Abstract
Background
Increased serum and intrahepatic interferon γ inducible protein 10 (IP‐10) levels in patients with chronic hepatitis C (CHC) have been described.
Aim
To analyse the possible association of serum IP‐10 levels with different outcomes to antiviral therapy.
Patients
A total of 137 CHC patients treated with peginterferon plus ribavirin.
Methods
Serum IP‐10 levels were determined by enzyme linked immunosorbent assay before therapy, after 12 weeks of treatment, and 24 weeks after cessation of therapy. Variables significantly associated with a sustained virological response (SVR) on univariate analysis were included in a multivariate logistic regression model.
Results
Pretreatment serum IP‐10 levels in patients with SVR were significantly lower than in non‐responders (NR) (332.4 (222.1) v 476.8 (305.3) pg/ml, respectively; p = 0.004). Serum IP‐10 concentrations significantly decreased in patients with SVR (pretreatment: 332.4 (222.1) pg/ml; post‐treatment: 170.2 (140.1) pg/ml; p<0.001) but not in NR (pretreatment: 476.8 (305.3) pg/ml; post treatment: 387.3 (268.1) pg/ml; p = 0.06). By multivariate analysis, non‐1 genotype (odds ratio (OR) 3.5 (95% confidence interval (CI) 1.1–10.4); p = 0.003) and low viral load at baseline (OR 0.34 (95% CI 0.14–0.79); p = 0.01) were independent predictors of SVR in all patients. When multivariate analysis was restricted to patients with genotype 1, only baseline viral load (OR 0.38 (95% CI 0.155–0.96); p = 0.04) and pretreatment serum IP‐10 levels (OR 0.99 (95% CI 0.996–0.999); p = 0.03) were identified as predictive factors of SVR.
Conclusion
Pretreatment serum IP‐10 behaves as a predictive factor of SVR to peginterferon plus ribavirin therapy in genotype 1 infected patients.
Keywords: IP‐10, chronic hepatitis C, virological response, peginterferon, ribavirin
Hepatitis C virus (HCV) infection affects more than 170 million people worldwide, being the most frequent cause of chronic liver disease in Western countries.1,2 Chronic HCV infection leads to a wide spectrum of liver disease, ranging from mild chronic hepatitis to end stage cirrhosis and hepatocellular carcinoma.3,4 The current treatment of choice for chronic hepatitis C (CHC) is based on a combination of pegylated interferon (IFN)‐α and ribavirin, reaching rates of sustained virological response (SVR) in different pivotal clinical trials of 54–61%.5,6,7 However, approximately 20% of patients infected with HCV genotype 2 or 3 and 50% of genotype 1 infected patients fail to eradicate the virus.
The molecular basis underlying failure of antiviral therapy in CHC is not fully understood but clinical and experimental studies indicate that the risk of treatment failure is related to multiple factors, including both viral and host factors.8,9 As therapeutic efficacy of IFN‐α plus ribavirin is likely due to their antiviral and immunomodulatory properties, it is conceivable that viral and host immune factors play an equally important role influencing IFN‐α plus ribavirin combination therapy. Low viral load and non‐1 genotype are well known predictors of SVR10,11 whereas less is known of the predictive value of host immune factors influencing SVR. In this regard, it has been reported that increased serum levels of cytokines, such as tumour necrosis factor α,12 interleukin (IL)‐1β,13 and IL‐10,14 as well as chemokines, such as IL‐8,15,16 correlated with a poor response to antiviral therapy in CHC patients. Recent data from our group provided evidence that intrahepatic and serum levels of a T cell specific chemokine termed interferon γ inducible protein 10 (IP‐10) were elevated in HCV infected patients, and that serum IP‐10 concentrations were higher in non‐responders (NR) than in responders to antiviral therapy.17 The aim of this study was to analyse the possible association between serum IP‐10 levels and virological response to peginterferon plus ribavirin therapy, in comparison with known predictors of SVR such as HCV genotype and viral load.
Materials and methods
Patient population
Consecutive naïve patients from four Spanish hospitals were studied and treated according to standard clinical practice. All patients (n = 137; 77 men (56.2%) and 60 women (43.8%); mean age 42 (9.7) years (range 19–68)) had persistently elevated serum alanine aminotransferase (ALT) levels, were HCV RNA positive, and had biopsy proven chronic hepatitis six months prior to initiation of therapy with peginterferon (alfa‐2a 99 patients; alfa‐2b 38 patients) plus ribavirin. Genotype distributions were as follows: genotype 1, n = 103 (75.2%); genotype 2, n = 9 (6.6%); genotype 3, n = 25 (18.2%). Characteristics of the patients included in this study are detailed in table 1.
