Abstract
Background and aims
Interleukin (IL)‐15 is a member of the IL‐2 family, stimulating dendritic cells, natural killer (NK) cells, NK T cells and memory CD8+ T cells. IL‐15 levels were elevated in the intestinal mucosa of inflammatory bowel diseases. Here we investigated the involvement of IL‐15 in the pathogenesis of acute and chronic dextran sulphate sodium (DSS) induced colitis.
Methods
IL‐15 knockout (KO) mice and control C57BL/6 mice were used to induce colitis with DSS in their drinking water. Survival rate, clinical activity of diseases, extent of tissue damage, leucocyte population, and cytokine production of lamina propria (LP) cells of the large intestines were assessed.
Results
IL‐15 KO mice exhibited resistance to DSS induced acute colitis, as reflected by lower lethality, weight loss, clinical scores, and histological scores compared with those in control mice (p<0.05). The proportions of CD44high CD8+ T cells and NK cells in LP cells and levels of interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, and IL‐12p40 in culture supernatants of LP cells were reduced in IL‐15 KO mice (p<0.05). In vivo depletion of CD8+ T cells and NK cells decreased levels of IFN‐γ, TNF‐α, and IL‐12p40 in culture supernatants of LP cells in C57BL/6 mice (p<0.01). In chronic colitis, weight loss and clinical scores were improved and levels of IFN‐γ, TNF‐α, and IL‐12p40 in culture supernatants of LP cells were also reduced in IL‐15 KO mice (p<0.05).
Conclusions
IL‐15 plays an important role in the pathogenesis of both acute and chronic colitis induced by DSS in mice.
Keywords: interleukin 15, colitis, dextran sodium sulphate, mice
Human inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are characterised by inflammation in the large and/or small intestine associated with uncontrolled innate and adaptive immunity against normal constituents, including commensal bacteria and various microbial products.1,2,3,4,5 Imbalance of proinflammatory cytokines in innate immunity has been demonstrated to play a crucial role in the pathogenesis of IBD.6 Dysregulated CD4+ T cells in adaptive immunity have also been postulated to play an important role in the pathogenesis of IBD.7,8,9,10 Responding T cells exhibit a T helper type 1 (Th1) phenotype capable of producing interferon (IFN)‐γ in CD whereas Th2 cytokines are closely associated with UC.7,8,9,10 Trinitrobenzene sulphonic acid induced colitis,11 colitis in interleukin (IL)‐2 deficient mice,12 and recombination activating gene deficient mice transferred with CD4+ CD45RBhigh T cells13 are used as Th1 colitis animal model resembling CD whereas oxazolone induced colitis in mice resembles UC with Th2‐like responses.14,15 Dextran sulphate sodium (DSS) induced colitis, one of most popular murine colitis models, was originally considered a T cell independent model because it occurs even in T cell deficient severe combined immunodeficiency mice.16 However, this was based on the finding of acute colitis induced by oral administration of DSS for seven days, whereas in chronic colitis induced by multiple cycles of DSS or in the recovery phase of exaggerated colitis induced by DSS, T cells play an important role in worsening the diseases.17,18 Thus DSS induced colitis is mediated by different mechanisms such as innate and adaptive immunity at different phases of the disease.
IL‐15 uses β‐ and γ‐chains of the IL‐2 receptor for signal transduction and is produced by activated macrophages/dendritic cells (DCs) and intestinal epithelial cells.19,20,21,22,23 IL‐15 can stimulate macrophages/DCs, natural killer (NK) cells, NK T cells, mucosal γδ Τ cells, and memory CD8+ T cells to proliferate and/or exhibit cytokine production or cytotoxic activity.24,25,26,27 Thus IL‐15 is a pleiotropic cytokine that plays an important role in both innate and adaptive phases of immune responses. Kirman and Nielsen reported that the number of IL‐15 expressing cells increased in active UC.28 We have also shown that IL‐15 activity was elevated in rectal mucosa in active IBD and even in inactive UC.29 Ohta et al have recently reported that overexpression of IL‐15 in intestinal epithelial cells induced chronic inflammation of the small intestine accompanied by production of IFN‐γ by NK1.1+ Tc1 cells in the lamina propria (LP) of the small intestine.30 Thus these reports suggest that mucosal IL‐15 is involved in the pathogenesis of IBD as one of the important mediators in activation of innate and adaptive immunity in intestinal mucosa. To our knowledge, the role of IL‐15 in the pathogenesis of acute and chronic colitis using IL‐15 knockout (IL‐15 KO) mice has not been examined previously. In this study, we assessed the survival rate, clinical activity of diseases, extent of tissue damage, leucocyte population, and cytokine production of LP cells of the large intestines.
