Abstract
Background and aims
The intestinal microbiota play a pivotal role in the inflammation associated with Crohn's disease through their interaction with the mucosal immune system. Some bifidobacteria species are immunoregulatory and induce increased dendritic cell interleukin 10 (IL‐10) release in vitro. Fructo‐oligosaccharides (FOS) increase faecal and mucosal bifidobacteria in healthy volunteers. The aim of this study was to assess the effect of FOS administration on disease activity, bifidobacteria concentrations, and mucosal dendritic cell function in patients with moderately active Crohn's disease.
Patients and methods
Ten patients with active ileocolonic Crohn's disease received 15 g of FOS for three weeks. Disease activity was measured using the Harvey Bradshaw index. Faecal and mucosal bifidobacteria were quantified by fluorescence in situ hybridisation, and mucosal dendritic cell IL‐10 and Toll‐like receptor (TLR) expression were assessed by flow cytometry of dissociated rectal biopsies.
Results
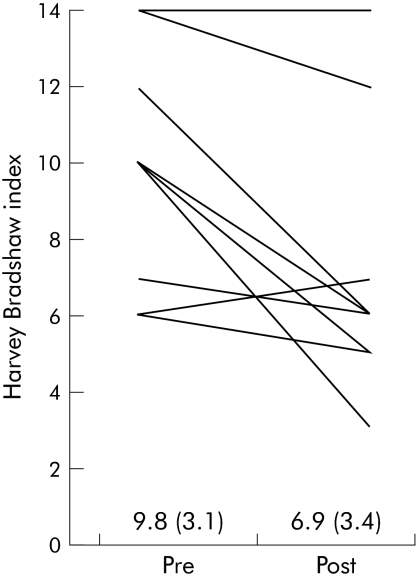
FOS induced a significant reduction in the Harvey Bradshaw index from 9.8 (SD 3.1) to 6.9 (3.4) (p<0.01). There was a significant increase in faecal bifidobacteria concentration from 8.8 (0.9) log10 to 9.4 (0.9) log10 cells/g dry faeces (p<0.001). The percentage of IL‐10 positive dendritic cells increased from 30 (12)% to 53 (10)% (p = 0.06). Finally, the percentage of dendritic cells expressing TLR2 and TLR4 increased from 1.7 (1.7)% to 36.8 (15.9)% (p = 0.08) and from 3.6 (3.6)% to 75.4 (3.4)% (p<0.001), respectively.
Conclusions
FOS supplementation increases faecal bifidobacteria concentrations and modifies mucosal dendritic cell function. This novel therapeutic strategy appears to decrease Crohn's disease activity in a small open label trial and therefore warrants further investigation.
Keywords: fructo‐oligosaccharide, oligofructose, inulin, prebiotic, Crohn's disease
The intestinal inflammation associated with Crohn's disease is characterised by an imbalance within the mucosal immune system leading to an abnormal Th1 T cell driven inflammatory response. The intestinal microbiota play a crucial role in perpetuating this inflammation in both animal models and patients with Crohn's disease.1 Thus in transgenic murine models of Crohn's disease, animals kept under germ free conditions do not develop inflammation until bacteria are introduced.2 Likewise, the number of mucosal adherent bacteria is increased in patients with Crohn's disease compared with healthy subjects, and diversion of the bacterial component of the faecal stream induces clinical improvement.3,4,5 There is also evidence for an immunoregulatory role for the commensal microbiota which protect against intestinal inflammation and upregulate epithelial defence mechanisms in animal models of colitis.6,7,8 In support of these differential effects of bacteria on intestinal inflammation, there are marked differences in both faecal and mucosal microbiota in patients with Crohn's disease compared with healthy subjects. Although results vary due to differences in both patient groups and the methods of quantifying the intestinal microbiota, higher concentrations of bacteroides and lower bifidobacteria have been demonstrated in both the faeces and mucosa of patients with Crohn's disease. Furthermore, entering disease remission is associated with changes in faecal bacteroides and Clostridum coccoides.3,9,10
Recent interest has focused on the mechanisms by which bacteria exert these differential effects on intestinal inflammation. It is likely that intestinal dendritic cells (DC), linking the innate and adaptive immune systems, are involved in early bacterial recognition.11 Gut DC have tissue specific properties which likely reflect adaptations to their microbe rich environment and are able to interact with bacteria both directly by passing dendrites through epithelial cell tight junctions into the gastrointestinal lumen and indirectly with bacteria that have been processed by M cells.12,13 DC recognise and respond to bacteria using pattern recognition receptors such as Toll‐like receptors (TLR) which bind microbial signature molecules termed pathogen associated molecular patterns. Subpopulations of DC differ in their expression of TLRs and the repertoire of microbial components to which they are able to respond.14 This responsiveness gives mucosal DC that have been modulated by bacteria the ability to activate and influence the functional differentiation of naive T cells.12 On contact with bacteria they secrete cytokines and express costimulatory molecules that in part determine whether a Th1, Th2, or regulatory T cell response predominates. DC are involved in the active maintenance of non‐responsiveness to self‐antigens and to generation of oral tolerance to fed antigens.15,16 By extension they are likely to be involved in limiting responses to the commensal microbiota.
The importance of the intestinal microbiota in the regulation of intestinal inflammation raises the possibility of its modulation as a therapy for Crohn's disease. Antibiotic therapy induces broad spectrum changes in the intestinal microbiota but has only a modest therapeutic effect predominantly in patients with ileocolonic and perianal Crohn's disease.17 Manipulating the microbiota with probiotics ameliorates disease in animal models of colitis and may have some benefit in maintaining surgically induced remission in patients with Crohn's disease.18,19 In studies using cells from healthy donors, the probiotic VSL#3 induced interleukin (IL)‐10 expression in both peripheral and intestinal CD11c+ (myeloid) DC after in vitro culture.20 Individual bacterial strains within VSL#3 displayed distinct immunomodulatory properties with the most marked immunoregulatory being induced by bifidobacteria. Culture with bifidobacteria increased peripheral blood DC IL‐10 production, decreased costimulatory CD80 expression, and decreased interferon γ release from cocultured allogenic CD4+ T cells.20
The growth of intestinal bifidobacteria can be stimulated by dietary supplementation with prebiotics.21 For example, fructo‐oligosaccharides (FOS), which are non‐digestible polymers of fructose found naturally in artichokes, leeks, asparagus, onions, and bananas, stimulate the growth of both faecal and mucosal bifidobacteria in healthy subjects.22,23,24 In animal models of colitis, FOS decreased disease activity, enhanced luminal bifidobacteria, and inhibited intracellular transcription factors such as nuclear factor κB.25 Rats fed a mixture of FOS and a probiotic bifidobacterium demonstrated enhanced IL‐10 release from Peyer's patches and increased caecal secretory IgA levels compared with controls.26 Despite the potential of FOS in patents with Crohn's disease there is surprisingly little evidence that FOS have such clinical, microbiological, and immunological effects in this group.19
The aim of this study was to investigate the clinical, microbiological, and immunological effects of FOS in patients with moderately active Crohn's disease with the hypothesis that FOS supplementation would result in an increase in faecal and mucosal bifidobacteria and induce immunoregulatory DC responses leading to a reduction in disease activity.
