned to receive 50% (PEN) or 100% (TEN) of their energy requirement as elemental formula for six weeks. The PEN group was encouraged to eat an unrestricted diet while those receiving TEN were not allowed to eat. The primary outcome was achievement of remission (PCDAI <10). Secondary analyses of changes in erythrocyte sedimentation rate (ESR), C reactive protein, albumin, and platelets were performed to look for evidence of anti‐inflammatory effects.
Results
Remission rate with PEN was lower than with TEN (15% v 42%; p = 0.035). Although PCDAI fell in both groups (p = 0.001 for both), the reduction was greater with TEN (p = 0.005). Moreover, the fall in PCDAI with PEN was due to symptomatic and nutritional benefits. With both treatments there were significant improvements in relation to abdominal pain, “sense of wellbeing”, and nutritional status. However, only TEN led to a reduction in diarrhoea (p = 0.02), an increase in haemoglobin and albumin, and a fall in platelets and ESR.
Conclusions
TEN suppresses inflammation in active Crohn's disease but PEN does not. This suggests that long term nutritional supplementation, although beneficial to some patients, is unlikely to suppress inflammation and so prevent disease relapse.
Keywords: Crohn's disease, enteral nutrition, elemental diet, children
Active Crohn's disease can be effectively treated by providing all of the patient's nutritional requirements in the form of a nutritionally balanced liquid formula.1,2 Such total enteral nutrition (TEN) with liquid formula has been widely used but its value as a primary treatment is debated. Corticosteroid therapy appears to be more effective in inducing remission.3,4,5,6 A significant disadvantage with TEN is its non‐acceptability to some patients. It requires consumption of a large volume of formula each day for about six weeks, and the patient is not allowed to eat throughout that time. Consequently, some patients are unable or unwilling to tolerate it. This is a major reason for treatment failure. Despite this, TEN induces remission in about 60% of patients, and it avoids the risks of corticosteroid toxicity.3,4,5 It is frequently used in children with Crohn's disease because excessive corticosteroid usage can suppress growth. Growth impairment is very common in paediatric Crohn's disease, even without corticosteroid usage.7,8
TEN does not merely alleviate symptoms and enhance the patient's nutritional status. It is clear that it actually suppresses intestinal inflammation.9,10,11 The mechanism of action is unknown. Originally it was thought that a reduction in dietary antigen exposure might be responsible, most patients being treated with an “elemental diet” containing amino acids or oligopeptides as the nitrogen source. More recently, however, studies have shown that whole protein (polymeric) formulas are also effective.12,13,14 The rationale for requiring that patients completely desist from eating normal foods during the treatment period can therefore be questioned. There are no randomised trials that have addressed this important issue. Partial enteral nutrition (PEN) with a liquid formula with continued eating of normal foods would be more acceptable to patients, and might be an effective treatment for active disease. Moreover, there are studies suggesting that such nutritional supplementation may prolong remission in patients with quiescent Crohn's disease.15,16,17,18 If PEN could be shown to suppress inflammation in active Crohn's disease, this would also lend support to its proposed use in maintaining remission.
The aim of this study was to compare PEN with conventional TEN for induction of remission in children with active Crohn's disease. Evidence was sought for an anti‐inflammatory response to these treatments.
Patients and methods
Subjects
Patients were recruited from three tertiary paediatric gastroenterology centres in the UK over an 18 month period. Children with active Crohn's disease involving the small bowel and/or colon were eligible for participation. The diagnosis of Crohn's disease was based on conventional criteria, including clinical, radiological, endoscopic, and histological findings. All had moderate to severe disease activity, with a paediatric Crohn's disease activity index (PCDAI) >20, justifying the use of TEN.19 Patients with newly diagnosed disease and those with established disease but stable medical treatment were eligible.
Treatment allocation
Following an initial assessment (see below) children were randomly assigned to one of two treatment groups. Randomisation was by numbered sealed envelope, concealment being maintained until after assignment. Randomisation was stratified for each of the three centres. The allocation sequence was generated by an external source (Research Division, SHS International Ltd, Liverpool, UK). Participants were assigned to their groups by the lead dietician.
The PEN group were to receive a liquid formula to provide 50% of their daily estimated average requirement (EAR) for energy for a treatment period of six weeks. In addition, they were encouraged to eat an unrestricted normal diet.
