Abstract
Background
Recent studies in animals have shown that ghrelin stimulates upper gastrointestinal motility through the vagus and enteric nervous system. The aim of the present study therefore was to simultaneously investigate the effect of administration of ghrelin on upper gastrointestinal motility and to elucidate its mode of action by measuring plasma levels of gastrointestinal hormones in humans.
Materials and methods
Nine healthy volunteers (four males; aged 22–35 years) underwent combined antroduodenal manometry and proximal stomach barostat study on two separate occasions at least one week apart. Twenty minutes after the occurrence of phase III of the migrating motor complex (MMC), saline or ghrelin 40 μg was administered intravenously over 30 minutes in a double blind, randomised, crossover fashion. Ghrelin, motilin, pancreatic polypeptide, glucagon, and somatostatin were measured by radioimmunoassay in blood samples obtained at 15–30 minute intervals. The influence of ghrelin or saline on MMC phases, hormone levels, and intraballoon volume was compared using paired t test, ANOVA, and χ2 testing.
Results
Spontaneous phase III occurred in all subjects, with a gastric origin in four. Administration of ghrelin induced a premature phase III (12 (3) minutes, p<0.001; gastric origin in nine, p<0.05), compared with saline (95 (13) minutes, gastric origin in two). Intraballoon volumes before infusion were similar (135 (13) v 119 (13) ml; NS) but ghrelin induced a longlasting decrease in intraballoon volume (184 (31) v 126 (21) ml in the first 60 minutes; p<0.05). Administration of ghrelin increased plasma levels of pancreatic polypeptide and ghrelin but motilin, somatostatin, and glucagon levels were not altered.
Conclusions
In humans, administration of ghrelin induces a premature gastric phase III of the MMC, which is not mediated through release of motilin. This is accompanied by prolonged increased tone of the proximal stomach.
Keywords: ghrelin, gastric motility, appetite control, gastrointestinal hormones, manometry
Ghrelin is a 28 amino acid motilin related peptide that was first derived from rat stomach1 and is the natural ligand for the growth hormone secretagogue (GHS) receptor. Ghrelin has strong growth hormone releasing activity but also acts as a starvation signalling molecule in the periphery that stimulates food intake and decreases fat utilisation.2,3 Animal studies revealed that ghrelin has distinct effects on gastrointestinal motility. In the anaesthetised rat, ghrelin stimulates gastric acid secretion and motility4 although others failed to observe enhanced acid secretion.5 In conscious rats, ghrelin accelerates gastric emptying, enhances small bowel transit, and can overcome postoperative ileus.6 Ghrelin is also able to enhance gastric emptying in mice.5,7 Recent observations have shown stimulation of interdigestive motility by ghrelin in rats.8,9 Both vagally mediated and vagus independent influences on gastrointestinal motility have been reported. In rats, the motor effects in vivo are blocked by vagotomy but once brain regulation is eliminated a local mechanism becomes operational.4,8 In vitro studies have provided evidence that in addition to known vagus nerve dependent mechanisms, the activity of ghrelin is mediated via the enteric nervous system. Ghrelin increases electrically evoked cholinergic neural responses in rat stomach strips10,11 and Xu et al provided morphological evidence for the presence of ghrelin and ghrelin receptors in myenteric neurones of guinea pig ileum.12 In the same species, calcium imaging studies have revealed that ghrelin activates a subset of myenteric neurones through the GHS‐R1a receptor.13 Apart from its action on the vagus nerve and on myenteric neurones, ghrelin may also affect plasma levels of gastrointestinal hormones that have been shown to be involved in the control of interdigestive motility in humans.14,15,16,17,18
These observations suggest a potential role for ghrelin in the control of motility of the stomach and of the small bowel in several species. To date, the effects of ghrelin on gastrointestinal motility in humans have not been studied. The aim of this study, therefore, was to investigate the influence of exogenously applied ghrelin on the interdigestive motor activity of the proximal gastrointestinal tract in humans. In addition, because ghrelin is structurally related to the gastrointestinal hormone, motilin, involved in the regulation of interdigestive motility, we simultaneously investigated whether the effect of ghrelin is mediated through release of motilin. Finally, as influences or interactions with the effects of ghrelin have been reported for other gastrointestinal hormones which are involved in the control of interdigestive motility (pancreatic polypeptide, somatostatin, glucagon),17,18 we also measured the influence of ghrelin on plasma levels of these peptides.
Materials and methods
Subjects
Nine healthy volunteers (five females; aged 22–35 years) participated in the study. None of the subjects had symptoms of or a history of gastrointestinal disease. None of the subjects was taking any medication or had any drug allergies. From a previous study, all were known to be Helicobacter pylori negative. Informed consent was obtained from each participant. The ethics committee of the University Hospital had previously approved the protocol.