Materials and methods
Mice
Seven to ten week old C57BL/6 mice or C57BL/6‐background IL‐15 KO mice were purchased from Charles River Japan, Inc. (Yokohama, Japan) or Taconic (Germantown, New York, USA), respectively. This study adhered to the Declaration of Helsinki and the institutional guidelines of Kyushu University.
Induction of acute or chronic colitis by DSS
For acute colitis induction by DSS, mice were administered 2% or 3% (weight/volume) DSS (molecular weight 36–50 kDa; ICN Biomedicals, Aurora, Ohio, USA) in their drinking water. In chronic colitis by DSS, mice were administered 2% DSS on days 0–5, 10–15, and 20–25. To assess the extent of colitis, body weight, stool consistency, and blood in the stool were monitored daily using the modified method of Cooper and colleagues.31 Diarrhoea was scored as follows: 0, normal; 2, loose stools; 4, watery diarrhoea. Blood in stool was scored as follows: 0, normal; 2, slight bleeding; 4, gross bleeding. Weight loss was scored as follows: 0, none; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, >15%. Disease activity index was the average of these scores: (combined score of weight loss, stool consistency, and bleeding)/3. Some mice were sacrificed on day 5 or 30. Tissues of the caecum and colon were removed and cleaned. Sections were taken for cell culture, flow cytometry (FCM), and histology.
Histology
The middle parts of the caecum and colon were removed and fixed with 10% neutral buffered formalin and embedded in paraffin. After cutting in round slices, thin tissue sections were stained with haematoxylin and eosin. Histology was scored as follows: epithelium (E), 0 = normal morphology; 1 = loss of globlet cells; 2 = loss of globlet cells in large areas; 3 = loss of crypts; 4 = loss of crypts in large areas; and infiltration (I), 0 = no infiltrate; 1 = infiltrate around the crypt basis; 2 = infiltrate reaching the L muscularis mucosae; 3 = extensive infiltration reaching the L muscularis mucosae and thickening of the mucosa with abundant oedema; 4 = infiltration of the L submucosa.32 Total histological score was given as E+I.
Antibodies and reagents
Fluorescein isothiocyanate (FITC) conjugated anti‐CD3ε (145‐2C11), anti‐CD44 (IM7), and anti‐CD11c (N418) monoclonal antibodies (mAbs), phycoerythrin (PE) conjugated anti‐T cell receptor (TCR) γδ (UC7‐13D5), anti‐NK1.1 (PK136), and anti‐CD8α (53‐6.7) mAbs, and biotin conjugated anti‐Gr‐1 (RB6‐8C5) mAbs were purchased from e‐Bioscience (San Diego, California, USA). PE conjugated anti‐CD11b (M1/70), cytochrome conjugated anti‐TCRβ (H57‐597), and anti‐CD4 (H129.19) mAbs, allophycocyanin (APC) conjugated streptavidin, and biotin‐conjugated anti‐Gr‐1 (RB6‐8C5) mAbs were purchased from BD PharMingen (San Diego, California, USA). Anti‐asialo GM1 and control IgG were purchased from Wako Pure Chemicals (Osaka, Japan).
In vivo depletion by treatment with antibodies
For in vivo cell depletion, 400 μg of anti‐CD8 mAb, 200 μg of anti‐asialo GM1 Ab, or 400 μg isotype control IgG was injected intraperitoneally into mice once a week from two days before administration of DSS. Anti‐CD8 mAb was obtained by growing hybridoma cells, 2.43 cells, in serum free medium (Nissui Pharmacia Co. Ltd, Tokyo, Japan).