The conventional TEN group were to receive sufficient liquid formula to provide at least 100% of their daily EAR for energy. If necessary, the volume given was increased above EAR to alleviate hunger and to promote adequate weight gain. The amount of formula was increased stepwise, the final volume being achieved within a week. Again, the duration of treatment was six weeks. Children in this group were not allowed to eat normal foods during the treatment period. As is usual with TEN, they were allowed small amounts of fat free and protein free drinks and boiled sweets.
The liquid formula used for both groups was Elemental O28 Extra (SHS International Ltd) (table 1). This is a nutritionally complete formula based on amino acids, glucose polymer, and blended vegetable oil (35% medium chain triglycerides). The formula was constituted by dissolving a powder in water to a final concentration of 0.85–1.2 kcal/ml. If desired, it could be flavoured or chilled to enhance palatability. If necessary it could be administered by nasogastric tube infusion.
Table 1 Composition of Elemental O28 Extra.
| Presentation | 100 g sachets of powder (flavoured or unflavoured) diluted to 20–27.5 g /100 ml |
|---|---|
| Nutrient source | |
| Nitrogen | Amino acids |
| Carbohydrate | Glucose syrup |
| Fat | Blended vegetable oil (35% MCT) |
| Composition per 100 g | 443 (flavoured 427) |
| Energy (kcal) | 12.5 |
| Protein (g) | 59.0 (flavoured 55) |
| Carbohydrate (g) | 17.5 |
| Fat (g) | |
| Linoleic acid (% energy) | 4 |
| Alpha linolenic (% energy) | 1 |
| Omega‐6:omega‐3 fatty acid ratio | 4:1 |
| Vitamin A (μg) | 330 |
| Vitamin D(μg) | 2.5 |
| Vitamin E (mg) | 6.1 |
| Vitamin C (mg) | 28.3 |
| Vitamin K (μg) | 25.0 |
| Thiamin (mg) | 0.6 |
| Riboflavin (mg) | 0.6 |
| Niacin (mg) | 4.2 |
| Vitamin B6 (mg) | 0.8 |
| Folic acid (μg) | 83.3 |
| Vitamin B12 (μg) | 1.7 |
| Biotin (μg) | 18.0 |
| Sodium (mg) | 305.0 |
| Potassium (mg) | 466.0 |
| Calcium (mg) | 245.0 |
| Phosphorus (mg) | 200.0 |
| Magnesium (mg) | 81.6 |
| Iron (mg) | 4.2 |
| Zinc (mg) | 4.2 |
| Copper (mg) | 0.4 |
| Manganese (mg) | 0.6 |
| Iodine (μg) | 33.3 |
Patient assessment and monitoring
Each child was formally assessed at the time of recruitment and after six weeks of treatment, or sooner if withdrawn early from the allocated treatment. In addition to clinical assessment, anthropometric measurements (weight, height, mid‐upper arm circumference, triceps, and subscapular skin fold thickness) were obtained. PCDAI was determined. Laboratory blood investigations included full blood count and platelets, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and serum albumin.
A paediatric dietician maintained regular contact with the subjects throughout the study period in order to provide advice and support with adhering to the allocated treatment regimen. Three day prospective food diaries were maintained at three weeks and six weeks to record the amount of formula being taken, and the amount and composition of food being eaten. Food intake was measured using a three day weighed food intake record. All food items were weighed to the nearest 2 g using electronic digital scales. Macronutrient and micronutrient intakes were determined using Microdiet Software based on McCance and Widdowson's “The composition of foods” version 5 (Downlee Systems Ltd, UK), with supplementary data from food manufacturers.
Patients were withdrawn from their allocated treatment if there were persistent severe symptoms, if they had not experienced a marked improvement in symptoms after three weeks, or if the patient and/or family asked to withdraw.
Power calculation and data analysis
The primary end point was the proportion achieving remission with the assigned treatment. Remission was defined as a PCDAI <10 at the time of finishing PEN or TEN. Assuming a 65% remission rate with TEN, it was calculated that recruitment of a total of 50 patients would provide 90% power (p<0.05, two tailed test) to detect a 25% difference in remission rate between the treatments. Secondary analyses were performed on changes in anthropometry, PCDAI subscores, and blood indices to explore the possible mechanisms of action of the two treatments. No end point analyses were performed other than those presented. All outcome analysis was based on “intention to treat”. Statistical comparisons were made using paired or unpaired t tests, the Mann Whitney rank sum test, or the signed rank sum test as appropriate. Categorical comparisons were made using the χ2 test.