Table 1 Baseline characteristics of the patients studied.
| Age (y) | 42 (9.7) |
| Sex | |
| Male | 77 (56.2%) |
| Female | 60 (43.8%) |
| ALT (U/l) | 117.2 (81.6) |
| HCV | |
| Genotype | |
| 1 | 103 (75.2%) |
| Non‐1 | 34 (24.8%) |
| Low viral load (<5×105 IU/ml) | 52 (37.9%) |
| High viral load (⩾5×105 IU/ml) | 85 (62.1%) |
| Grade score (Ishak) | |
| 0–6 | 78 (56.9%) |
| 7–14 | 59 (43.1%) |
| Stage score (Ishak) | |
| 0–3 | 106 (77.3%) |
| 4–6 | 31 (22.7%) |
Data are n (%) or mean (SD).
ALT, alanine aminotransferase; HCV, hepatitis C virus;
Written informed consent was obtained from each patient, and approval for the study was granted by the institution's ethics committee. Patients with active alcohol consumption >80 g/day were excluded and no patient had been treated with antiviral drugs. In addition, they were seronegative for hepatitis B surface antigen (HBsAg) and for antibodies to human immunodeficiency virus (HIV).
Treatment outcomes
Patients were treated on a genotype based strategy for 24 weeks (non‐1 genotypes: 2 and 3) or 48 weeks (genotype 1). Ninety nine patients received 180 μg of peginterferon alfa‐2a (Pegasys; Hoffmann‐LaRoche, Basel, Switzerland) weekly plus ribavirin (Hoffmann‐LaRoche); 38 patients were treated with peginterferon alfa‐2b (PEG‐Intron; Schering‐Plough, Kenilworth, New Jersey) adjusted by weight (1.5 μg/kg per week) plus ribavirin (Rebetol; Schering‐Plough). In all cases, the dose of ribavirin was adjusted according to body weight (<75 kg, 1000 mg/day; >75 kg, 1200 mg/day). SVR was defined as undetectable HCV RNA in serum 24 weeks after cessation of therapy. Patients with reappearance of HCV RNA after conclusion of treatment or serum HCV RNA positivity during therapy were considered as NR.
Virological tests
HBsAg and antibodies to HIV were assessed by means of immunoenzymatic assays (Murex, Dartford, UK). Quantification of serum HCV RNA levels was performed according to a polymerase chain reaction assay (Amplicor HCV Monitor Test, version 2.0; Roche Diagnostics, Branchburg, New Jersey, USA). The lower limit of detection was 50 IU/ml. HCV genotyping was performed with a line probe hybridisation assay (INNO‐LiPA HCV II; Innogenetics, Zwijnaarden, Belgium), according to the manufacturer's instructions.
Liver histology
Biopsy specimens from all patients, obtained by the percutaneous route under ultrasonographic control, were read and scored by local pathologists at the university hospitals participating in this study. The Ishak score18 was used for grade of inflammation and necrosis (range 0–18, with higher scores indicating more severe abnormalities) and for stage of fibrosis (range 0–6: 0, no fibrosis; 1, fibrous expansion of some portal areas; 2, fibrous expansion of most portal areas; 3, occasional bridging; 4, marked bridging; 5, incomplete cirrhosis; and 6, definite cirrhosis).
Assay for serum IP‐10 levels
All serum samples assessed were stored at −70°C, shipped on dry ice, and thawed only for performing this laboratory investigation. A commercially available solid phase sandwich enzyme linked immunosorbent assay (ELISA) was used, according to the manufacturer's instructions, for quantitative measurement of IP‐10 (human IP‐10 immunoassay kit; BioSource Europe SA, Nivelles, Belgium) in the serum of all patients included in this study before therapy, after 12 weeks of treatment, and 24 weeks after therapy. The minimum detectable level of IP‐10 for this ELISA is <2 pg/ml, and intra and interassay coefficients of variations are <5% and <7%, respectively.