Materials and methods
Patient selection
The trial was approved by the Harrow Local Research Ethics Committee and informed consent was obtained prior to any study related procedure. Patients at St Mark's Hospital between the ages of 18 and 80 years with moderately active colonic or ileocolonic disease (Harvey Bradshaw Index (HBI) >5) were included.27 Diagnosis was confirmed using standard clinical, radiological, and histological criteria.28 Exclusion criteria were a positive stool culture for an enteropathogen; severe disease or imminent need for surgery; significant hepatic, renal, endocrine, respiratory, neurological, or cardiovascular disease; pregnancy or lactation; previous pan‐proctocolectomy; short bowel syndrome; or pure anal disease. In order to ensure stable maintenance therapy, patients were excluded where they had a change in dose of azathioprine/methotrexate during the preceding 12 weeks or oral steroids/5‐aminosalicylic acid during the preceding four weeks, or a total steroid dose in excess of 10 mg prednisolone or equivalent per day. These permitted medications were continued at the same dose throughout the study. Patients were also excluded if they had used any antibiotics, probiotics, or commercially available prebiotic preparations during the preceding four weeks; any rectal preparations during the preceding two weeks; and any non‐steroidal anti‐inflammatory drug during the preceding week.
Trial protocol
The trial consisted of a one week baseline period (week 1) followed by a three week intervention (weeks 2–4). Patients meeting the inclusion criteria recorded symptoms for one week, after which they returned; disease activity was calculated, and patients with a HBI >5 continued in the study. A patient and physician global assessment score was recorded. A fresh faecal sample was collected for analysis of faecal microbiota and an unprepared flexible sigmoidoscopy was performed during which 10 biopsies were taken from non‐inflamed rectal mucosa for analysis of mucosal microbiota and immunology. Finally, a blood sample was collected for measurement of full blood count, C reactive protein (CRP), and routine biochemistry.
Patients received 15 g/day of FOS (Prebio 1; Nestlé, Switzerland) for consumption as a dietary supplement for three weeks. FOS contained a mixture of oligofructose (70%) and inulin (30%) provided in 15 g sachets to be dissolved in water or food. This dose was selected as it induces proliferation of faecal and mucosal bifidobacteria without inducing excessive gastrointestinal symptoms in healthy subjects.23 Patients continued on their normal diet and were advised not to consume any products containing probiotic or prebiotic compounds. Patients recorded gastrointestinal symptoms for a further three weeks. They continued on any concurrent medication with no dose modification.
After three weeks, the same investigator reviewed the patients, and disease activity and global assessment scores were determined. CRP, full blood count, and biochemistry were repeated. A fresh faecal sample and mucosal biopsies were collected as before. Any unused FOS was returned and weighed as a covert measure of compliance.
Outcome measures
The outcome measures in this study were tolerability of FOS, disease activity, physician and patient global assessment score, faecal and mucosal microbiota, mucosal DC IL‐10 production, and TLR expression. Ingestion of FOS can be associated with gastrointestinal symptoms such as abdominal bloating and flatulence.23 Patients recorded the incidence and severity of acid reflux, rumbling gut, abdominal bloating, abdominal pain, and flatulence using the scale 0 (absent), 1 (mild), 2 (moderate), and 3 (severe).29 The sum of the daily scores was compared for each week of the trial. Disease activity was measured using the HBI and Crohn's disease activity index (CDAI).30 Induction of clinical remission was defined as a reduction in HBI to 5 or less. HBI was used as the primary disease activity index as subjective criteria such as abdominal pain and general well being which may be affected by FOS have less weighting than in the CDAI. Physician and patient global assessment scores were recorded on an analogue scale from 0–100 (0 = worst possible).28
Microbiological assessment
Constituents of the faecal and mucosal microbiota were quantified using fluorescence in situ hybridisation. Faecal and mucosal microbiota were fixed and hybridised with indocarbocyanin labelled oligonucleotide DNA probes targeting total bacteria, bifidobacteria, Clostridium coccoides, and Bacteroides‐Prevotella (Microsynth, Switzerland; see table 1), as detailed below. These bacterial groups were chosen because they have been shown to be altered in patients with Crohn's disease compared with controls or between patients with active and inactive disease.3,9,10
Table 1 Oligonucleotide probes used for fluorescent in situ hybridisation.
| Target bacterial group | Probe | Sequence (5′‐3′) |
|---|---|---|
| Universal bacteria | EUB (Amann31) | ‐GCT GCC TCC CGT AGG AGT‐ |
| Bifidobacteria | Bif 164 (Langendijk45) | ‐CAT CCG GCA TTA CCA CCC‐ |
| Clostridium coccoides‐Eubacterium rectale cluster | EREC 482 (Franks31) | ‐GCT TCT TAG TCA RGT ACC G‐ |
| Bacteroides‐Prevotella cluster | Bac 303 (Manz47) | ‐CCA ATG TGG GGG ACC TT‐ |
Faecal bacteria were harvested by centrifugation, fixed overnight in 4% (w/v) paraformaldehyde, and washed in phosphate buffered saline (PBS). Fixed bacteria were spotted on 3‐aminopropyltriethoxysilane treated eight well slides, air dried, and serially dehydrated in 60%, 80%, and 96% (v/v) ethanol. Fixed bacteria were hybridised with the oligonucleotide probes according to a previously published protocol.31 Briefly, probes were diluted to a concentration of 4.5 ng/μl in sterile hybridisation buffer (0.9 M NaCl; 0.02 M Tris/HCl; 0.01% (w/v) sodium dodecyl sulphate) and 10 μl added to each well which was then incubated at 46°C overnight in a light free saturated humidity chamber. Slides were washed to remove unbound probe prior to quantification.