Cell preparation
LP cells in the large intestine were isolated using a previously described method with slight modifications?33 Briefly, gut pieces were cut into 2 mm samples and the epithelium was eliminated by stirring, first twice for 10 minutes in phosphate buffered saline containing 3 mM EDTA at 37°C and then twice for 15 minutes in RPMI (Sigma Chemical Co., St. Louis, Missouri, USA) containing 1% fetal bovine serum (FBS), 1 mM EGTA, and 1.5 mM MgCl2. Gut pieces were collected and stirred at 37°C for 90 minutes in RPMI containing 20% FBS, 100 U/ml collagenase (C2139; Sigma‐Aldrich Corp.), and 5 U/ml DNase 1 (Sigma‐Algrich Corp.). Halfway through the incubation and at the end of the incubation, the suspension was dissociated by multiple aspirations through a syringe for two minutes. The pellet was washed and in some experiments LP cells were purified to LP lymphocytes on a 45%/66.6% discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient at 600 g for 20 minutes.
FCM analysis
For FCM analysis, isolated cells were preincubated with an Fcγ receptor blocking mAb (CD16/32; 2.4G2) for 15 minutes at 4°C. These cells were then incubated with saturating amounts of FITC, PE, Cy‐Chrome, APC, and biotin conjugated Abs for 30 minutes at 4°C. To detect biotin conjugated mAb, cells were stained with Cy‐Chrome or APC conjugated streptavidin. Stained cells were analysed using a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, California, USA . Data were analysed with CellQuest software (BD Biosciences).
Cell culture
Four ×105 LP cells from non‐treated mice or DSS treated mice were cultured without stimulation for 24 hours at 37°C under 5% CO2 in 96 well flat bottomed plates (Falcon, Becton Dickinson Ltd., Oxford, UK) in a volume of 0.2 ml RPMI containing 10% FBS. After incubation for 24 hours, supernatants were collected to estimate cytokine production. Cytokine activity was assayed by enzyme linked immunosorbent assay (ELISA) using an ELISA Development Kit for mouse IFN‐γ, tumour necrosis factor (TNF)‐α, IL‐1β, IL‐6, IL‐10, and IL‐12p40 (Genzyme Diagnostics, Cambridge, Massachusetts, USA).
Statistical analysis
Differences in survival rates were evaluated by the log rank test (Mantel‐Cox). Disease activity index and histological scores were statistically analysed using the Mann‐Whitney U test. Differences in parametric data were evaluated by the Student's t test. Differences of p<0.05 were considered statistically significant.
Results
Improvement in DDS induced acute colitis in IL‐15 KO mice
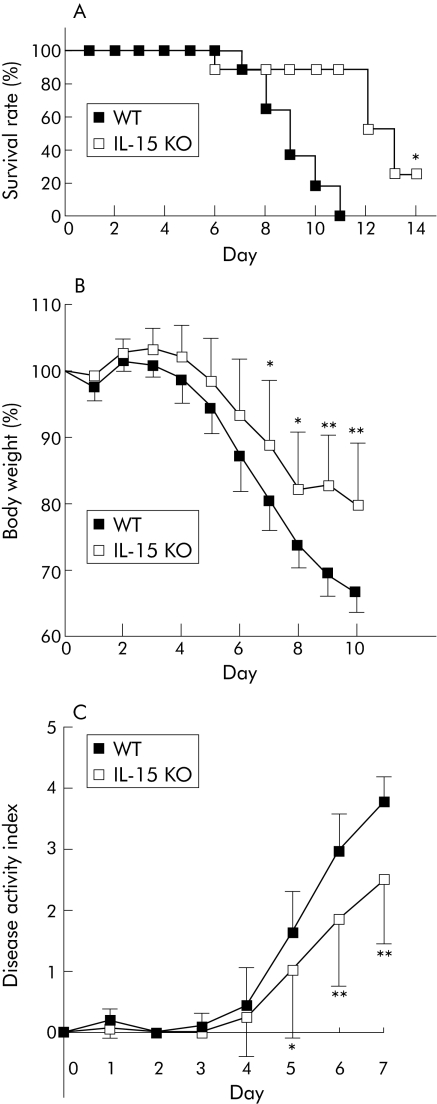
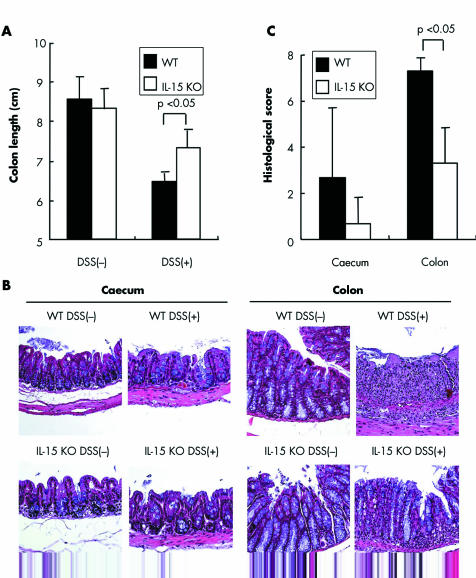