Results
Comparison of groups at enrolment
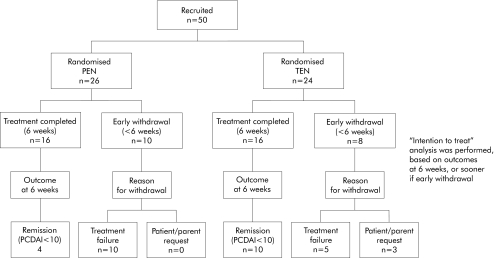
A total of 50 children were recruited, with 26 randomised to PEN and 24 to TEN (fig 1). The two groups were similar at the time of enrolment (table 2). There was no significant difference between the groups in terms of PCDAI, weight/height standard deviation score (SDS), or disease distribution (small bowel, colon).
Figure 1 Flow diagram showing progression of subjects through the trial stages. PEN, partial enteral nutrition; TEN, total enteral nutrition; PCDAI, paediatric Crohn's disease activity index.
Table 2 Patient characteristics at enrolment.
| PEN (n = 26) | TEN (n = 24) | |
|---|---|---|
| Age (y)* | 12.9 (6.8–15.9) | 12.0 (3.8–16.0) |
| Sex (% males) | 50% | 66% |
| Newly diagnosed | 80% | 66% |
| PCDAI* | 40 (22.5–70) | 42.5 (22.5–72.5) |
| Weight/height SDS* | −0.97 (−2.01 to +2.5) | −0.5 (−2.3 to +1.3) |
| Disease location (% subjects) | ||
| Small bowel | 15% | 17% |
| Colon | 12% | 17% |
| Small bowel and colon | 73% | 66% |
| Laboratory indices* | 10.7 (8.7–13.3) | 10.6 (7.7–14.1) |
| Haemoglobin (g/dl) | ||
| Platelets (×109/l) | 495 (166–952) | 522 (263–1329) |
| Albumin (g/l) | 32 (17–45) | 32 (16–46) |
| ESR (mm/hr) | 52 (5–141) | 53 (10–115) |
| CRP (mg/l) | 30 (0–158) | 23 (0–138) |
PEN, partial enteral nutrition; TEN, total enteral nutrition; PCDAI, paediatric Crohn's disease activity index; SDS, standard deviation score; ESR, erythrocyte sedimentation rate; CRP, C reactive protein.
*Values are median (range).
Comparison of nutrition intake in the groups
Dietary energy intake in the two groups based on food diaries completed during the trial is summarised in table 3. Median intake in the PEN group was 126% of EAR and in the TEN group 111% of EAR. The PEN group achieved the target of consuming at least 50% of EAR for energy from normal food. Their overall energy intake was therefore slightly greater than the TEN group. In the PEN group, 47% of energy was provided as liquid formula (range 39–58%). In the TEN group, median total energy intake as liquid formula was 98% (range 89–100%) with just 2% (range 0–11%) from other permitted foods and drinks.
Table 3 Comparison of energy intakes in the study groups.
| PEN | TEN | |
|---|---|---|
| Total energy intake (kcal/day) | 2380 (1890–3165) | 2190 (1660–3415) |
| Total energy intake as proportion of EAR | 126% (96–142) | 111% (93–153) |
| Proportion of total energy intake as formula | 47% (39–58) | 98% (89–100) |
| Energy intake from formula as proportion of EAR | 51% (48–69) | 111% (93–146) |
PEN, partial enteral nutrition; TEN, total enteral nutrition; EAR, estimated average requirement.
Results are expressed as median (range). Values are based on the three day food diary completed at three and six weeks; those withdrawing from the study were included only if enteral nutrition continued for at least three weeks.
Protein, carbohydrate, and fat intake as a proportion of total energy were very similar in both groups (table 4). Both received a comparable proportion of their energy as fat—37% in the PEN group and 35% in the TEN group. Children in the TEN group received 1% of total energy intake from omega‐3 fatty acids with an omega‐6/omega‐3 ratio of 4 to 1. The omega‐3 fatty acid content in Elemental 028 Extra is greater than that present in a typical child's diet, and so the omega‐6/omega‐3 ratio would have been higher in the PEN group. In both groups intake of micronutrients met or exceeded the recommended nutrient intake (table 4).