Mucosal biopsies were immediately snap frozen in liquid nitrogen and stored at −80°C prior to further processing. Then, 5 μm cryostat sections were cut, placed onto poly L‐(+) lycine coated glass slides, and fixed in 4% (w/v) paraformaldehyde. A representative section was stained with haematoxylin and eosin to confirm correct orientation of the tissue. Slides were washed in PBS, incubated in sterile hybridisation buffer at 46°C for one hour, and then in hybridisation buffer containing 200 ng of the relevant probe at 46°C overnight. Slides were then washed to remove unbound probe prior to quantification.32
Hybridised faecal and mucosal microbiota were quantified by viewing the slides under an Axioplan 2 imaging microscope (Zeiss, Germany) equipped with an HBO‐100 fluorescent lamp (Osram, Germany) and filterset number 15 (Zeiss). In each case the microscopist was unaware of whether the slides were from patients during the baseline or FOS period. Faecal bacterial concentrations were expressed as cells per gram of dry faeces in order to standardise comparisons between samples. Mucosal bacterial concentrations were expressed as cells/mm of epithelial surface.
Immunological assessment
Antibodies
Antibodies with the following specificities and fluorochrome labels were used: CD3‐PC5 (UCHT‐1), CD14‐PC5 (MIP9), CD16‐PC5 (B73.1), CD19‐PC5 (4G7), CD56‐PC5 (N901), and CD8‐PC5 (B9.11) from Beckman Coulter (High Wycombe, UK); TLR2‐FITC (TL2.1) and TLR4‐FITC (HTA125) from Serotec (Oxford, UK); and CD11c‐PE (B‐ly6), HLA‐DR‐APC (G46‐6), and CD8‐FITC/PE/APC (SK1) from BD Biosciences (Oxford, UK). Intracellular cytokine staining used: IL‐10‐PE (JES3‐9D7; Serotec), IL‐12p40/p70‐PE (C11.5; BD Biosciences), and IL‐6 PE (#1936; R&D Minneapolis, Minnesota, USA).
Isolation of lamina propria mononuclear cells
The method used was described in detail by Bell and colleagues.33 Eight mucosal biopsies (approximately 60 mg) were taken per patient. Biopsies were collected in transport medium: RPMI‐1640 Dutch modification (Sigma‐Aldrich, Dorset, UK) supplemented with 10% fetal calf serum (FCS), gentamicin (25 μg/ml), and penicillin/streptomycin (100 U/ml)(complete medium). Mucus and faeces were removed from the tissue using 1 mM dithiothreitol (Sigma‐Aldrich) in Hanks' balanced salt solution (HBSS; GibcoBRL, Paisley, UK) for 20 minutes in T25 flasks. The epithelium was removed using two 30 minute treatments with 1 mM EDTA in calcium and magnesium free HBSS at 37°C with gentle agitation. Biopsy samples were washed in HBSS between each treatment. Tissue was digested by agitation in a solution of 1 mg/ml collagenase D (Roche Diagnostics Ltd, East Sussex, UK) in RPMI‐1640 HEPES (Sigma Aldrich Co. Ltd, Irvine, UK) containing 2% FCS and 20 μg/ml DNAase I (Roche Diagnostics Ltd) for 120 minutes at 37°C. After incubation, lamina propria mononuclear cells (LPMC) released from tissue samples were passed through a cell strainer and washed in complete medium. Cells were assessed for viability by their ability to exclude trypan blue.
Cell surface labelling
LPMC were suspended in PBS containing 2% FCS, 1 mM EDTA, and 0.02% sodium azide (FACS buffer). A minimum of 50 000 LPMC were used per antibody labelling. Antibodies were added at predetermined optimal concentrations. Cell surface labelling of LPMC was performed on ice for 20 minutes and cells were then washed twice by centrifugation in FACS buffer (300 g, 10 minutes, 4°C). Paraformaldehyde (500 μl of a 1.0%w/v solution in 0.85% saline) was added and samples were stored at 4°C until acquisition on the flow cytometer within 24 hours.
Intracellular cytokine staining
LPMC were placed in wells at 2.5×105 cells per well in complete medium (96 well U bottom Falcon; BD Biosciences). Paired cultures, one incubated with monensin (3 μmol/l) to maintain cytokines within the cells and the other incubated without monensin, were cultured for four hours at 37°C in a humidified atmosphere of 5% CO2 in air. No exogenous stimulus was added to these in vitro cultures. Cells were then labelled for surface markers, fixed with Leucoperm A (100 μl; Serotec), and permeabilised with Leucoperm B (100 μl; Serotec). Anti‐cytokine antibodies IL‐10‐PE, IL‐12‐PE, or IL‐6‐PE (5 μl) were added for 30 minutes, cells were washed, and finally cells were resuspended in 1% paraformaldehyde.
Flow cytometry
Data were acquired using a FACSCalibur flow cytometer. Using multicolour analysis, DC were identified as an HLA‐DR+ lineage− (CD3−, CD14−, CD16−, CD19−, CD34−, CD56−) population and within this gate the CD11c+ population was assessed. Analysis of TLR positive and cytokine positive cell populations used WinList version 5.0 flow cytometry software (Verity, Topsham, Maine, USA). TLR positive CD11c+ DC were defined by subtracting staining of the appropriate isotype control from staining of the TLR using the super enhanced Dmax (SED) normalised subtraction facility in the Winlist 5.0 software package.
The proportion of cytokine positive cells was determined using SED normalised subtraction: staining of cells cultured in the absence of monensin was subtracted from staining of cells cultured in the presence of monensin, giving a measure of ongoing cytokine production.20 This technique allows positive cells to be resolved in situations where distribution histograms overlap. The use of the same antibody to label cells from both monensin treated and untreated cultures gives this technique a high degree of sensitivity for detecting small changes in antibody binding. Specificity of antibody labelling was confirmed in competition experiments with unlabelled antibodies.20
Statistical analysis
Continuous data were compared at baseline and following FOS supplementation using a paired two tailed t test. Continuous data were compared between patients in remission and not in remission using an independent two tailed t test. Faecal bacterial concentrations were log transformed prior to statistical analysis due to their log normal distribution in population samples. Correlations were assessed using Spearman's rank correlation test. Patient and physician global assessment and CRP were compared using the Wilcoxon signed rank test. Values of p<0.05 were regarded as statistically significant.