Acute colitis was induced in IL‐15 KO and control mice by 2% or 3% DSS. Survival rates were significantly increased in IL‐15 KO mice compared with those in control mice after 3% or 2% DSS administration (p<0.05) (fig 1A and data not shown). IL‐15 KO mice showed significant protection against 2% and 3% DSS induced acute colitis, as indicated by the weight loss and clinical scores for weight loss, bleeding, and diarrhoea (p<0.05) (fig 1B, 1C, and data not shown). Macroscopic examinations on day 5 after DDS administration revealed that shortening of colon length of IL‐15 KO mice was significantly inhibited compared with that of control mice (p<0.05) (fig 2A). On histological examination, crypt damage, ulceration, and infiltration of inflammatory cells were observed in the colons of DSS treated control mice (fig 2B). On the other hand, histological analysis of colons from DSS treated IL‐15 KO mice showed greatly reduced numbers of infiltrating cells, degree of mucosal injury, and oedema. The histological score of the colons was significantly lower in IL‐15 KO mice than in control mice on day 5 after DSS administration (p<0.05) (fig 2C).
Figure 1 Time course of survival rate, body weight, and disease activity of dextran sulphate sodium (DSS) induced acute colitis in interleukin (IL)‐15 knockout (KO) mice. (A) Survival rates of IL‐15 KO mice and wild‐type (WT) mice administered 3% DSS were monitored every day. Data are the sum of results of two independent experiments (n = 11 per group). (B, C) IL‐15 KO mice and control mice were administered 2% DSS. Body weight (B) and disease activity index (C) were monitored every day and the values for body weight are expressed as percentage of body weight on day 0. Each point represents the mean (SD). Data are the sum of results of two independent experiments (n = 11 per group). Statistically significant differences are shown: *p<0.05, ** p<0.01.
Figure 2 Evaluation of dextran sulphate sodium (DSS) induced colocaecal damage in interleukin (IL)‐15 knockout (KO) mice. (A) The caecum and colon were obtained from DSS treated IL‐15 KO mice, DSS treated wild‐type (WT) mice, and IL‐15 KO mice and WT mice not treated with DSS. We measured colon length in these mice. Results are representative of two independent experiments (n = 6 per group). (B) The caecum and colon were dissected for histological analysis with haematoxylin and eosin staining (original magnification 200 x). (C) Histological scores of the caecum and colon in DSS treated mice are shown (n = 6 per group). Results are representative of two independent experiments. Statistically significant differences are shown.