Study technique
Recording of antroduodenal intraluminal pressures was performed using a nine lumen polyvinyl catheter (outer diameter 6 mm) with a latex bag at its end that could be filled with mercury to facilitate its progression. Seven manometry sensors were located on the probe at 5 cm intervals. The probe was introduced via the mouth and positioned under fluoroscopic control (the manometry ports have a radiopaque marker point) in order to have the most distal of three proximal sensors at the pylorus or just proximal to it. The other sensors were located in the duodenum. The remaining two channels were used for filling and emptying the mercury bag (fig 1A). This catheter assembly allowed at least one recording site to be kept in the distal antrum during the whole test and thus the migrating motor complex (MMC) was adequately detected in the distal antrum and in the duodenum.
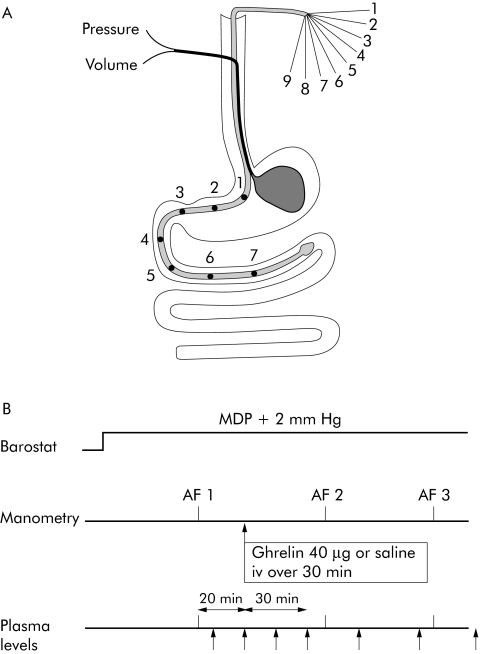
Figure 1 (A) Schematic representation of the position of the antroduodenal manometry catheter and gastric barostat balloon in healthy subjects. Numbers represent manometry channels and side holes. (B) Schematic outline of the study protocol. AF, activity front. MDP, minimal distending pressure.
Once the manometry assembly was adequately positioned, a double lumen polyvinyl tube (Salem sump tube 14 Ch; Sherwood Medical, Petit Rechain, Belgium) with an adherent plastic bag (1200 ml capacity; 17 cm maximal diameter), finely folded, was introduced through the mouth and secured to the subject's chin with adhesive tape. The position of the bag in the gastric fundus was checked fluoroscopically.
The barostat polyvinyl tube and the manometry catheter were then connected to a combined barostat/manometry device (PC Polygraph and Synectics Visceral Stimulator, Stockholm, Sweden). The manometry channels were continuously perfused with water by means of a low compliance pneumohydraulic infusion pump (Arndorfer Medival Specialties Inc., Greendale, Wisconsin, USA) at a flow rate of 0.4 ml/min, and were connected to external pressure transducers (Siemens Elema 746, Siemens, Iselin, New Jersey, USA). Pressures were recorded with a polygraph (PC Polygraph, Synetics, Stockholm, Sweden). The pressure and volume in the barostat bag were continuously monitored. All data were transferred to a personal computer through a fibreoptic interface. Individual channels were displayed on computer screen and stored for later analysis.
Study design
All subjects were studied twice after an overnight fast with at least one week interval. The study protocol is summarised in fig 1B. The barostat and manometry assembly was introduced as described above and secured to the subject's chin with adhesive tape. To unfold the bag it was inflated with a fixed volume of 300 ml of air for two minutes, with the study subject in the recumbent position, and again deflated completely. Subjects were then positioned in a comfortable sitting position with the knees bent (80°) and the trunk upright in a specifically designed bed. After a 30 minute adaptation period, minimal distending pressure (MDP) was first determined by increasing intrabag pressure by 1 mm Hg every three minutes until a volume of 30 ml or more was reached.19,20 This pressure level equilibrates intra‐abdominal pressure. Subsequently, intra‐balloon pressure was set at MDP+2 mm Hg for the entire study. Motility was recorded in the fasting state until the appearance of phase III of the MMC. Twenty minutes after the passage of a gastric activity front, an infusion of saline or ghrelin 40 μg (ghrelin; Clinalfa, Switzerland) over 30 minutes was administered. The dose of ghrelin was chosen based on the available literature at the time the study was designed; maximum doses of up to 10 μg/kg have been reported.21,22,23 Effects on growth hormone secretion occur with doses as low as 0.2 μg/kg. Robust effects on cardiac output occur at 1 μg/kg. As it is unclear to what extent cardiac and endocrine effects are related to gastrointestinal motor effects, we studied the effects of dose of 20 and 40 μg/kg in a pilot study in two healthy volunteers. As only the latter induced prompt changes in gastrointestinal motility, this dose was then chosen for the placebo controlled study. The order of saline and ghrelin treatment was randomised by drawing cards from a box of cards determining the sequence. A study nurse who was otherwise not involved in the study administered the medication. Half of the subjects received saline first; the other half received ghrelin first. The motility research technician was unaware of the order of administration. Motility recording continued until the passage of two more activity fronts or for a maximum of five hours after the start of the infusion.