Results
Patient cohort
Ten patients (eight males, two female) with a mean age of 40 years (range 29–46) with active ileocolonic or colonic Crohn's disease were recruited to the study. Mean HBI at baseline was 9.8 (SD 3.1). Baseline demographic data and concomitant medication are shown in table 2. All 10 patients completed the one week baseline period and the three week intervention.
Table 2 Baseline demographic data for patients recruited to the trial.
| Patient group (n = 10) | |
|---|---|
| Age (y) (median (range)) | 40 (29–56) |
| Sex (male:female) | 8:2 |
| Smoking (n) | 3 |
| Disease characteristics (n) | |
| Ileocolonic | 7 |
| Colonic | 3 |
| Perianal disease | 2 |
| HBI (mean (SD) [range]) | 9.8 (3.1) [6–14] |
| Concomitant medication (n) | |
| 5‐aminosalicylate | 4 |
| Steroids | 5 |
| Azathioprine/6 mercaptopurine* | 2 |
| Methotrexate | 0 |
| None | 3 |
| Previous surgery | |
| Ileocaecal resection (right hemicolectomy) | 5 |
| Proctocolectomy and distal colostomy | 1 |
HBI, Harvey Bradshaw index.
*At least six months' duration.
Compliance and tolerability
Compliance with 15 g FOS supplementation was measured by weighing the amount of FOS returned at the end of the trial. In total, 91 (15)% of the prescribed FOS was consumed, with 60% of patients consuming all 15 g each day. This equates to an average FOS consumption of 13.7 (2.3) g/day. Gastrointestinal symptoms were reported on most days both during baseline and FOS supplementation (table 3). The only significant changes between the baseline week and the last week of FOS supplementation were an increase in the incidence of “gut rumbling” (baseline 4.0 days v 5.4 days after FOS; p = 0.049) and an increase in the severity of flatulence (8.0 (3.7) at baseline compared with 11.1 (3.2) after FOS; p = 0.009). No other side effects or serious adverse events were reported.
Table 3 Comparison of the incidence and severity of symptoms at baseline and during fructo‐oligosaccharide (FOS) supplementation.
| Baseline (week 1) | FOS week 3 | p Value (paired t test) | |
|---|---|---|---|
| Incidence* | |||
| Acid reflux | 3.0 (3.4) | 2.7 (2.9) | 0.304 |
| Rumbling gut | 4.0 (2.9) | 5.4 (1.9) | 0.049 |
| Abdominal bloating | 5.1 (2.8) | 5.2 (2.6) | 0.826 |
| Abdominal pain | 6.3 (1.6) | 6.1 (1.8) | 0.396 |
| Flatulence | 5.8 (1.6) | 6.6 (0.5) | 0.104 |
| Severity† | |||
| Acid reflux | 4.4 (5.1) | 4.3 (4.7) | 0.742 |
| Rumbling gut | 6.0 (4.9) | 7.4 (3.8) | 0.232 |
| Abdominal bloating | 7.0 (5.2) | 8.4 (5.7) | 0.457 |
| Abdominal pain | 11.0 (4.3) | 10.8 (4.9) | 0.534 |
| Flatulence | 8.0 (3.7) | 11.1 (3.2) | 0.009 |
*Mean (SD) number of days subjects reported each symptom over a seven day period.
†Mean (SD) cumulative score subjects reported for each symptom over a seven day period using the score: 0 = absent; 1 = mild; 2 = moderate; and 3 = severe.
Disease activity
There was a significant fall in mean HBI from 9.8 (SD 3.1) at baseline to 6.9 (3.4) after FOS (p = 0.01) (fig 1). Four patients went into clinical remission, as defined by a fall in HBI to 5 or less. The mean reduction in HBI of those patients entering clinical remission was 4.5 (range 1–7) compared with the overall mean reduction in HBI of 3 (range 0–7). There was no significant difference in mean reduction of HBI when comparing patients with or without a prior right hemicolectomy (n = 5 per group). Mean CDAI fell from 250.9 (SD 77.8) to 220.6 (127.8) (p = 0.39). In addition, there were significant increases in both the physician and patient global assessment score. Mean physician scores increased from 65.5 (SD 11.7) at baseline to 77.5 (13.4) after FOS supplementation (p<0.01). Likewise, the patient global score increased from 58.6 (18.2) at baseline to 73.1 (14.5) after FOS (p<0.005). Finally, there was a trend towards a reduction in CRP (35 (46) to 24 (28)) although this did not reach statistical significance (p = 0.12).
Figure 1 Fructo‐oligosaccharide (FOS) supplementation resulted in a reduction in disease activity. Ten patients with active Crohn's disease on stable medication received 15 g/day FOS for three weeks. Disease activity (Harvey Bradshaw index (HBI)) was recorded at baseline and after three weeks of FOS supplementation. HBI values for each patient before and after FOS are shown. Differences between mean HBI values were assessed using a paired two tailed Student's t test (9.8 (SD 3.1) at baseline and 6.9 (3.4) after FOS; p = 0.01).
Microbiological results
Previous studies have demonstrated that 15 g FOS per day leads to an increase in both faecal and mucosal bifidobacteria throughout the colon in healthy volunteers.23,24 Faecal samples were available at baseline and following FOS supplementation for eight of the 10 patients. There was a significant increase in the concentration of faecal bifidobacteria following FOS supplementation (baseline 8.8 (0.9) v FOS 9.4 (0.9) log10 cells/g dry faeces; p = 0.005). Although studies in healthy subjects have demonstrated an inverse association between baseline concentrations of bifidobacteria and the magnitude of the bifidogenic effect of FOS, this was not confirmed in patients with Crohn's disease (Pearson correlation coefficient −0.033; p = 0.419).34 Likewise, there was no correlation between the magnitude of bifidogenesis and a reduction in disease activity, as measured by the HBI. There were no significant changes in concentrations of total bacteria, bacteroides, or C coccoides‐E rectale (table 4). Finally, prior right hemicolectomy did not alter the bifidogenic effect of FOS in this patient group (data not shown).