Statistical analyses
All parametric values are expressed as means (SD). Different groups were compared using the Mann‐Whitney U test or the Student's t test for continuous variables and by the χ2 test for categorical data. The Spearman coefficient was used to evaluate correlations. Logistic regression was used in the univariate and multivariate analysis to determine factors associated with SVR. Variables included in the analyses were age, sex, liver histology (grade and stage), ALT, baseline serum IP‐10 level, viral load, and genotype. Statistical analyses were performed by SPSS 11.0 for Windows (SPSS Inc., Chicago, Illinois, USA) and by BMDP 1.1 for Windows (BMDP Statistical Software LTD., Cork, Ireland). Sensitivity, specificity, receiver operating characteristic (ROC) curves, and area under the curve (AUC) were carried out using MedCalc (Mariakerke, Belgium) software. If not otherwise stated, all tests were two tailed and p values lower than 0.05 were considered significant.
Results
From the 137 patients treated, 79 (57.7%) achieved an SVR whereas 58 (42.3%) were NR.
Baseline features associated with sustained virological response
Baseline characteristics significantly associated with SVR in all patients were non‐1 genotype (p = 0.003), a low viral load (p = 0.01), and pretreatment serum IP‐10 levels (p = 0.004) by univariate comparisons of SVR and NR (table 2). Age, sex, serum ALT levels, grade of necroinflammatory activity, and stage of fibrosis were not significantly associated with SVR. Furthermore, no significant advantage of treatment with peginterferon alfa‐2a or alfa‐2b for SVR could be observed.
Table 2 Patient characteristics at baseline and response to treatment.
| Variable | SVR (n = 79) | NR (n = 58) | p Value |
|---|---|---|---|
| Age (y) | 40.9 (9.9) | 43.3 (9.3) | 0.84 |
| Sex | |||
| Male | 44 (57.1%) | 33 (42.9%) | 0.15 |
| Female | 35 (58.3%) | 25 (41.7%) | |
| ALT (U/l) | 122.5 (73.2) | 110.5 (92.6) | 0.78 |
| HCV genotype | |||
| 1 | 52 (50.5%) | 51(49.5%) | 0.003 |
| Non‐1 | 27 (79.4%) | 7 (20.6%) | |
| HCV viral load | |||
| Low (<5×105 IU/ml) | 40 (76.9%) | 12 (23.1%) | 0.01 |
| High (⩾5×105 IU/ml) | 39 (45.9%) | 46 (54.1%) | |
| Grade score (Ishak) | |||
| 0–6 | 43 (55.1%) | 35 (44.9%) | 0.46 |
| 7–14 | 36 (61.1%) | 23 (38.9%) | |
| Stage score (Ishak) | |||
| 0–3 | 64 (60.4%) | 42 (39.6%) | 0.11 |
| 4–6 | 15 (48.4%) | 16 (51.6%) | |
| Serum IP‐10 (pg/ml) | 332.4 (222.1) | 476.8 (305.3) | 0.004 |
SVR, sustained virological responders; NR, non‐responders; ALT, alanine aminotransferase; HCV, hepatitis C virus; IP‐10, interferon γ inducible protein 10.
Data are n (%) or mean (SD).
From multivariate logistic regression analysis of all patients, non‐1 genotype (p = 0.003) and a low baseline viral load (p = 0.01) were independent predictors for SVR whereas the significance of pretreatment serum IP‐10 disappeared. In contrast, when analysis was performed in the cohort of genotype 1 infected patients, pretreatment serum IP‐10 levels and baseline viral load were independent predictors for SVR (p = 0.03 and p = 0.04, respectively) (table 3).
Table 3 Independent baseline factors for sustained virological response (logistic regression analysis).
| Variable | All HCV patients | Genotype 1 patients | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Non‐1 genotype | 3.53 (1.10–11.41) | 0.003 | ||
| Low HCV viral load (<5×105 IU/ml) | 0.34 (0.14–0.79) | 0.01 | 0.38 (0.155–0.961) | 0.04 |
| Serum IP‐10 (pg/ml) | 0.99 (0.99–1.001) | 0.07 | 0.99 (0.996–0.999) | 0.03 |
HCV, hepatitis C virus; IP‐10, interferon γ inducible protein 10; OR, odds ratio; CI, confidence interval.