Blood samples for motilin, pancreatic polypeptide, glucagon, and somatostatin radioimmunoassay were taken at −20, 0, 15, and 30 minutes, and then at 30 minute intervals for up to 150 minutes after the start of the infusion. Blood samples were collected in Li‐heparin tubes containing aprotinin (500 kallikrein inhibitory units/ml), centrifuged, and stored at −80°C until assayed.
Data analysis
Analysis was done on coded traces by one of the investigators (JT). As described above, immediately after insertion, the position of the probes was checked fluoroscopically. In addition, during the study, identification of the most distal antral recording site was checked visually by identifying the channel displaying up to three waves per minute (antral waves) just proximal to the channel displaying up to 12 waves per minute (duodenal waves) or to the channel displaying a mixture of antral and duodenal waves. In the present study, which did not involve administration of a meal, corrections for catheter movement were not necessary. The different phases of the MMC were identified according to previously described criteria.24,25,26 The number of contractions per minute, wave amplitude and duration, and motility index were determined during the infusion period for both the antrum and duodenum with the help of a semi‐automated computer aided procedure, which used a software program developed in our laboratory.27
Gastric tone was measured by calculation of mean intrabag volume for consecutive five minute intervals during the long distending periods at a pressure of MDP+2 mm Hg.
Determination of gastrointestinal hormones in plasma
Plasma levels of gastrointestinal hormones were measured by radioimmunoassay. Ghrelin levels were measured after extraction of plasma samples on Sep‐Pak C18 cartridges using human 125I‐[Tyr0]‐ghrelin (13–28)‐OH as tracer and an antibody raised in rabbits against human [Cys0]‐ghrelin (13–28)‐OH. The assay does not distinguish between endogenous and exogenously applied ghrelin. Motilin and pancreatic polypeptide were measured as previously described28,29 using 125I‐nle13‐human motilin or 125I‐human pancreatic polypeptide (NEN Life Science Products, Boston, Massachusetts, USA) as label and a rabbit anti‐nle13‐motilin or a rabbit antihuman pancreatic polypeptide serum (Peninsula Laboratories, San Carlos, California, USA). Somatostatin measurements were performed with (3‐[125I]iodotyrosyl11)somatostatin‐14(Tyr11) (Amersham Biosciences Europe, Freiburg, Germany) as tracer and an antibody raised in rabbits against somatostatin,1,2,3,4,5,6,7,8,9,10,11,12,13,14 as described by Chayvialle and colleagues.30 Plasma glucagons levels were assayed using an RIA kit (Euro‐Diagnostica AB, Malmö, Sweden).
Statistical analysis
Among the recording channels, the most distal antral and most distal duodenal channels were chosen for evaluation of motor activity of the antrum and duodenum. For each site, manometric parameters (that is, number of contractions per minute, wave amplitude and duration, and motility index) were calculated on recordings obtained after saline and ghrelin therapy. Data were compared by t test, χ2 testing, and ANOVA. For comparison of changes in plasma levels of gastrointestinal hormones, the area under the curve (AUC) was calculated and comparisons between AUCs were made using the paired t test. Time dependent changes in plasma hormone levels after saline or ghrelin infusion were compared by two way ANOVA analysis with repeated measurement on two factors and univariate testing of significance for planned comparisons (Statistica, Statsoft Inc, Tulsa, Oklahoma, USA). Results are expressed as mean (SD). A p value <0.05 was considered significant.
Results
Conduct of the study
All subjects completed the study. Administration of ghrelin was well tolerated and did not induce any specific sensations or adverse symptoms.
Phases of the migrating motor complex
Phase III activity, before drug administration, was readily identified in all nine volunteers, occurring on average 59 (16) minutes after the start of the recording. The activity front had a gastric origin in four subjects (44%) and a small intestinal origin in five subjects (56%). After administration of saline, the next activity front occurred 75 (9) minutes after the end of the infusion, and had a gastric origin in two subjects (22%). When ghrelin was administered, the next activity front occurred 12 (3) minutes after the end of the infusion (p<0.005 compared with saline) and had a gastric origin in all subjects (p<0.001). A representative tracing is shown in fig 2. The characteristics of the activity fronts (duration and propagation velocity) after saline and after ghrelin did not differ significantly (table 1). The characteristics of gastric and intestinal contractions before and after infusion are summarised in table 2. The stimulatory effect was most pronounced in the stomach where the number of contractions as well as their amplitude and duration were significantly increased after ghrelin. In the small bowel, ghrelin mainly increased contraction frequency.