Table 4 Effect of fructo‐oligosaccharides (FOS) on faecal and mucosal microbiota in patients with Crohn's disease.
| Faecal (log10 cells/g dry faeces) (n = 8) | Mucosal (cells/mm) (n = 10) | p Value | ||||
|---|---|---|---|---|---|---|
| Baseline | FOS | p Value | Baseline | FOS | ||
| Universal bacteria | 10.1 (0.7) | 10.3 (0.6) | 0.329 | 636 (273) | 569 (287) | 0.614 |
| Bifidobacteria | 8.8 (0.9) | 9.4 (0.9) | <0.0005 | 299 (186) | 326 (211) | 0.761 |
| C coccoides‐E rectale | 9.6 (0.8) | 9.6 (0.5) | 0.824 | 20 (20) | 22 (23) | 0.842 |
| Bacteroides‐Prevotella | 9.6 (0.8) | 9.8 (0.7) | 0.329 | 75 (49) | 84 (43) | 0.696 |
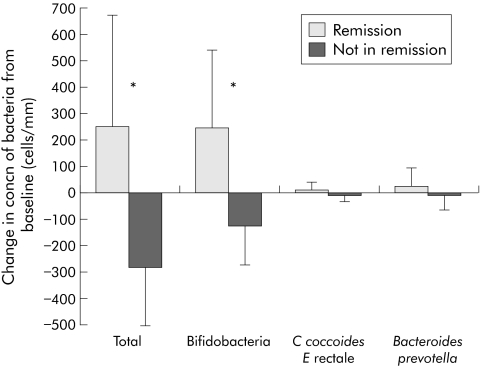
Despite increases in faecal bifidobacteria after FOS supplementation, there was no corresponding change in mucosal bifidobacteria (baseline 299 (186) v FOS 326 (211) cells/mm mucosa, n = 10; p = 0.761). Likewise, there were no increases in mucosal concentrations of total bacteria, C coccoides‐E rectale or bacteroides following FOS supplementation (table 4). However, there were strikingly different changes in mucosal microbiota following FOS supplementation between patients who entered disease remission (n = 4) and those who did not (n = 6). Patients who entered disease remission had an increase in mucosal concentration of total bacteria (remission +254 (417) v active −280 (227); p = 0.029) and bifidobacteria (remission +250 (293) v active −121 (151); p = 0.029) (fig 2).
Figure 2 Patients who entered clinical remission with fructo‐oligosaccharides (FOS) had increased mucosal bifidobacteria. Mucosal biopsies were collected at baseline and after three weeks of FOS supplementation (n = 10). Mucosal microbiota were assessed by fluorescence in situ hybridisation. Mean change in mucosal microbiota after FOS in patients who entered clinical remission (n = 4) was compared with those that had persistent disease activity (n = 6). Results are expressed as mean (SD) change in the number of bacteria/mm epithelial surface. Differences were assessed using an independent two tailed Student's t test (*p<0.05).
Immunological results
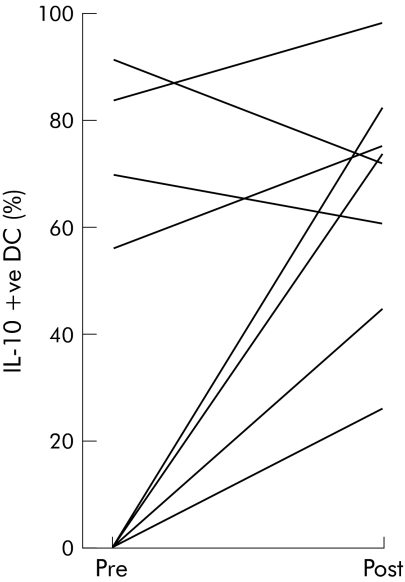
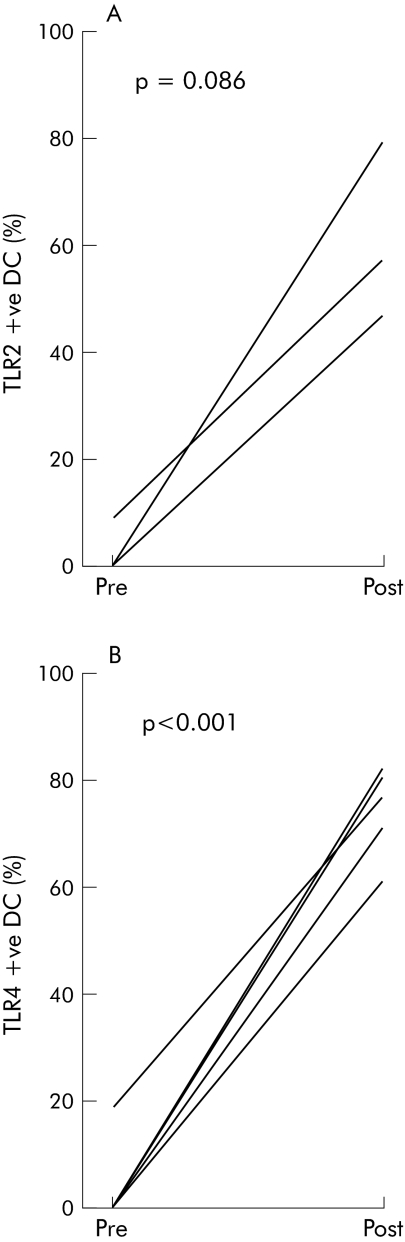
Histological analysis of rectal biopsies showed no evidence of active inflammation at any biopsy site. Figure 3 shows the percentage of IL‐10 positive CD11c+ DC in each of the Crohn's disease patients before and after dietary FOS supplementation. There was an increase in the percentage of IL‐10 positive CD11c+ DC following FOS supplementation (baseline 30.1 (38) v FOS 53.3 (33); p = 0.06) In contrast, FOS supplementation had no significant effect on the number of IL‐6 or IL‐12 positive CD11c+ intestinal DC (data not shown). We investigated whether the immunoregulatory DC responses documented above were associated with alterations in TLR2 or TLR4 expression. DC from five patients were analysed; insufficient cells were obtained from the remainder. The proportion of TLR+ myeloid DC before and after FOS supplementation is shown in fig 4. At baseline the percentage of CD11c+ intestinal DC expressing TLR2 and TLR4 was 1.7 (3.4)% and 3.6 (7.1)%. However, after FOS supplementation there was a marked increase in DC expression of both TLR2 and TLR4 (36.8 (32)%, p = 0.086; and 75.4 (7.9)%, p<0.001, respectively).