Table 4 Comparison of macronutrient and micronutrient intakes in the study groups.
| PEN | TEN | |
|---|---|---|
| Macronutrient (% energy) | ||
| Protein | 12% | 11% |
| Fat | 37% | 35% |
| Carbohydrate | 51% | 54% |
| Micronutrients (% RNI) | ||
| Iron | 163% | 212% |
| Calcium | 131% | 130% |
| Folate | 180% | 254% |
| Zinc | 189% | 272% |
| Vitamin B1 | 273% | 323% |
| Vitamin B2 | 235% | 285% |
| Vitamin C | 356% | 451% |
| Vitamin A | 215% | 314% |
| Vitamin E | 289% | 511% |
| Vitamin B12 | 606% | 841% |
PEN, partial enteral nutrition; TEN, total enteral nutrition; RNI, recommended nutrient intake.
Withdrawal from the allocated treatment before completion of the intended six week period was necessary in 10/26 (39%) in the PEN group and in 8/24 (33%) in the TEN group (fig 1). In three patients, all on TEN, withdrawal was at the request of patient and parent. In the remaining 15 withdrawals this was necessary because the allocated nutritional treatment failed. All patients withdrawn from nutritional therapy received a course of corticosteroid therapy and entered remission.
PCDAI response to the interventions
Based on intention to treat analysis, remission (PCDAI <10) was achieved in only 15% (4/26) of the PEN group compared with 42% (10/24) of the TEN group (p = 0.035) (fig 1). In both groups there was a significant reduction in PCDAI at the time of finishing PEN or TEN (p<0.001 for each) but the mean reduction with TEN was greater than with PEN (−26 v −13; p = 0.005) (table 5).
Table 5 Changes in paediatric Crohn's disease activity index (PCDAI) score and in symptom subscores.
| PEN mean (95% CI) | TEN mean (95% CI) | Group comparison | |
|---|---|---|---|
| PCDAI score | −13 (−7 to −19) p = 0.001 | −26 (−19 to −33) p = 0.001 | p = 0.005 |
| Abdominal pain subscore | −2 (−0.2 to −3.8) p = 0.02 | −4 (−2.4 to −5.6) p = 0.001 | NS |
| “Wellbeing” subscore | −3 (−1 to −5) p = 0.002 | −5.7 (−4 to −7.4) p = 0.001 | NS |
| Diarrhoea subscore | −0.7 (−1.7 to +3) NS | −3 (−1.4 to −4.6) p = 0.02 | p = 0.04 |
PEN, partial enteral nutrition; TEN, total enteral nutrition; 95% CI, 95% confidence intervals.
Analysis of specific changes
To explore the factors responsible for reductions in PCDAI, changes in individual PCDAI clinical symptom parameters in the two groups were examined (table 5). Analysis of the PCDAI symptom subscores showed a significant reduction in abdominal pain and an improvement in “wellbeing” in both groups, with no significant difference between the groups. Only the TEN group had a significant reduction in diarrhoea.
The anthropometric changes in the groups were compared (table 6). In both groups there was a significant increase in weight, weight for height SDS, and subscapular skin fold thickness. Those in the PEN group had a significant increase in triceps skin fold thickness while those in the TEN group had a significant increase in mid‐upper arm circumference. There were no significant differences between the groups in relation to any of these parameters.
Table 6 Change in nutritional parameters.
| PEN mean (95%CI) | TEN mean | Group comparison | |
|---|---|---|---|
| Weight (kg) | +3.0 (+2 to +4) p = 0.001 | +3.6 (+2.5 to +4.7) p = 0.001 | NS |
| Weight/height SDS | +0.53 (+0.28 to +0.78) p<0.001 | +0.7 (+0.5 to +0.9) p<0.001 | NS |
| Mid‐upper arm circumference (mm) | +9 (0 to +18) NS | +15 (+8 to +22) p<0.001 | NS |
| Triceps skin fold (mm) | +1.3 (+0.3 to +2.3) p = 0.014 | +1.3 (−0.2 to +2.8) NS | NS |
| Subscapular skin fold (mm) | +1.2 (0 to +2.4) p = 0.04 | +1.3 (+0.6 to +2) p = 0.001 | NS |
PEN, partial enteral nutrition; TEN, total enteral nutrition; SDS, standard deviation score; 95% CI, 95% confidence intervals.