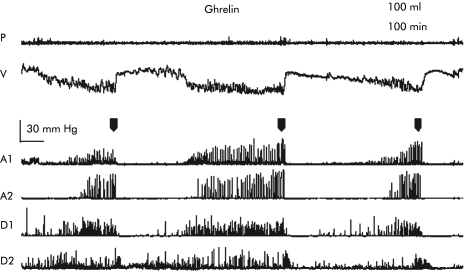
Figure 2 Effect of intravenous ghrelin on proximal gastric tone and antroduodenal motility in healthy volunteers. Two antral and two duodenal recording sites are shown. All traces begin at the start of the recording. P, intraballoon pressure; V, intraballoon volume. A1, 2 and D1, 2, first and second antral or duodenal manometry channel, respectively. Administration of ghrelin caused a premature phase III with gastric origin (indicated by black arrowheads), and this was accompanied by an increase in proximal gastric tone, shown as a decrease in isobaric balloon volume.
Table 1 Characteristics of migrating motor complex (MMC) activity after administration of placebo and ghrelin in nine healthy volunteers.
| Variable | Placebo | Ghrelin | p Value |
|---|---|---|---|
| MMC cycle duration (min) | 103 (9) | 66 (14) | 0.0001 |
| Phase I duration (min) | 19 (4) | 13 (3) | <0.05 |
| Phase II duration (min) | 81 (10) | 51 (12) | <0.001 |
| Phase III duration (min) | 3.1 (0.4) | 3.7 (0.9) | NS |
| Phase III propagation velocity (cm/min) | 4.9 (2.1) | 5.1 (1.8) | NS |
| Phase III (% gastric onset) | 22% (2/9) | 100% (9/9) | <0.001 |
Results are mean (SEM).
Durations of the different phases of the MMC were measured at the most distal duodenal channel as this was the most distal point of propagation; propagation velocity of phase III was measured at the duodenal level.
Table 2 Characteristics of gastric and intestinal contractions during infusion of placebo and ghrelin in nine healthy volunteers.
| Variable | Placebo | Ghrelin | p Value |
|---|---|---|---|
| Stomach | |||
| No of contractions | 35 (4) | 46 (5) | 0.04 |
| Contraction amplitude (mm Hg) | 32 (4) | 53 (6) | 0.004 |
| Duration of contractions (s) | 21 (2) | 30 (4) | 0.03 |
| Motility index (min×mm Hg) | 3.5 (0.2) | 4.4 (0.4) | 0.01 |
| Small bowel | |||
| No of contractions | 29 (5) | 56 (9) | 0.03 |
| Contraction amplitude (mm Hg) | 33 (4) | 37 (5) | 0.5 |
| Duration of contractions (s) | 16 (2) | 22 (5) | 0.3 |
| Motility index (min×mm Hg) | 3.1 (0.3) | 4.2 (0.3) | 0.02 |
Results are mean (SEM).
Characteristics of contractions were measured at the most distal gastric and the most distal small intestinal channel; motility index is shown as average per minute.
Intragastric balloon volume
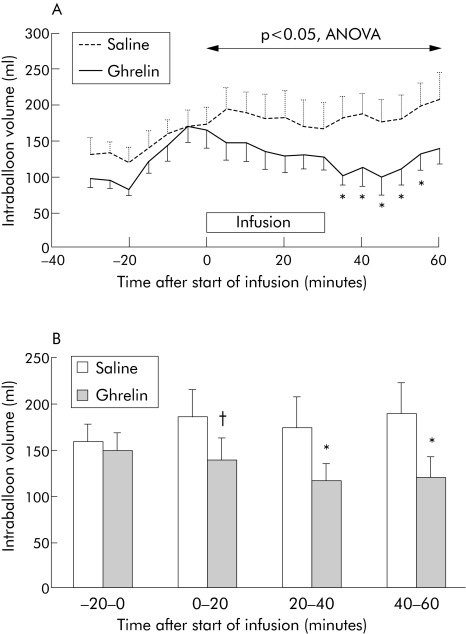
Before infusion, intraballoon volume fluctuated with the phases of the MMC, as illustrated in fig 2. During phase III activity, occurring between 30 and 20 minutes prior to the start of infusion, intraballoon volumes were low, but increased again during the following phase I activity (figs 2, 3). During the whole pre‐infusion period, intraballoon volumes did not differ between the treatment arms (130 (13) v 116 (12) ml; NS). During phase I, prior to the infusion, intraballoon volumes did not differ significantly between the groups (160 (18) v 150 (20) ml; NS) (fig 3). During infusion of saline, intraballoon volume did not change significantly. However, during infusion of ghrelin, intraballoon volumes decreased and were significantly lower compared with infusion of saline. Significance for five minute interval values was reached from 30 to 55 minutes after administration. Analysis of variance confirmed that the lowered intraballoon volumes persisted for the remainder of the study (p<0.05, ANOVA).