Figure 3 Fructo‐oligosaccharide (FOS) supplementation increased intestinal CD11c+ dendritic cell (DC) interleukin 10 (IL‐10) production. Lamina propria mononuclear cells were dissociated from mucosal biopsies taken from non‐inflamed rectum before and after FOS (n = 10). DC were identified as an HLA‐DR+ lineage− (CD3−, CD14−, CD16−, CD19−, CD34−, CD56−) population and within this gate the CD11c+ population was assessed. The proportion of IL‐10 positive cells was determined after intracellular cytokine staining using super enhanced Dmax normalised subtraction. Staining of cells cultured in the absence of monensin was subtracted from staining of cells cultured in the presence of monensin, giving a measure of ongoing cytokine production. Percentage of IL‐10 positive CD11c+ DC for each patient before and after FOS is shown.
Figure 4 Fructo‐oligosaccharides (FOS) increased CD11c+ dendritic cell (DC) toll‐like receptor (TLR)2 and TLR4 expression. Lamina propria mononuclear cells were dissociated from mucosal biopsies taken from non‐inflamed rectum before and after FOS (n = 10). DC were identified as an HLA‐DR+ lineage− (CD3−, CD14−, CD16−, CD19−, CD34−, CD56−) population and within this gate the CD11c+ population was assessed. TLR positive CD11c+ DC were defined by subtracting staining of the appropriate isotype control from staining of the TLR (n = 5/TLR). The percentage of TLR2 (A) and TLR4 (B) CD11c+ DC for each patient is shown at baseline and after FOS. Differences were assessed using a paired two tailed Student's t test.
Discussion
We have reported the first trial of FOS as a primary therapy for moderately active Crohn's disease. This therapeutic strategy is based on the evidence that FOS stimulate the growth of both faecal and mucosal bifidobacteria in healthy subjects and that these bacteria induce immunoregulatory mucosal DC responses in vitro.20,23,24 We demonstrated a significant reduction in disease activity in patients with moderately active Crohn's disease after FOS supplementation. In addition, FOS induced a marked increase in the concentration of faecal bifidobacteria and were associated with a significant increase in mucosal bifidobacteria in those patients that entered disease remission compared with those who did not. Finally, there was an increase in the percentage of lamina propria DC expressing IL‐10 and significant upregulation of TLR2 and TLR4 expression.
Dietary supplementation with FOS is associated with gastrointestinal symptoms such as bloating and flatulence in healthy subjects. Despite our concern that patients with Crohn's disease would experience increased gastrointestinal symptoms compared with healthy controls, the intervention was well tolerated. Patients reported an increase in only the incidence of gut rumbling and the severity of flatulence, the magnitude of which was consistent with that observed in healthy subjects.23,24,29 Compliance with the study intervention was in excess of 90% and complete compliance was recorded in six out of the 10 patients. The lowest recorded FOS intake (8.7 g per day, in one patient) is still in excess of amounts that are bifidogenic in healthy subjects.23 No other adverse events were reported.
Despite the increase in gastrointestinal symptoms associated with FOS supplementation there was still a significant reduction in HBI during the study period. This was an open label trial and caution should be used when interpreting the results of clinical efficacy due to the potential for a placebo response. However, in a recent meta‐analysis, study duration and number of study visits were positively associated with placebo effect.35 Our study had a short duration and only three study visits in order to minimise placebo response rates. The magnitude of the clinical response and percentage of patients entering clinical remission were similar to those seen in other recent reports of novel therapies in moderately severe Crohn's disease.36 In view of these preliminary findings, the efficacy of FOS supplementation in patients with Crohn's disease requires confirmation in an appropriately powered, double blind, placebo controlled trial.
Dietary supplementation with 15 g FOS per day leads to a significant increase in both faecal and mucosal bifidobacteria in healthy subjects.23,24 However, it was not previously known whether FOS are bifidogenic in patients with Crohn's disease who may have alterations in both intestinal microbiota and gastrointestinal transit times, which may affect colonic fermentation capacity. Using fluorescence in situ hybridisation, our results demonstrated that FOS increased the concentration of bifidobacteria in the faeces of patients with active ileocolonic Crohn's disease. This was specific for bifidobacteria, as no increase in total bacteria, C coccoides‐E rectale or Bacteroides‐Prevotella was detected. This shows, for the first time, the prebiotic effect of FOS in patients with Crohn's disease. Previous work has determined that the increase in bifidobacteria is inversely proportional to their concentration at baseline.23 However, this correlation was not observed in our patient group. Whether this difference results from the small patient group studied in our trial, or is a consequence of the altered concentrations of bifidobacteria seen in patients with Crohn's disease, is not clear.
There was no correlation between the magnitude of the increase in faecal bifidobacteria measured in individual patients and the reduction in their disease activity. However, there was a significant connection between the effect of FOS on disease activity and its effect on the total concentration of mucosal bifidobacteria. Thus patients who experienced clinical remission after FOS had a significant increase in mucosal bifidobacteria compared with those that had persistent disease activity. This result suggests that the clinical benefit of FOS relates to its ability to affect the mucosal microbiota. However, it is also possible that the increase in mucosal bifidobacteria is a consequence of the reduction in disease activity rather than the cause. It is of course also possible that the clinical and immunological effects of FOS relate to a change in an as yet unidentified component of the mucosal microbiota. Further studies, which measure the qualitative effects of FOS supplementation on faecal and mucosal microbiota, are required to investigate this possibility.
We did not assess whether FOS was fermented in the small bowel or its impact on the small intestinal microbiota in patients with Crohn's disease. Studies in ileostomists consuming oligofructose (15.5 g/day) or inulin (17 g/day) have demonstrated 89% recovery in the ileostomy effluent.37 Factors responsible for the high degree of resistance to digestion in the small bowel include the absence of human gastrointestinal enzymes able to hydrolyse the glycosidic bonds in FOS, and the rapid transit of small bowel contents. It is not known whether patients with Crohn's disease have alterations in small bowel FOS fermentation compared with healthy subjects. Theoretically, increased small bowel transit would tend to decrease fermentation although alterations in small bowel microbiota post hemicolectomy might also impact on this process. We have reported side effects before and after FOS in our patient group. The only factor that increased in severity was flatulence (suggestive of colonic fermentation); there was no increase in bloating or abdominal pain and the side effect profile was similar to reports in healthy subjects.23,24,29 This suggests that excessive small bowel fermentation does not occur in this patient group.