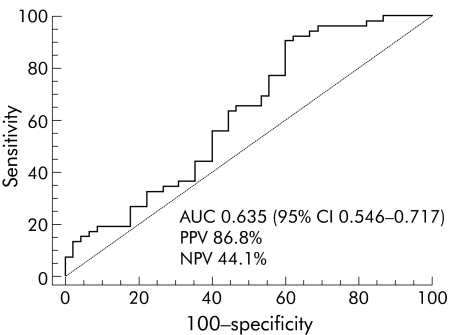
To identify a suitable threshold for baseline serum IP‐10 levels, an ROC curve for predicting SVR for genotype 1 infected patients was calculated, and showed an AUC of 0.635 (95% 95% confidence interval 0.546–0.771) (fig 1). The resulting threshold for predicting SVR was 594.1 pg/ml, with a positive predictive value and a negative predictive value of 86.8% and 44.1%, respectively.
Figure 1 Receiver operating characteristic (ROC) curve of pretreatment serum interferon γ inducible protein 10 levels in genotype 1 infected patients and therapeutic response. The curve shows an area under the curve (AUC) of 0.635 (95% confidence interval 0.546–0.771), with a positive predictive value (PPV) and a negative predictive value (NPV) of 86.8% and 44.1%, respectively.
Serum IP‐10 levels and virological response
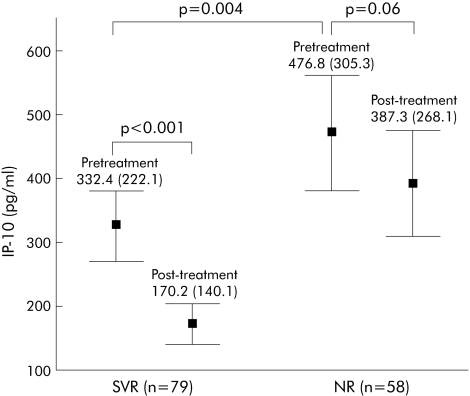
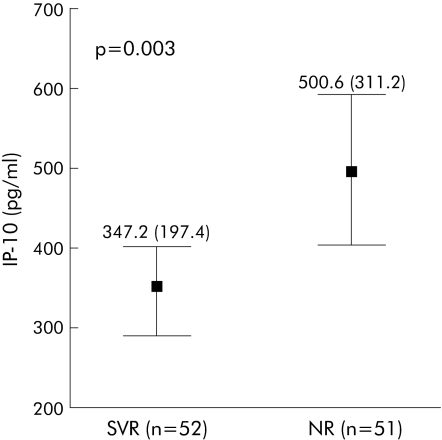
Taking into account the overall HCV study cohort, in patients with an SVR, significantly lower pretreatment serum IP‐10 levels (332.4 (222.1)) were observed compared with NR (476.8 (305.3); p = 0.004) (fig 2). Considering only patients infected with genotype 1, those who achieved an SVR also showed significantly lower baseline IP‐10 concentrations (347.2 (197.4)) than patients with NR (500.6 (311.2); p = 0.003) (fig 3). In contrast, no significant difference was observed in serum IP‐10 levels after 12 weeks of treatment in patients with SVR (289.3 (197.6)) or in NR (323.8 (200.2); p = 0.14). As expected from our previous work,17 serum IP‐10 concentrations were significantly decreased 24 weeks after completion of therapy in patients with an SVR (pretreatment: 332.4 (222.1) pg/ml; post‐treatment: 170.2 (140.1) pg/ml; p<0.001) but not in NR (pretreatment: 476.8 (305.3) pg/ml; post‐treatment: 387.3 (268.1) pg/ml; p = 0.06) (fig 2).
Figure 2 In the overall hepatitis C virus study cohort (n = 137), in patients with a sustained virological response (SVR), significantly lower pretreatment serum interferon γ inducible protein 10 (IP‐10) levels (332.4 (222.1)) were observed compared with non‐responders (NR) (476.8 (305.3); p = 0.004). In addition, serum IP‐10 concentrations were significantly decreased 24 weeks after cessation of therapy in patients with an SVR (n = 79) (pretreatment: 332.4 (222.1) pg/ml; post‐treatment: 170.2 (140.1) pg/ml, p<0.001) but not in NR (n = 58) (pretreatment: 476.8 (305.3) pg/ml; post‐treatment: 387.3 (268.1) pg/ml; p = 0.06). Values are means (SD).