Figure 3 (A) Mean volume of the isobaric barostat balloon before and after administration of saline or ghrelin. Administration of ghrelin was associated with a prolonged decrease in intraballoon volume. *p<0.05 compared with saline. (B) Intraballoon volumes before and after the administration of saline or ghrelin, shown as 20 minute averages. A significantly lower volume was observed between 20 and 60 minutes after the start of ghrelin infusion. *p<0.05, †p = 0.07 compared with saline.
Gastrointestinal hormone plasma levels
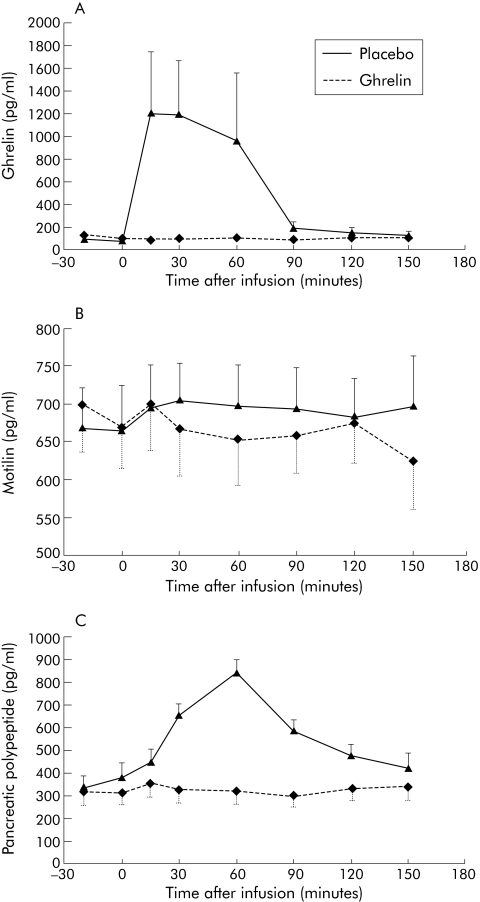
Ghrelin plasma levels did not rise after administration of saline. Infusion of ghrelin induced a significant rise in ghrelin plasma levels (AUC 15954 (2331) v 84058 (28640) min×pg/ml; p<0.04). A more than 10‐fold increase in plasma ghrelin level was observed between 15 and 60 minutes after the start of infusion, as illustrated in fig 4A. Administration of saline or ghrelin was not associated with a change in plasma motilin levels (AUC 108551 (8895) (saline) v 112971 (7565) (ghrelin) min×pg/ml; NS), and no significant differences were found between both treatments (fig 4B). Plasma levels for motilin at the time of the ghrelin induced activity front did not differ between administration of ghrelin or saline (637 (54) v 705 (61) pg/ml; NS).
Figure 4 Plasma levels of ghrelin (A), motilin (B), and pancreatic polypeptide (C) before and after administration of placebo or ghrelin. After administration of ghrelin, ghrelin and pancreatic polypeptide plasma levels were significantly increased (p<0.05, two way ANOVA) whereas motilin plasma levels were not significantly altered.
Levels of somatostatin (AUC 31593 (1883) (saline) v 31992 (2588) (ghrelin) min×pg/ml; NS) and of glucagon (AUC 21195 (1550) (saline) v 21029 (1147) (ghrelin) min×pg/ml; NS) were not altered. Administration of ghrelin induced a significant increase in pancreatic polypeptide plasma levels (AUC 52530 (10932) (saline) v 91518 (15521) (ghrelin) min×pg/ml; p<0.03). Significant increases in pancreatic polypeptide levels occurred from 30 to 90 minutes after the start of ghrelin infusion (fig 4C).
Discussion
Ghrelin is a 28 amino acid peptide that was isolated from the stomach and found to be the natural ligand for the GHS receptor. Up until then, only synthetic analogues, such as GHRP‐6, were available. Apart from its effects on growth hormone secretion and energy balance, thereby counteracting the hormone leptin, distinct effects on gastrointestinal motility in animals have been described.