Recent evidence suggests that DC mediate both the proinflammatory and immunoregulatory effects of the intestinal microbiota on the mucosal immune system. Over a number of years, our group has developed flow cytometry techniques that enable the identification and functional study of the small numbers of DC present in human gut biopsies.20,33 We reported that bifidobacteria promote IL‐10 and inhibit IL‐12 expression when cultured in vitro with lamina propria DC.20 Furthermore, allogeneic CD4+ T lymphocytes release less interferon γ on stimulation if they have been precultured with bifidobacteria treated DC than control DC. This finding suggests that bifidobacteria promote immunoregulatory responses within the mucosal immune system via an interaction with lamina propria DC. However, the immunological effects of FOS have not been studied in vivo either in healthy controls or in the setting of intestinal inflammation.
We demonstrated that 15 g FOS per day increased the percentage of lamina propria DC releasing IL‐10, but had no effect on IL‐6 or IL‐12. Our hypothesis is that this effect is dependent on the bifidogenic effect of FOS acting via TLRs or other pattern recognition receptors. The interaction between bifidobacteria and pattern recognition receptors could occur at the level of the DC itself or at another site with an indirect effect on DC. Bifidobacteria contain a number of TLR ligands, including peptidoglycan and DNA, but the components responsible for triggering IL‐10 production by DC in vitro have yet to be identified. We cannot exclude alternative explanations for the effects of FOS supplementation on DC such as alterations in colonic short chain fatty acid release resulting from colonic fermentation as FOS has been reported to increase faecal butyrate levels in an in vitro fermentation system.38 Butyrate influences DC maturation and cytokine production in vitro, altering the balance of IL‐12 and IL‐10 production in favour of the latter.39 Short chain fatty acids such as butyrate are putative ligands for PPARγ, a nuclear receptor expressed by DC among other cells which appears to have an anti‐inflammatory role in the intestine.40,41,42
In addition, more DC expressed TLR2 and/or TLR4 following FOS supplementation. This increase in TLR expression may be a result of enhanced recognition of bifidobacteria by DC. Signalling through TLRs is often associated with initiation of inflammatory responses. Indeed, increased TLR2 and TLR4 expression has been observed in tissue sections from inflamed intestinal tissue and more TLR2+ and TLR4+ DC are found in inflamed colonic tissue from both Crohn's disease and ulcerative colitis patients than in normal colon from of healthy controls.43,44 However, recent evidence has also identified a protective role for TLRs in the gut: activation of TLRs by commensal microbiota is essential for intestinal homeostasis and protection against gut injury.7 These effects are associated with production of cytoprotective and reparative factors, including IL‐6, KC‐1, and heat shock proteins. Therefore, it is possible that triggering of DC in the lamina propria via TLR recognition of bacterial products may signal loss of epithelial integrity and initiate a response to enhance epithelial restitution. Initiation of cytoprotective mechanisms in conjunction with production of immunoregulatory IL‐10 may be beneficial in the context of Crohn's disease. These pathways provide a plausible link between the bifidogenesis associated with FOS ingestion, modulation of DC, and a reduction in disease activity. The mechanism by which the prebiotic regulates DC expression of TLRs remains to be determined.
The DC analysed in these studies were sampled from rectal tissue that was endoscopically and histologically non‐inflamed, suggesting that the changes observed in DC on FOS ingestion are unlikely to be simply markers of altered gross inflammatory activity. However, IL‐10 production by DC was more variable between individual patients than we had previously observed in analysis of DC from the colons of healthy individuals, possibly reflecting a variable degree of underlying immunological activity in the “non‐involved” tissue from patients with active Crohn's disease.
In summary, FOS was well tolerated by patients with active Crohn's disease and led to a significant improvement in disease activity. This novel therapeutic strategy increased intestinal bifidobacteria and enhanced lamina propria DC IL‐10 production and TLR expression. Placebo controlled trials are required to confirm the therapeutic efficacy and to explore in detail the microbiological and immunological effects of FOS supplementation.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Abbreviations
CRP - C reactive protein
DC - dendritic cells
FISH - fluorescence in situ hybridisation
FOS - fructo‐oligosaccharides
GALT - gut associated lymphoid tissue
HBI - Harvey Bradshaw index
IL - interleukin
TLR - toll‐like receptor
CDAI - Crohn's disease activity index
PBS - phosphate buffered saline
FCS - fetal calf serum
HBSS - Hanks' balanced salt solution
LPMC - lamina propria mononuclear cells
SED - super enhanced Dmax
Footnotes
This work was supported by an unrestricted grant from Nestle, UK.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Sartor R B. Intestinal micro‐flora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol 200117324–330. [DOI] [PubMed] [Google Scholar]
- 2.Sellon R K, Tonkonogy S, Schultz M.et al Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin‐10‐deficient mice. Infect Immun 1998665224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swidsinski A, Ladhoff A, Pernthaler A.et al Mucosal flora in inflammatory bowel disease. Gastroenterology 200212244–54. [DOI] [PubMed] [Google Scholar]
- 4.Harper P G, Lee E C, Kettlewell M G W.et al Role of faecal stream in the maintenance of Crohn's colitis. Gut 198526279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasoli R, Kettlewell M G W, Mortensen N J.et al Response to fecal challenge in defunctioned colonic Crohn's disease: prediction of long term response. Br J Surg 199077616–617. [DOI] [PubMed] [Google Scholar]
- 6.Madsen K L, Doyle J S, Jewell L D.et al Lactobacillus species prevents colitis in interleukin 10 gene‐deficient mice. Gastroenterology 19991161107–1114. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff‐Nahoum S, Paglino J, Eslami‐Varzaneh F.et al Recognition of commensal microflora by toll‐like receptors is required for intestinal homeostasis. Cell 2004118229–241. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson A J, Uhr T. Induction of protective sIGA by dendritic cells carrying commensal bacteria. Science 20043031662–1665. [DOI] [PubMed] [Google Scholar]