Reduced cell accumulation in the colon mucosa of IL‐15 KO mice in DSS induced acute colitis
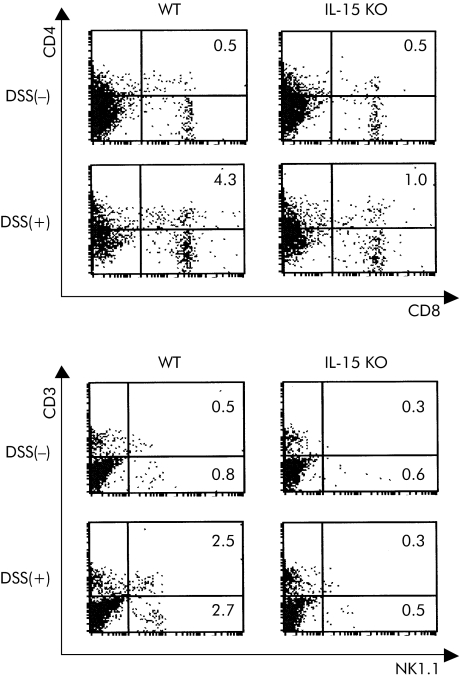
Populations of LP cells in the large intestines from IL‐15 KO mice after DDS administration were analysed by flow cytometer. A typical result is shown in fig 3 and data from at least three mice are summarised in table 1. Proportions of NK1.1+ CD3ε− cells, NK1.1+ CD3ε+ cells, and CD44high CD8+ T cells were significantly increased in DSS treated control mice compared with those in non‐treated control mice (p<0.05), but these significant increases were not observed in DSS treated IL‐15 KO mice. There were no significant differences between the ratios of the T cell subpopulations bearing CD4, TCRαβ, or TCRγδ in IL‐15 KO mice and control mice on day 5 after DSS administration. There were also no significant differences between both groups after DSS administration for the ratios of CD11b+ Gr‐1+ cells, CD11b+ Gr‐1− cells, or CD11c+ cells, which were regarded as polymorphonuclear leucocytes, macrophages, and DCs, respectively. These results suggest that IL‐15 plays an important role in induction of effector/memory CD8+ T cells and NK cells in LP cells in the large intestine after DSS administration.
Figure 3 Flow cytometry (FCM) analysis of lamina propria (LP) cells in the large intestine from dextran sulphate sodium (DSS) treated interleukin (IL)‐15 knockout (KO) mice. LP cells in the large intestine of DSS treated IL‐15 KO mice, DSS treated wild‐type (WT) mice, and IL‐15 KO mice and WT mice not treated with DSS were stained with various monoclonal antibodies (CD3, NK1.1, CD4, or CD8). The results of FCM are presented as typical profiles after an analysis gate had been set on lymphocytes using forward and side scatter.
Table 1 Populations of lamina propria (LP) cells in the large intestines of C57BL/6 and interleukin (IL)‐15 knockout (KO) mice treated (+) or not treated (−) with 2% dextran sulphate sodium (DSS).
| DSS(−) WT | DSS(−) IL‐15KO | DSS(+) WT | DSS(+) IL‐15KO | |
|---|---|---|---|---|
| Whole LP cell number | ||||
| (×106 cells/mouse) | 1.4 (0.4) | 1.3 (0.4) | 1.1 (0.4) | 1.5 (0.6) |
| T cell subset (%) | ||||
| CD8+ | 26 (1) | 15 (3)* | 24 (7) | 11 (3)* |
| CD44high CD8+ | 0.6 (0.1) | 0.5 (0.1) | 4.1 (1.4)† | 1.1 (0.6)* |
| CD4+ | 57 (4) | 73 (1)* | 60 (2) | 74 (11) |
| CD44high CD4+ | 3.4 (0.4) | 4.6 (0.2)* | 17 (4)† | 11 (5) |
| TCR αβ | 88 (0) | 89 (1) | 93 (1) | 89 (7) |
| TCR γδ | 6.2 (1.1) | 3.8 (1.5) | 4.4 (1.0) | 5.3 (4.8) |
| NK1.1+ CD3ε+ | 0.5 (0.0) | 0.2 (0.1)* | 1.7 (0.7)† | 0.2 (0.1)* |
| Other cell subset (%) | ||||
| NK1.1+ CD3ε− | 0.8 (0.2) | 0.5 (0.2) | 3.1 (1.1)† | 0.5 (0.2)* |
| CD11b+ Gr‐1+ | 0.4 (0.1) | 0.4 (0.1) | 2.4 (0.1)† | 2.6 (0.4)† |
| CD11b+ Gr‐1− | 3.8 (0.5) | 2.9 (0.8) | 5.3 (1.0) | 5.0 (2.5) |
| CD11c+ | 0.7 (0.1) | 0.7 (0.2) | 1.8 (0.2)† | 1.6 (0.0)† |
Populations of LP cells were analysed by fluorescence activated cell sorter. Percentages of T cell subsets, except CD44high CD8+, CD44high CD4+ cells, and NK1.1+ CD3ε+, were calculated in CD3ε+ and lymphocyte gated LP lymphocytes. Percentages of subsets of CD44high CD8+, CD44high CD4+ cells, NK1.1+ CD3ε+, and NK1.1+ CD3ε− cells were calculated in lymphocyte gated LP lymphocytes. Percentages of other cell subsets were calculated in total surviving LP cells.