In the present study, we evaluated the influence of 40 μg of ghrelin on upper gastrointestinal motility in the fasting state in healthy volunteers. This dose was chosen based on the available literature and after two pilot studies with 20 μg had no clear effect. Elevated ghrelin plasma levels were obtained after infusion of ghrelin but not of saline. We observed that ghrelin induced a premature activity front with a gastric origin in all subjects. This was accompanied by an increase in proximal gastric tone which persisted for the remainder of the recording. Infusion of ghrelin was associated with a rise in plasma levels of pancreatic polypeptide but no changes in motilin, glucagon, or somatostatin plasma levels.
Ghrelin has a 27% amino acid identity with motilin, another important gastrointestinal hormone,1 and their respective receptors display a remarkable 52% identity.31 Together they constitute a new subfamily within class A of rhodopsin‐like GPCR. The absence of an increase in motilin plasma levels in our study indicates that the effect of ghrelin on induction of a premature phase III activity front was not mediated via release of motilin. The possibility that ghrelin exerts its effect partially via cross activation of the motilin receptor cannot be fully excluded however. Several studies in different models suggest that, in spite of the structural homology with motilin, ghrelin does not activate the motilin receptor. Indeed, receptor binding studies on membrane preparations from rabbit antrum revealed that ghrelin interacts very weakly with the motilin receptor.32 In cultured CHO cells, expressing the human motilin receptor and the Ca2+ indicator apoaequorin, ghrelin did not induce receptor activation, as measured by Ca2+ luminescence studies.33 Also, in guinea pig small intestine, ghrelin induced Ca2+ transients in myenteric neurones in situ in guinea pig small intestine, independent of motilin receptor activation.13 In rats, intracerebroventricular or intravenous injection of ghrelin induced fasted motor activity in the stomach and duodenum. This effect was blocked by injection of D‐lys3‐ghrelin, a GHS receptor antagonist.8
The increase in pancreatic polypeptide plasma levels is in line with previous reports that ghrelin stimulates pancreatic polypeptide release in humans which may be, to some extent, caused by vagal stimulation.17 However, induction of phase III activity is unlikely to occur through release of pancreatic polypeptide as this hormone is not involved in the regulation of the MMC.29,34 In contrast with the study of Arosio and colleagues,17 who used a higher dose of ghrelin than that in the present study, we did not observe any change in plasma somatostatin levels.
The effect of ghrelin in our study cannot be attributed exclusively either to direct GHS receptor stimulation in the stomach or duodenum or to stimulation of vagal efferent pathways. In the mouse, peripherally administered human ghrelin was shown to cross the blood‐brain barrier.35 It is unclear whether the same is true in humans. There is little doubt that the vagal nerve plays a pivotal role in ghrelin induced feeding behaviour, growth hormone release, and food deprivation induced increases in plasma ghrelin levels as these effects can be blocked by vagotomy and by selective chemical blocking of vagal afferents with capsaicin.36,37 In addition, expression of the ghrelin receptor in the rat nodose ganglion has been confirmed using reverse transcription‐polymerase chain reaction, direct sequencing, in situ hybridisation histochemistry, and electrophysiology.36,37,38 Although Fujino and colleagues8 showed that peripheral ghrelin may stimulate fasted motor activity in rats by activating neuropeptide Y neurones in the brain through receptors on vagal afferents, they also provided evidence for a direct peripheral action of ghrelin on gastroduodenal motility. Indeed, induction of phase III fasted motor activity in the stomach and duodenum of conscious rats by intravenously injected ghrelin could not be blocked by intracerebroventricular injection of the ghrelin antagonist D‐Lys3‐GHRP‐6 or by vagotomy. These studies are strengthened by other in vitro studies showing the presence of the GHS receptor in myenteric neurones in humans and rodents.10,11,12,13
Finally, the exact role of ghrelin in the regulation of fasted gastrointestinal motor activity remains to be elucidated. Plasma ghrelin levels during infusion were higher than those occurring after an overnight fast.23 In addition, we studied only one dose of this hormone but cost issues prohibited an extensive dose ranging study. These observations suggest that pharmacological effects were observed in the present study, and there is no evidence for involvement of ghrelin in the control of normal interdigestive motility in humans. So far, unlike motilin,39 fluctuation of plasma ghrelin levels in synchrony with phase III activity fronts has not been reported. Ghrelin levels display a diurnal rhythm in humans, in phase with plasma leptin levels, with a peak at night during fasting, and lowest concentrations in the morning after breakfast. The fact that plasma levels increase nearly twofold before a meal and drop within one hour postprandially rather suggests a role for ghrelin in meal initiation.40 A possible association between ghrelin levels and gastric emptying has been suggested,30 although studies with ghrelin knockout mice could not indicate a difference in gastric emptying parameters between wild‐type and ghrelin knockout mice, indicating that either ghrelin does not play a physiological role in the regulation of gastric emptying or that its effect is compensated by other peptides.41 The strong stimulatory effects of ghrelin on gastric motility observed in the present study suggest a potential therapeutic application of ghrelin agonists in the treatment of gastroparesis. Recent studies, using the same dose of 40 μg of ghrelin intravenously/30 min, as in the present study, or using a constant infusion of 5 pmol/kg/min, have established gastroprokinetic actions of ghrelin in idiopathic and in diabetic gastroparesis.42,43
In conclusion, intravenous administration of ghrelin induces a premature phase III activity and a prolonged increase in proximal gastric tone in healthy volunteers. The effect is direct and not mediated through release of other gastrointestinal hormones. The exact role of ghrelin in the physiological regulation of upper gastrointestinal motility has yet to be clarified.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Acknowledgements
R Bisschops and J Tack are supported by the FWO, Vlaanderen, Belgium.