- 9.Seksik P, Rigottier L, Gramet G.et al Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 200352237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamboli C P, Neut C, Desreumaux P.et al Dysbiosis in inflammatory bowel disease. Gut 2004531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stagg A J, Hart A L, Knight S C.et al The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut 200352522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagg A J, Kamm M A, Knight S C. Intestinal dendritic cells increase T cell expression of alpha4 beta7 integrin. Eur J Immunol 2002321445–1454. [DOI] [PubMed] [Google Scholar]
- 13.Rescigno M, Urbano M, Valzasina B.et al Dendritic cells express tight junction proteins and penetrate gut epithelial mono‐layers to sample bacteria. Nat Immunol 20012361–367. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki N, Ho S, Antonenko S.et al Subsets of human dendritic cell precursors express different toll‐like receptors and respond to different microbial antigens. J Exp Med 2001194863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheinecker C, McHugh R, Shevach E M.et al Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med 20021961079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viney J L, Mowat A M, O'Malley J M.et al Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol 19981605815–5825. [PubMed] [Google Scholar]
- 17.Sutherland L, Singleton J, Sessions J.et al Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 1991321071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm Bowel Dis 20006107–115. [DOI] [PubMed] [Google Scholar]
- 19.Sartor R B. Therapeutic manipulation of the enteric micro flora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 20041261620–1633. [DOI] [PubMed] [Google Scholar]
- 20.Hart A L, Lammers K, Brigidi P.et al Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004531602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings J, MacFarlane G. Gastrointestinal effects of prebiotics. Br J Nutr 200287S145–S151. [DOI] [PubMed] [Google Scholar]
- 22.Van Loo J, Coussement P, de Leenheer L.et al On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit Rev Food Sci Nutr 199535525–552. [DOI] [PubMed] [Google Scholar]
- 23.Gibson G R, Beatty E R, Wang X.et al Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995108975–982. [DOI] [PubMed] [Google Scholar]
- 24.Langlands S J, Hopkins M J, Coleman N.et al Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut 2004531610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holma R, Juvonen P, Asmawi M.et al Galacto‐oligosaccharides stimulate the growth of Bifidobacteria but fail to attenuate inflammation in experimental colitis in rats. Scand J Gastroenterol 2002371042–1047. [DOI] [PubMed] [Google Scholar]
- 26.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium modulates intestinal immune function in rats. J Nutr 2004134153–156. [DOI] [PubMed] [Google Scholar]
- 27.Harvey R F, Bradshaw J M. A simple index of Crohn's‐disease activity. Lancet 19801514. [DOI] [PubMed] [Google Scholar]
- 28.Forbes A.Inflammatory bowel disease: a clinicians' guide, 2nd Edn. London: Edward Arnold 2001
- 29.Van Munster I P, de Boer H M, Jansen M C.et al Effect of resistant starch on breath‐hydrogen and methane excretion in healthy volunteers. Am J Clin Nutrition 199459626–630. [DOI] [PubMed] [Google Scholar]
- 30.Best W R, Becktel J M. The Crohn's disease activity index as a clinical instrument. In: Pena A, Weterman IT, Booth CC, et al, eds. Recent advances in Crohn's disease. The Hague: Martinus Nijhoff, 19817–12.
- 31.Amann R I, Binder B J, Olson R J.et al Combination of 16S rRNA‐targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 1990561919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mylonaki M, Rayment N B, Rampton D S.et al Molecular characterization of rectal mucosa‐associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis 200511481–487. [DOI] [PubMed] [Google Scholar]
- 33.Bell S J, Rigby R, English N.et al Migration and maturation of human colonic dendritic cells. J Immunol 20011664958–4967. [DOI] [PubMed] [Google Scholar]
- 34.Rycroft C E, Jones M R, Gibson G R.et al A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol 200191878–887. [DOI] [PubMed] [Google Scholar]
- 35.Su C, Lichtenstein G R, Krok K.et al A meta‐analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology 20041261257–1269. [DOI] [PubMed] [Google Scholar]
- 36.Leiper K, Morris A I, Rhodes J M. Open label trial of oral clarithromycin in active Crohn's disease. Aliment Pharmacol Ther 200014801–806. [DOI] [PubMed] [Google Scholar]
- 37.Ellegard L, Andersson H, Bosaeus I. Inulin and oligofructose do not influence the absorption of cholesterol, or the excretion of cholesterol, Ca, Mg, Zn, Fe, or bile acids but increases energy excretion in ileostomy subjects. Eur J Clin Nutr 1997511–5. [DOI] [PubMed] [Google Scholar]
- 38.Probert H M, Apajalahti J H, Rautonen N.et al Polydextrose, lactitol, and fructo‐oligosaccharide fermentation by colonic bacteria in a three‐stage continuous culture system. Appl Environ Microbiol 2004704505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millard A L, Mertes P M, Ittelet D.et al Butyrate affects differentiation, maturation and function of human monocyte‐derived dendritic cells and macrophages. Clin Exp Immunol 2002130245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinoshita M, Suzuki Y, Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem Biophys Res Commun 2002293827–831. [DOI] [PubMed] [Google Scholar]
- 41.Desreumaux P, Dubuquoy L, Nutten S.et al Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator‐activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 2001193827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubuquoy L, Jansson E A, Deeb S.et al Impaired expression of peroxisome proliferator‐activated receptor gamma in ulcerative colitis. Gastroenterology 20031241265–1276. [DOI] [PubMed] [Google Scholar]
- 43.Hausmann M, Kiessling S, Mestermann S.et al Toll‐like receptors 2 and 4 are up‐regulated during intestinal inflammation. Gastroenterology 20021221987–2000. [DOI] [PubMed] [Google Scholar]
- 44.Hart A L, Omar Al‐Hassi H, Rigby R J.et al Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology 200512950–65. [DOI] [PubMed] [Google Scholar]
- 45.Langendijk P S, Schut F, Jansen G J.et al Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus‐specific 16S rRNA‐targeted probes and its application in fecal samples. Appl Environ Microbiol 1995613069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franks A H, Harmsen H J, Raangs G C.et al Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group‐specific 16S rRNA‐targeted oligonucleotide probes. Appl Environ Microbiol 1998643336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manz W, Amann R, Ludwig W.et al Application of a suite of 16S rRNA‐specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga‐flavobacter‐bacteroides in the natural environment. Microbiology 19961421097–1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.