Figure 3 In genotype 1 infected patients (n = 103), those who achieved a sustained virological response (SVR) also showed significantly lower baseline interferon γ inducible protein 10 (IP‐10) concentrations (347.2 (197.4)) than non‐responders (NR) (500.6 (311.2); p = 0.003). Values are means (SD).
Association of pretreatment serum IP‐10 with baseline parameters
Pretreatment IP‐10 was significantly associated with HCV genotype, as serum levels of this chemokine were significantly higher in genotype 1 patients (421.8 (266.2)) than in those infected with non‐1 genotypes (304.1 (261.3); p = 0.04) (table 4). No significant association of sex, viral load, or grade of necroinflammatory activity from the liver biopsy obtained before initiation of antiviral therapy with pretreatment IP‐10 levels in HCV patients was detected. In contrast, in HCV patients with advanced fibrosis (stage scores 4–6), significantly higher pretreatment serum IP‐10 concentrations (510.7 (313.4) pg/ml) were observed compared with those with mild fibrosis (stage scores 0–3) (353.6 (234.1) pg/ml; p = 0.005). This association was also significant when the analysis was performed in the subgroup of genotype 1 infected patients.
Table 4 Association of pretreatment serum interferon γ inducible protein 10 (IP‐10) with baseline characteristics of hepatitis C virus (HCV) patients.
| Variable | Serum IP‐10 levels (pg/ml)) | |||
|---|---|---|---|---|
| All HCV patients (n = 137) | p Value | Genotype 1 patients (n = 103) | p Value | |
| Sex | ||||
| Male | 348.5 (253.3) (n = 77) | 0.09 | 374.1 (265.2) (n = 64) | 0.07 |
| Female | 428.2 (277.6) (n = 60) | 458.5 (254.3) (n = 39) | ||
| HCV genotype | ||||
| 1 | 421.8 (266.2) (n = 103) | 0.04 | ||
| Non 1 | 304.1 (261.3) (n = 34) | |||
| Viral load | ||||
| Low | 365.9 (236.5) (n = 52) | 0.13 | 371.1 (214.3) (n = 37) | 0.08 |
| High | 414.5 (285.1) (n = 85) | 451.9 (291.5) (n = 66) | ||
| Grade score | ||||
| 0–6 | 362.9 (241.6) (n = 78) | 0.11 | 395.2±243.7 (n = 56) | 0.39 |
| 7–14 | 421.6 (283.1) (n = 59) | 425.6±275.9 (n = 47) | ||
| Stage score | ||||
| 0–3 | 353.6 (234.1) (n = 106) | 0.005 | 376.1 (239.5) (n = 78) | 0.02 |
| 4–6 | 510.7 (313.4) (n = 31) | 525.7 (292.1) (n = 25) | ||
Values are mean (SD).
Discussion
IP‐10, also termed CXCL10, belongs to the CXC chemokine family and exerts its chemotactic function on different cell types following binding to its specific receptor CXCR3.19,20 It has been shown that the majority of liver infiltrating T cells in patients with CHC expressed CXCR3,21 and that the amount of hepatic IP‐10 mRNA and protein was markedly increased in these patients17,22 and correlated strongly with serum IP‐10 concentrations,23 suggesting that this chemokine could play an important role in the pathogenesis of chronic HCV infection.
The main finding of the present study was the association of serum IP‐10 levels with SVR to peginterferon plus ribavirin therapy in patients with CHC infected by genotype 1, showing that pretreatment IP‐10 concentrations lower than 594.1 pg/ml had a positive predictive value of 86.8% in this study population. Noteworthy, after successful antiviral therapy with an SVR, serum IP‐10 concentrations decreased to levels lower than baseline whereas they were unchanged in NR, suggesting that HCV itself may be responsible for elevated serum IP‐10 concentrations found in HCV infected patients. Supporting this notion, we have recently demonstrated that HCV proteins such as NS5A and core, alone or in combination with proinflammatory cytokines, can induce IP‐10 gene expression and secretion in human hepatocyte derived cells.24 The biological meaning of this HCV mediated IP‐10 induction is still unknown but it has been reported that expression of the HCV NS5A protein in human cells was found to induce a CXC chemokine termed IL‐8, and enhanced IL‐8 was associated with inhibition of the antiviral effects of IFN‐α in vitro.25 Whether IP‐10 interferes with IFN‐α signalling in HCV infected hepatocytes has yet to be defined.