The various laboratory indices were compared as objective indicators of disease activity (table 7). In the PEN group there were no significant changes in haemoglobin, platelet count, ESR, CRP, or albumin. By comparison, the TEN group experienced a significant rise in haemoglobin and albumin and a significant reduction in platelet count and ESR.
Table 7 Changes in laboratory blood indices.
| PEN mean (95%CI) | TEN mean (95% CI) | Group comparison | |
|---|---|---|---|
| Haemoglobin (g/dl ) | −0.3 (−6 to 0) p = 0.056 | +0.8 (+0.3 to +1.3) p = 0.011 | p<0.001 |
| Platelets (×109/l) | −26 (−81 to +29) p = 0.39 | −146 (−210 to −82) p<0.001 | p = 0.006 |
| ESR ( mm/h) | −4 (−17 to +8) p = 0.46 | −26 (−42 to −10) p = 0.004 | p = 0.003 |
| CRP (mg/l ) | −6 (−26 to +14) p = 0.55 | −13 (−47 to +21) p = 0.06 | p = 0.36 |
| Albumin (g/l ) | +1.7 (−0.1 to +3.5) p = 0.054 | +5.0 (+3 to +7) p<0.001 | p = 0.019 |
PEN, partial enteral nutrition; TEN, total enteral nutrition; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; 95% CI, 95% confidence intervals.
Discussion
In this study, conventional TEN was associated with a significantly higher remission rate than PEN (42% v 15%). This difference was seen despite a relatively low remission rate with TEN. Most published studies have reported remission rates in the order of 60–70% with TEN.5 This may be explained by various factors, such as patient inclusion criteria and the relatively stringent definition of remission (PCDAI <10) employed in this study compared with others.14,20 Both TEN and PEN led to a reduction in PCDAI, although the reduction with TEN was significantly greater. Moreover, the reduction with PEN was explicable on the basis of weight gain, reduction in abdominal pain, and improved “wellbeing”. These effects, though beneficial, are not necessarily indicative of an anti‐inflammatory action in the intestine. TEN led to a significant reduction in diarrhoea, while PEN did not. Moreover, TEN was associated with a significant rise in haemoglobin and serum albumin concentration, and reduction in platelet count and ESR, but no such changes occurred with PEN.
The effectiveness of TEN in patients with active Crohn's disease was a fortuitous discovery. In the 1970s malnourished patients with poorly controlled Crohn's disease were treated with an elemental (amino acid based) formula and it was noted that some experienced disease remission.21 Consequently, for many years patients with Crohn's disease were treated with amino acid or oligopeptide based formulas, on the assumption that the therapeutic effect depended on the absence of whole protein in the diet. It was believed that dietary antigen stimulation might have had a role in promoting the inflammatory process. More recently, however, studies have shown that polymeric whole protein formulas are also effective.6,12,13,14 The possibility that active Crohn's disease might be treated using a nutritional regimen in which patients are allowed to eat had not previously been investigated in a randomised controlled trial.
An alternative to the antigen hypothesis has been the suggestion that differences in fat content or composition might be important, suppressing inflammation in the gut.22 In one randomised controlled trial there was no difference between liquid formulas of low and high fat content.23 In agreement with this, the current study used diets of similar fat content, and yet the remission rate was higher with TEN. Polyunsaturated fatty acids (PUFAs) are potent modulators of immunity and inflammation.24 In a recent double blind trial, feeds containing mainly oleic acid (a monounsaturated fatty acid) or linoleic acid (an omega‐6 PUFA) were compared in patients with active Crohn's disease.25 The hypothesis was that the oleic acid based formula should be more effective. Linoleic acid is the precursor of arachidonic acid, and leukotrienes derived from arachidonic acid have a proinflammatory effect.24 Contrary to expectations, the linoleic acid formula was associated with a higher remission rate. Omega‐3 PUFAs share a common enzyme pathway with omega‐6 PUFAs and so tend to competitively inhibit their biological actions. In the current study, compared with the PEN group the TEN group received fat with a lower omega‐6/omega‐3 PUFA ratio. As TEN proved more effective, this is not consistent with the suggestion that omega‐6 PUFAs might be responsible for the efficacy of the treatment's. The findings in this study do not, however, support or refute the possible importance of fat composition in explaining the anti‐inflammatory effects of TEN.