Results are representative of the means (SD) of three experiments. Each group consisted of at least three mice.
WT, wild type; TCR, T cell receptor.
*p<0.05 between DSS(−)WT and DSS(−) IL‐15KO or DSS(+) WT and DSS(+) IL‐15KO.
†p<0.05 between DSS(−) WT and DSS(+) WT or DSS(−) IL‐15KO and DSS(+) IL‐15KO.
Aberrant cytokine production by LP cells of IL‐15 KO mice in DSS induced acute colitis
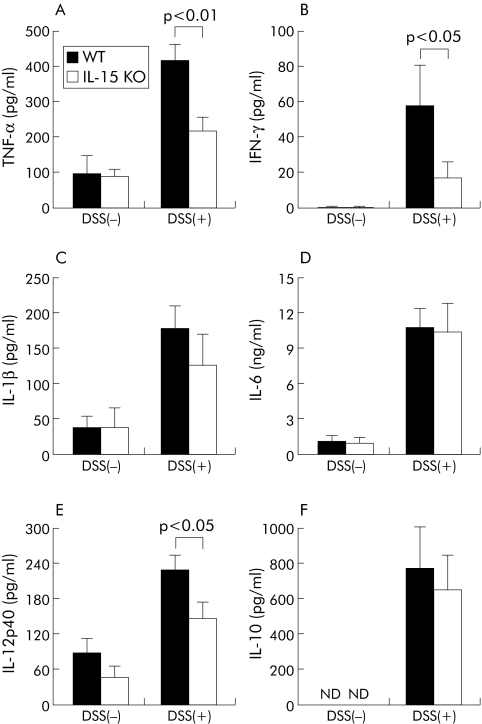
Spontaneous release of several cytokines was evaluated in culture supernatants of LP cells from the large intestines of DSS treated IL‐15 KO mice. As shown in fig 4A–F, levels of cytokines were significantly increased in both DSS treated IL‐15 KO mice and DSS treated control mice compared with those in non‐treated IL‐15 KO mice and non‐treated control mice, respectively (p<0.01). There were no significant differences between levels of IL‐1β, IL‐6, or IL‐10 production in IL‐15 KO mice and control mice on day 5 after DSS administration. In contrast, levels of IFN‐γ, TNF‐α, and IL‐12p40 were significantly lower in DSS treated IL‐15 KO mice than in DSS treated control mice (p<0.05, p<0.01, and p<0.05, respectively).
Figure 4 Cytokine production of lamina propria (LP) cells in the large intestine of dextran sulphate sodium (DSS) treated interleukin (IL)‐15 knockout (KO) mice. LP cells in the large intestine of DSS treated IL‐15 KO mice, DSS treated wild type (WT) mice, and in IL‐15 KO mice and WT mice not treated with DSS were prepared and cultured without stimulation for 24 hours at 37°C. Concentrations of tumour necrosis factor (TNF)‐α (A), interferon (IFN)‐γ (B), IL‐1β (C), IL‐6 (D), IL‐12p40 (E), and IL‐10 (F) in culture supernatants of LP cells were measured by enzyme linked immunosorbent assay. Each group consisted of at least three mice. Results are representative of three independent experiments. Statistically significant differences between DSS treated IL‐15 KO mice and DSS treated WT mice are shown. ND, not detectable.