Based on our results, IP‐10 may have a role in failure of antiviral therapy in patients with chronic HCV infection, as NR had significantly higher serum IP‐10 levels than patients with an SVR. Similarly, persistently elevated IP‐10 concentrations have been correlated with failure of highly active antiretroviral therapy in HIV patients.26 However, the mechanism by which IP‐10 modulates the efficacy of peginterferon plus ribavirin therapy remains elusive, but it is conceivable that it is related to its biological functions.
CXCR3/IP‐10 interactions have been associated with a number of viral infections in animals and humans,27,28,29 serving both to modulate viral infection and to recruit effector T cells to sites of infection. Analysing IP‐10 effects on viral replication, Lane and colleagues30 have shown that IP‐10 stimulated HIV replication in primary human lymphocytes and macrophages in vitro as well as neutralising endogenous IP‐10 or blocking CXCR3 binding reduced HIV replication in these same cells. They also observed that IP‐10 was active in the range 5–125 ng/ml, which is likely to be physiologically relevant as cerebrospinal fluid levels of IP‐10 of up to 40 ng/ml have been detected in HIV infected individuals.26 While serum IP‐10 concentrations ranged between 0.02 and 1.19 ng/ml in our HCV study cohort, the amount of chemokine available in liver tissue where HCV replication occurs is likely to be much higher. We found that IP‐10 concentrations in the culture supernatant of cytokine activated HCV transfected human hepatocytes were in the range 58.18–158.96 ng/ml,24 an scenario mimicking the intrahepatic microenvironment during chronic HCV infection in vivo. There is no evidence to date that IP‐10 might contribute to treatment failure by enhancing hepatocellular HCV replication, but it is interesting to note that genotype 1 infected patients with a high viral load had greater serum IP‐10 levels than those with a low viral load (451.9 (291.5) and 371.1 (214.3), respectively), but these differences were not statistically significant (p = 0.08).
How IP‐10 might induce treatment failure may also be explained on the basis of its important role in the recruitment of effector Th1 lymphocytes into the liver parenchyma of patients with chronic HCV infection,31,32 potentially contributing to the host immune response against the virus as well as to disease progression. Here, we confirmed our previous results17 showing that elevated serum IP‐10 levels in genotype 1 infected patients were significantly associated with a poor response to antiviral therapy. We hypothesised that elevated IP‐10 concentrations within the liver of HCV patients may lead to further accumulation of effector T cells secreting Th1‐type cytokines. Thus this vigorous immune pressure exerted on the virus would favour the outgrowth of variant viruses generating immune escape mutants. The extent to which viral escape mutants are generated during antiviral therapy should play a decisive role as a cause of HCV resistance. Whether or not this scenario exists in vivo and plays a role in treatment outcome remains unknown.
It is well known that hepatic stellate cells (HSC) are key fibrogenic cells33 and, therefore, those factors involved in activation, proliferation, and migration of these cells, such as cytokines, platelet derived growth factors, and chemokines, among others, could play a central role in the pathogenesis of liver fibrosis.34 CXCR3 has been found to be expressed on HSC and, more interestingly, IP‐10 induced migration of these cells in a dose dependent manner.35 These data in conjunction with our results showing that HCV patients with advanced fibrosis had significantly higher serum IP‐10 levels than those with mild fibrosis (510.7 (313.4) and 353.6 (234.1), respectively; p = 0.005), strongly suggest that IP‐10 could play an important role in the progression of hepatic fibrosis in chronic HCV infection.
Predicting response to antiviral therapy is a crucial point in the management of patients with CHC and, in this regard, HCV genotype is currently considered the major determinant of outcome to treatment.36 However, given the high proportion of patients with genotype 1 who still do not respond to antiviral therapy, searching for predictive factors in these groups of difficult‐to‐cure patients is needed. Here, we have provided evidence that pretreatment serum IP‐10 is a predictive factor of SVR to peginterferon plus ribavirin therapy in genotype 1 infected patients, identifying those with low serum IP‐10 as the subgroup of patients with the best profile of response to 48 weeks of antiviral therapy. Whether tailoring treatment on the basis of serum IP‐10 levels at baseline could improve the SVR rate of patients infected by HCV genotype 1 should be investigated in future prospective clinical trials.