In the past, the effect of “bowel rest” on active Crohn's disease was studied in adult patients.26 Patients were randomised to TEN, total parenteral nutrition, or a combination of parenteral nutrition and normal food, and remission rates in each group were not significantly different (approximately 65%). Although the study was underpowered, it was concluded that “bowel rest” was not an important factor in nutritional therapy for active Crohn's disease. Our study did not examine the effect of “bowel rest” but compared PEN with TEN.
Several studies have addressed the value of long term nutritional supplementation with PEN in Crohn's disease, and specifically examined its possible impact on disease activity. In 1983 a controlled trial of cross over design examined the effects of nutritional supplementation for a two month period in children with Crohn's disease.15 This regimen was associated with nutritional benefits. It was noted, however, that serum orosomucoid levels fell significantly, and the authors suggested that supplementation might have reduced disease activity. In a retrospective study of 47 children who had responded to a course of TEN, 28 who had continued with nasogastric nutritional supplementation were compared with 19 who had not.17 It was observed that supplementation was associated with a longer time interval to relapse. In a recent trial, 39 adults with quiescent Crohn's disease were randomised to receive oral supplementation or no supplementation (controls).18 Of those assigned to supplementation, 48% remained in remission at one year compared with 22% of controls. Such reports have led to a common perception that nutritional supplementation may have an important effect in suppressing bowel inflammation and thereby preventing disease relapse. However, they have various methodological weaknesses, including retrospective design and, in the case of the clinical trials, insufficient power with subgroup post hoc analysis. Importantly, the reported efficacy of supplementation may have reflected clinical benefits unrelated to suppression of inflammation.
This study was adequately powered to compare TEN with PEN supplementation in relation to remission rate, the primary outcome measure. Supplementation, amounting to 50% of EAR, was more intensive than in previous studies. Secondary analysis of changes in clinical symptoms, anthropometry, and laboratory blood indices were performed to explore the factors responsible for the PCDAI improvement observed in both groups, and to look for objective evidence of an anti‐inflammatory effect. The findings strongly suggest that while nutritional supplementation may be of clinical benefit, it does not suppress intestinal inflammation.
There are two possible explanations for the absence of an anti‐inflammatory effect with PEN. It could be attributed to a simple “dose effect”. By reducing the total amount of liquid formula the biological effect on the inflammatory process might have been reduced. Alternatively, the anti‐inflammatory effect might have been lost due to the continued intake of normal foods. This latter explanation appears more probable. The absence of any significant improvement in blood indices with PEN argues against a “dose effect” phenomenon.
Adherence to TEN is often difficult, and the treatment may have significant psychosocial consequences for both child and family. While most children accept TEN at first presentation, some may be reluctant to receive repeated courses of treatment when relapses occur, and most eventually receive corticosteroid therapy. PEN would be more acceptable than TEN, but this study shows it is not an effective treatment for active Crohn's disease. Moreover, given that PEN does not suppress inflammation it is unlikely that it can truly prevent disease relapse.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Acknowledgements
The authors thank colleagues at Birmingham Children's Hospital, Great Ormond Street Hospital for Children, London, and Booth Hall Children's Hospital, Manchester, for their help with this study. In particular we thank Professor IW Booth, Dr S Protheroe, Professor P Milla, Dr K Lindley, and Dr A Akobeng.