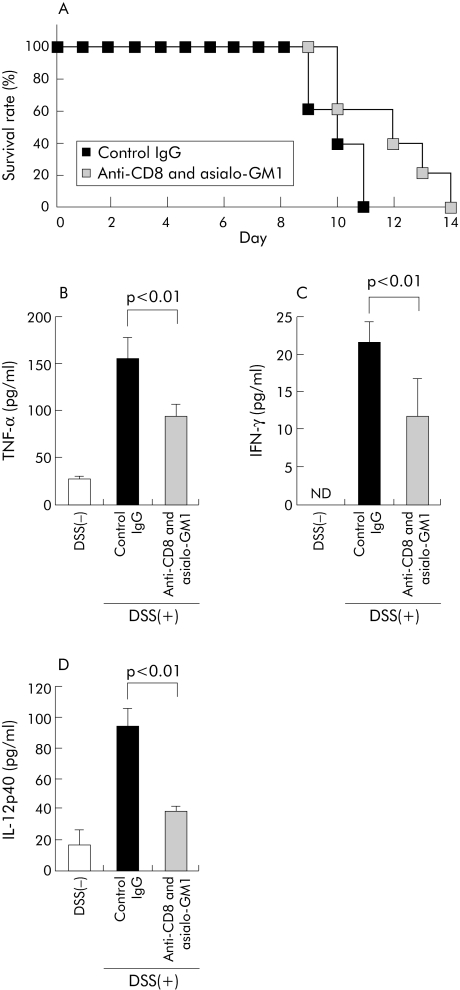
In vivo depletion of CD8+ T and NK cells ameliorated acute colitis induced by DSS
To elucidate the roles of CD8+ T cells and NK cells in acute colitis after DSS administration, we examined the effect of in vivo administration of anti‐CD8 mAb and/or asialo‐GM1 Ab on DSS induced acute colitis in C57BL/6 mice. C57BL/6 mice were injected intraperitoneally with anti‐CD8 mAb, anti‐asialoGM1 Ab, or control IgG once every week from two days before administration of DSS. We confirmed depletion of CD8+ T cells and/or NK1.1+ cells in LP cells by FCM analysis (data not shown). In vivo administration of either anti‐CD8 mAb or asialo‐GM1 Ab partially protected against acute DSS induced colitis, as assessed by survival time (data not shown). Furthermore, in vivo administration of both anti‐CD8 mAb and asialo‐GM1 Ab extended the survival period of mice with DSS induced colitis (fig 5A).
Figure 5 Effects of in vivo treatment with anti‐CD8 monoclonal antibody (mAb) and asialo‐GM1 Abs on dextran sulphate sodium (DSS) induced colitis in C57BL/6 mice. (A) For in vivo cell depletion, both anti‐CD8 mAb and anti‐asialo GM1 Ab or isotype control IgG was injected intraperitoneally into C57BL/6 mice once a week from two days before administration of DSS. Survival rates of these mice administered 2% DSS were monitored every day (n = 5 per group). (B–D) Lamina propria (LP) cells from anti‐CD8 and asialo‐GM1 treated mice or control IgG treated mice on day 5 after DSS administration or naïve C57BL/6 mice (DSS(−)) were prepared and cultured without stimulation for 24 hours at 37°C. Tumour necrosis factor (TNF)‐α (B), interferon (IFN)‐γ (C), and IL‐12p40 (D) concentrations in culture supernatants of LP cells were determined by enzyme linked immunosorbent assay (n = 4 per group). Statistically significant differences are shown. ND, not detectable.
We next examined spontaneous release of TNF‐α, IFN‐γ, and IL‐12p40 in culture supernatants of LP cells from both anti‐CD8 mAb and anti‐asialoGM1 Abs treated C57BL/6 mice or control IgG treated C57BL/6 mice on day 5 after DSS administration. Levels of IFN‐γ, TNF‐α, and IL‐12p40 were significantly lower in both anti‐CD8 mAb and anti‐asialoGM1 Ab treated mice than those in control IgG treated mice (p<0.01, p<0.01, and p<0.01 respectively) (fig 5B, C, D), suggesting that the decreased susceptibility of both anti‐CD8 mAb and anti‐asialoGM1 Abs treated C57BL/6 mice to DSS induced acute colitis is associated with inhibition of production of IFN‐γ, TNF‐α, and IL‐12p40. Taken together, these results suggest that CD8+ T cells and NK cells are partially responsible for the pathogenesis of acute DSS induced colitis.