Abbreviations
MMC - migrating motor complex
GHS - growth hormone secretagogue
MDP - minimal distending pressure
AUC - area under the curve
Footnotes
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Kojima M, Hosoda H, Date I.et al Ghrelin is a growth‐hormone releasing acylated peptide from the stomach. Nature 1999402656–660. [DOI] [PubMed] [Google Scholar]
- 2.Tschop M, Smiley D L, Heiman M L. Ghrelin induces adiposity in rodents. Nature 2000407908–913. [DOI] [PubMed] [Google Scholar]
- 3.Nakazato M, Murakami N, Date Y.et al A role for ghrelin in the central regulation of feeding. Nature 2001409194–198. [DOI] [PubMed] [Google Scholar]
- 4.Masuda Y, Tanaka T, Inomata N.et al Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 2000276905–908. [DOI] [PubMed] [Google Scholar]
- 5.Dornonville de la Cour C, Lindstrom E, Norlen P.et al Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 200412023–32. [DOI] [PubMed] [Google Scholar]
- 6.Trudel L, Tomasetto C, Rio M C.et al Ghrelin/motilin‐related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 2002282G948–G952. [DOI] [PubMed] [Google Scholar]
- 7.Kitazawa T, De Smet B, Verbeke K.et al Gastric motor effects of peptide and non‐peptide ghrelin agonists in mice in vivo and in vitro. Gut 2005541078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujino K, Inui Am Asakawa A, Kihara N.et al Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 2003550227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edholm T, Schmidt P T. Ghrelin stimulates interdigestive migrating myoelectrical complex in the rat small intestine: involvement of cholinergic neurons. Neurogastroenterol Motil. 2003;15: abstract 470, (Suppl 21)
- 10.Dass N B, Munonyara M, Bassil A K.et al Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience 2003120443–453. [DOI] [PubMed] [Google Scholar]
- 11.Depoortere I, De Winter B, Thijs T.et al Comparison of the prokinetic effects of ghrelin, GHRP‐6 and motilin in rats in vivo and in vitro. Gastroenterology 2004124580. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Depoortere I, Tomasetto C.et al Evidence for the presence of motilin, ghrelin and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept 2005124119–125. [DOI] [PubMed] [Google Scholar]
- 13.Bisschops R, Bugajski A, Depoortere I.et al Ghrelin induces calcium transients in guinea‐pig myenteric neurons through specific neuronal receptor activation (submitted)
- 14.Janssens J, Vantrappen G, Peeters T L. The activity front of the migrating motor complex of the human stomach but not of the small intestine is motilin‐dependent. Regul Pept 19836363–369. [DOI] [PubMed] [Google Scholar]
- 15.Peeters T L, Janssens J, Vantrappen G R. Somatostatin and the interdigestive migrating motor complex in man. Regul Pept 19835209–217. [DOI] [PubMed] [Google Scholar]
- 16.Larsen S, Osnes M, Christensen M S. The effect of glucagon, glucagon‐(1–21)‐peptide, and placebo on duodenal pressure activity in healthy subjects. Scand J Gastroenterol 198621634–640. [DOI] [PubMed] [Google Scholar]
- 17.Arosio M, Ronchi Cl, Gebbia C.et al Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Endocrinol Metab 200388701–704. [DOI] [PubMed] [Google Scholar]
- 18.Egido E M, Rodriguez‐Gallardo J, Silvestre R A.et al Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 2002146241–244. [DOI] [PubMed] [Google Scholar]
- 19.Tack J, Piessevaux H, Coulie B.et al Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 19981151346–1352. [DOI] [PubMed] [Google Scholar]
- 20.Tack J, Caenepeel P, Fischler B.et al Symptoms associated with hypersensitivity to gastric distension in functional dyspepsia. Gastroenterology 2001121526–535. [DOI] [PubMed] [Google Scholar]
- 21.Takaya K, Ariyasu H, Kanamoto N.et al Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 2000854908–4911. [DOI] [PubMed] [Google Scholar]
- 22.Peino R, Baldelli R, Rodriguez‐Garcia J.et al Ghrelin‐induced growth hormone secretion in humans. Eur J Endocrinol 2000143R11–R14. [DOI] [PubMed] [Google Scholar]