Abbreviations
CRP - C reactive protein
ESR - erythrocyte sedimentation rate
PCDAI - paediatric Crohn's disease activity index
PUFA(s) - polyunsaturated fatty acid(s)
PEN - partial enteral nutrition
EAR - estimated average requirement
SDS - standard deviation score
TEN - total enteral nutrition
Footnotes
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.O'Sullivan M, O'Morain C. Nutritional treatments in inflammatory bowel disease. Curr Treat Options Gastroenterol 20014207–213. [DOI] [PubMed] [Google Scholar]
- 2.Forbes A. Review article: Crohn's disease—the role of nutritional therapy. Aliment Pharmacol Ther 200216(suppl 4)48–52. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez‐Banares F, Cabre E, Esteve‐Comas M.et al How effective is enteral nutrition in inducing clinical remission in active Crohn's disease? A meta‐analysis of the randomized clinical trials. JPEN J Parenter Enteral Nutr 199519356–364. [DOI] [PubMed] [Google Scholar]
- 4.Messori A, Trallori G, D'Albasio G.et al Defined‐formula diets versus steroids in the treatment of active Crohn's disease: a meta‐analysis. Scand J Gastroenterol 199631267–272. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths A M, Ohlsson A, Sherman P M.et al Meta‐analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology 19951081056–1067. [DOI] [PubMed] [Google Scholar]
- 6.Zachos M, Tondeur M, Griffiths A M. Enteral nutritional therapy for inducing remission of Crohn's disease. Cochrane Database Syst Rev, issue 3. Oxford: Update Software 2001; CD000542, [DOI] [PubMed]
- 7.Markowitz J, Grancher K, Rosa J.et al Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 199316373–380. [DOI] [PubMed] [Google Scholar]
- 8.Spray C, Debelle G D, Murphy M S. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr 200190400–405. [PubMed] [Google Scholar]
- 9.Teahon K, Smethurst P, Pearson M.et al The effect of elemental diet on intestinal permeability and inflammation in Crohn's disease. Gastroenterology 199110184–89. [DOI] [PubMed] [Google Scholar]
- 10.Fell J M, Paintin M, Arnaud‐Battandier F.et al Mucosal healing and a fall in mucosal pro‐inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Aliment Pharmacol Ther 200014281–289. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson I R, Boulton P, Menzies I.et al Improvement of abnormal lactulose/rhamnose permeability in active Crohn's disease of the small bowel by an elemental diet. Gut 1987281073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigaud D, Cosnes J, Le Quintrec Y.et al Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: elemental versus polymeric diet. Gut 1991321492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giaffer M H, North G, Holdsworth C D. Controlled trial of polymeric versus elemental diet in treatment of active Crohn's disease. Lancet 1990335816–819. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Brown S, Kirkwood B.et al Polymeric versus elemental diet as primary treatment in active Crohn's disease: a randomized, double‐blind trial. Am J Gastroenterol 200095735–739. [DOI] [PubMed] [Google Scholar]
- 15.Harries A D, Jones L A, Danis V.et al Controlled trial of supplemented oral nutrition in Crohn's disease. Lancet 19831887–890.6132218 [Google Scholar]
- 16.Belli D C, Seidman E, Bouthillier L.et al Chronic intermittent elemental diet improves growth failure in children with Crohn's disease. Gastroenterology 198894603–610. [DOI] [PubMed] [Google Scholar]
- 17.Wilschanski M, Sherman P, Pencharz P.et al Supplementary enteral nutrition maintains remission in paediatric Crohn's disease. Gut 199638543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Kirkwood B, Brown S.et al Oral nutritional supplementation is effective in the maintenance of remission in Crohn's disease. Dig Liver Dis. 2000 Dec 32769–774. [DOI] [PubMed] [Google Scholar]
- 19.Hyams J S, Ferry G D, Mandel F S.et al Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr 199112439–447. [PubMed] [Google Scholar]
- 20.Ludvigsson J F, Krantz M, Bodin L.et al Elemental versus polymeric enteral nutrition in paediatric Crohn's disease: a multicentre randomized controlled trial. Acta Paediatr 200493327–335. [PubMed] [Google Scholar]
- 21.Voitk A J, Echave V, Feller J H.et al Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch Surg 1973107329–333. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez‐Banares F, Cabre E, Gonzalez‐Huix F.et al Enteral nutrition as primary therapy in Crohn's disease. Gut 199435(suppl)S55–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiper K, Woolner J, Mullan M M.et al A randomised controlled trial of high versus low long chain triglyceride whole protein feed in active Crohn's disease. Gut 200149790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsella J E, Lokesh B, Broughton S.et al Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition 1990624–44. [PubMed] [Google Scholar]
- 25.Gassull M A, Fernandez‐Banares F, Cabre E.et al Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn's disease: results of a double blind randomised multicentre European trial. Gut 200251164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg G R, Fleming C R, Jeejeebhoy K N.et al Controlled trial of bowel rest and nutritional support in the management of Crohn's disease. Gut 1988291309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.