It has recently been reported that insulin resistance correlated with a poor response to peginterferon plus ribavirin therapy in genotype 1 infected patients,37 suggesting that therapeutic interventions aimed at decreasing insulin resistance in these patients may be useful to improve the response rate to antiviral therapy. In this study, pretreatment serum IP‐10 was an independent predictor of SVR in genotype 1 infected patients, as significantly lower serum levels of this chemokine were observed in patients with SVR compared with NR. These results identify IP‐10 as a possible new target for improving virological response rate of these poor response patients.
In conclusion, our study found that pretreatment serum IP‐10 is an independent predictive factor of SVR in HCV patients infected by genotype 1. As IP‐10 may play a role in the mechanism of failure of antiviral therapy, interventions neutralising endogenous IP‐10 or blocking the function of its receptor, CXCR3, may provide new strategies to improve the treatment outcome of these difficult‐to‐cure patients.
Acknowledgements
This work was supported by grants from Instituto de Salud Carlos III (FIS: 01/0152, FIS: 03/0417 and RTIC: G03/015). An unrestricted grant to CG‐M from Schering‐Plough Biotech (Madrid, Spain) is also acknowledged. The authors thank Professor Piero Almasio (University of Palermo, Italy) for his helpful advice on statistics and Raquel Lorente for expert technical assistance.
Abbreviations
CHC - chronic hepatitis C
IFN - interferon
IP‐10 - interferon γ inducible protein 10
HCV - hepatitis C virus
HSC - hepatic stellate cells
NR - non‐responders
SVR - sustained virological response
IL - interleukin
ALT - alanine aminotransferase
HBsAg - hepatitis B surface antigen
HIV - human immunodeficiency virus
ELISA - enzyme linked immunosorbent assay
ROC - receiver operating characteristic
AUC - area under the curve
Footnotes
Conflict of interest: None declared.
References
- 1.Liang T J, Rehermann B, Seef L B.et al Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000132296–305. [DOI] [PubMed] [Google Scholar]
- 2.Seeff L B, Hoofnagle J H. The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis 20037261–287. [DOI] [PubMed] [Google Scholar]
- 3.Seeff L B. Natural history of hepatitis C. Hepatology 199726(suppl 1)21–8S. [DOI] [PubMed] [Google Scholar]
- 4.Lauer G M, Walker B D. Hepatitis C virus infection. N Engl J Med 200134541–52. [DOI] [PubMed] [Google Scholar]
- 5.Manns M P, McHutchison J G, Gordon S C.et al Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 2001358958–965. [DOI] [PubMed] [Google Scholar]
- 6.Fried M W, Schiffman M L, Reddy R.et al Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002347975–982. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis S J, Sette H, Jr, Morgan T R.et al Peginterferon alfa‐2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004140346–355. [DOI] [PubMed] [Google Scholar]
- 8.Pawlotsky J M. Mechanisms of antiviral treatment efficacy and failure in chronic hepatitis C. Antiviral Res 2003591–11. [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Hong F, Radaeva S. Host factors and failure of interferon‐α treatment in hepatitis C virus. Hepatology 200439880–890. [DOI] [PubMed] [Google Scholar]
- 10.Pawlotsky J M, Roudot‐Thoraval F, Bastie A.et al Factors affecting treatment responses to interferon‐alpha in chronic hepatitis C. J Infect Dis 19961741–7. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P. Predicting the therapeutic response in patients with chronic hepatitis C: the role of viral kinetic studies. J Antimicrob Chemother 20045315–18. [DOI] [PubMed] [Google Scholar]
- 12.Larrea E, García N, Qian C.et al Tumor necrosis factor alpha gene expresión and the response to interferon in chronic hepatitis C. Hepatology 199623210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishihara Y, Hayashi J, Yoshimura F.et al IL‐1 beta and TNF‐alpha produced by peripheral blood mononuclear cells before and during interferon therapy in patients with chronic hepatitis C. Dig Dis Sci 199641315–321. [DOI] [PubMed] [Google Scholar]
- 14.Cramp M E, Rossol S, Cohkshi S.et al Hepatitis C virus‐specific T‐cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 2000118346–355. [DOI] [PubMed] [Google Scholar]