- 23.Nagaya N, Kojima M, Uematsu M.et al Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 2001280R1483–R1487. [DOI] [PubMed] [Google Scholar]
- 24.Vantrappen G, Janssens J, Hellemans J.et al The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest 1977591158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilmer A, Tack J, Coremans G.et al 5‐hydroxytryptamine3 receptors are involved in the initiation of gastric phase 3 motor activity. Gastroenterology 1993105773–778. [DOI] [PubMed] [Google Scholar]
- 26.Tack J, Janssens J, Vantrappen G.et al Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 199210372–79. [DOI] [PubMed] [Google Scholar]
- 27.Wilmer A, Andrioli A, Coremans G.et al Ambulatory small intestinal manometry. Detailed comparison of duodenal and jejunal motor activity in healthy man. Dig Dis Sci 1997421618–1627. [DOI] [PubMed] [Google Scholar]
- 28.Bormans V, Peeters T L, Janssens J.et al In man, only activity fronts that originate in the stomach correlate with motilin peaks. Scand J Gastroenterol 198722781–784. [DOI] [PubMed] [Google Scholar]
- 29.Janssens J, Hellemans J, Adrian T E.et al Pancreatic polypeptide is not involved in the regulation of the migrating motor complex in man. Regul Pept 1982341–49. [DOI] [PubMed] [Google Scholar]
- 30.Chayvialle J A, Miyata M, Rayford P L.et al Effects of test meal, intragastric nutrients, and intraduodenal bile on plasma concentrations of immunoreactive somatostatin and vasoactive intestinal peptide in dogs. Gastroenterology 198079844–852. [PubMed] [Google Scholar]
- 31.McKee K K, Palyha O C, Feighner S D.et al Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol 199711415–423. [DOI] [PubMed] [Google Scholar]
- 32.Depoortere I, Thijs T, Thielemans L.et al Interaction of the growth hormone‐releasing peptides ghrelin and growth hormone‐releasing peptide‐6 with the motilin receptor in the rabbit gastric antrum. J Pharmacol Exp Ther 2003305660–667. [DOI] [PubMed] [Google Scholar]
- 33.Depoortere I, Thielemans l, Thijs T.et al Do ghrelin and the growth hormone releasing peptide, GHRP‐6, interact with the motilin receptor? Neurogastroenterol Motil 200113387 [Google Scholar]
- 34.Chung S A, Greenberg G R, Diamant N E. Relationship of postprandial motilin, gastrin and pancreatic polypeptide release to intestinal motility during vagal interruption. Can J Physiol Pharmacol 1992701148–1153. [DOI] [PubMed] [Google Scholar]
- 35.Banks W A, Tschop M, Robinson S M.et al Extent and direction of ghrelin transport across the blood‐brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 2002302822–827. [DOI] [PubMed] [Google Scholar]
- 36.Date Y, Murakami N, Toshinai K.et al The role of the gastric afferent vagal nerve in ghrelin‐induced feeding and growth hormone secretion in rats. Gastroenterology 20021231120–1128. [DOI] [PubMed] [Google Scholar]
- 37.Williams D L, Grill H J, Cummings D E.et al Vagotomy dissociates short‐ and long‐term control of circulating ghrelin. Endocrinology 20031445184–5187. [DOI] [PubMed] [Google Scholar]
- 38.Sakata I, Yamazaki M, Inoue K.et al Growth hormone secretagogue receptor expression in the cells of the stomach‐projected afferent nerve in the rat nodose ganglion. Neurosci Lett 2003342183–186. [DOI] [PubMed] [Google Scholar]
- 39.Peeters T L, Vantrappen G, Janssens J. Fasting plasma motilin levels are related to the interdigestive motility complex. Gastroenterology 198079716–719. [PubMed] [Google Scholar]
- 40.Cummings D E, Purnell J Q, Frqyo R S.et al A preprandial reise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001501714–1719. [DOI] [PubMed] [Google Scholar]
- 41.De Smet B, Depoortere I, Moreaux B.et al Gastric emptying and food intake in ghrelin knockout mice. Gastroenterology 2004126A90 [Google Scholar]
- 42.Tack J, Depoortere I, Bisschops R.et al Influence of ghrelin on gastric emptying and meal‐related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther 200522847–853. [DOI] [PubMed] [Google Scholar]
- 43.Murray C D R, Martin N M, Patterson M.et al Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 2005541693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.