- 15.Polyak S J, Khabar K S, Rezeiq M.et al Elevated levels of interleukin‐8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol 2001756209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihm U, Herrmann E, Sarrazin U.et al Association of serum interleukin‐8 with virologic response to antiviral therapy in patients with chronic hepatitis C. J Hepatol 200440845–852. [DOI] [PubMed] [Google Scholar]
- 17.Apolinario A, Diago M, Lo Iacono O.et al Increased circulating and intrahepatic T‐cell specific chemokines in chronic hepatitis C: Relationship with the type of virologic response to peginterferon plus ribavirin combination therapy. Aliment Pharmacol Ther 200419551–562. [DOI] [PubMed] [Google Scholar]
- 18.Ishak K, Baptista A, Bianchi L.et al Histological grading and staging of chronic hepatitis. J Hepatol 199522696–699. [DOI] [PubMed] [Google Scholar]
- 19.Luster A D. Chemokines: Chemotactic cytokines that mediate inflammation. N Engl J Med 1998338436–445. [DOI] [PubMed] [Google Scholar]
- 20.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood 2000953032–3043. [PubMed] [Google Scholar]
- 21.Apolinario A, Majano P L, Alvarez‐Perez E.et al Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol 2002972861–2870. [DOI] [PubMed] [Google Scholar]
- 22.Patzwahl R, Meier V, Ramadori G.et al Enhanced expression of interferon‐regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression‐substractive hybridization. J Virol 2001751332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihm S, Schweyer S, Ramadori G. Expression of the chemokine IP‐10 correlates with the accumulation of hepatic IFN‐γ and IL‐18 mRNA in chronic hepatitis C but not in hepatitis B. J Med Virol 200370562–570. [DOI] [PubMed] [Google Scholar]
- 24.Apolinario A, Majano P L, Lorente R.et al Gene expression profile of T cell‐specific chemokines in human hepatocyte‐derived cells: Evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat 20051227–37. [DOI] [PubMed] [Google Scholar]
- 25.Polyak S J, Khabar K S, Paschal D M.et al Hepatitis C virus nonstructural 5A protein induces interleukin‐8, leading to partial inhibition of the interferon‐induced antiviral response. J Virol 2001756095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stylianou E, Aukrust P, Bendtzen K.et al Interferon and interferon (IFN)‐inducible protein 10 during highly active anti‐retroviral therapy (HAART)‐possible immunosuppressive role of IFN‐alpha in HIV infection. Clin Exp Immunol 2000119479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedland J S. Chemokines in viral disease. Res Virol 1996147131–138. [DOI] [PubMed] [Google Scholar]
- 28.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol 199715675–705. [DOI] [PubMed] [Google Scholar]
- 29.Molesworth‐Kenyon S, Mates A, Yin R.et al CXCR3, IP‐10, and MIG are required for CD4+ T cell recruitment during the DTH response to HSV‐1 yet are independent of the mechanism for viral clearance. Virology 20053331–9. [DOI] [PubMed] [Google Scholar]
- 30.Lane B R, King S R, Bock P J.et al The C‐X‐C chemokine IP‐10 stimulates HIV‐1 replication. Virology 2003307122–134. [DOI] [PubMed] [Google Scholar]
- 31.Shields P L, Morland C M, Salmon M.et al Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C‐infected liver. J Immunol 19991636236–6243. [PubMed] [Google Scholar]
- 32.Harvey C E, Post J J, Palladinetti P.et al Expression of the chemokine IP‐10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol 200374360–369. [DOI] [PubMed] [Google Scholar]
- 33.Pinzani M, Marra F. Cytokine receptors and signaling during stellate cell activation. Semin Liver Dis 200121397–416. [DOI] [PubMed] [Google Scholar]
- 34.Friedman S L. Liver fibrosis‐from bench to bedside. J Hepatol 200338(suppl 1)S38–S53. [DOI] [PubMed] [Google Scholar]
- 35.Bonachi A, Romagnani P, Romanelli R G.et al Signal transduction by the chemokine receptor CXCR3. Activation of Ras/ERK, Src, and phosphatidylinositol 3‐kinase/AKT controls cell migration and proliferation in human vascular pericytes. J Biol Chem 20012769945–9954. [DOI] [PubMed] [Google Scholar]
- 36.Ferenci P. Predictors of response to therapy for chronic hepatitis C. Semin Liver Dis 200424(suppl 2)25–31. [DOI] [PubMed] [Google Scholar]
- 37.Romero‐Gomez M, Viloria M, Andrade R J.et al Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005128636–641. [DOI] [PubMed] [Google Scholar]