Improvement of DDS induced chronic colitis in IL‐15 KO mice
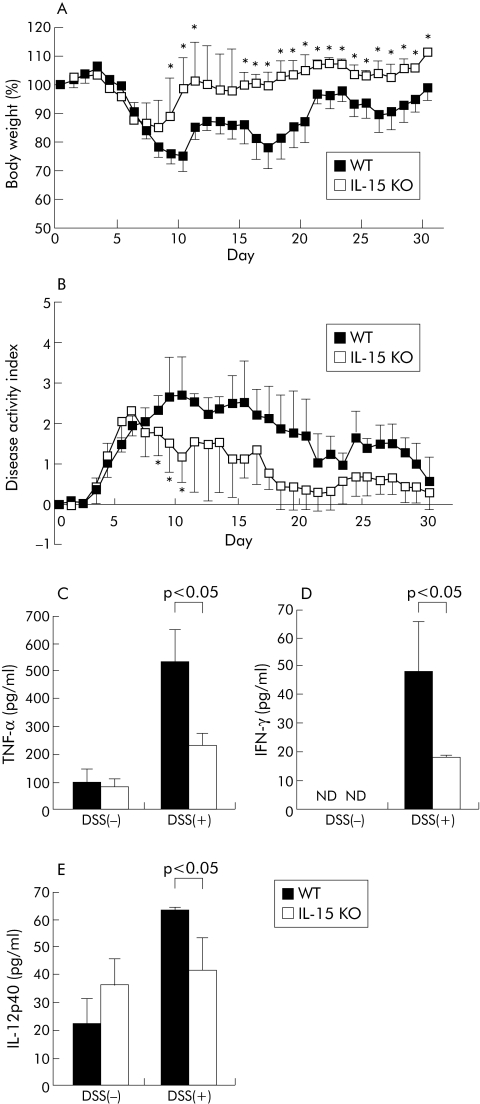
To investigate the involvement of IL‐15 in the pathogenesis in DSS induced chronic colitis, IL‐15 KO mice were administered 2% DSS on days 0–5, 10–15, and 20–25. As shown in fig 6A and 6B, DSS induced chronic colitis was attenuated in IL‐15 KO mice, as indicated weight loss and disease activity index of weight loss, bleeding, and diarrhoea (p<0.05). Disease activity index was improved, particularly in the first recovery phase (days 8–10) of exaggerated colitis induced by DSS. Proportions of NK1.1+ CD3ε− cells and CD44high CD8+ T cells in IL‐15 KO mice were significantly lower than control mice on day 30 (p<0.05) (data not shown).
Figure 6 Dextran sulphate sodium (DSS) induced chronic colitis in interleukin (IL)‐15 knockout (KO) mice. IL‐15 KO mice and wild‐type (WT) mice were administered 2% DSS on days 0–5, 10–15, and 20–25. Body weight (A) and disease activity index (B) were monitored every day and values for body weight are expressed as percentage of body weight on day 0. Each point represents the mean (SD) (n = 7 per group). (C–E) Lamina propria (LP) cells from IL‐15 KO mice or WT mice on day 30 after DSS administration or naïve IL‐15 KO mice or WT mice (DSS(−)) were prepared and cultured without stimulation for 24 hours at 37°C. Production of tumour necrosis factor (TNF)‐α (C), interferon (IFN)‐γ (D), or IL‐12p40 (E) in culture supernatants of LP cells was determined by enzyme linked immunosorbent assay (n = 4 per group). Statistically significant differences are shown: * p<0.05. ND, not detectable.
To examine cytokine production in DSS treated IL‐15 KO mice, spontaneous release of TNF‐α, IFN‐γ, and IL‐12p40 was evaluated in culture supernatants of LP cells from the large intestines of DSS treated mice on day 30. Levels of IFN‐γ, TNF‐α, and IL‐12p40 were significantly lower in IL‐15 KO mice than in control mice (p<0.05, p<0.05, and p<0.05 respectively) (fig 6C, D, E). These results suggest that the decreased susceptibility of IL‐15 KO mice to DSS induced chronic colitis is associated with inhibition of production of IFN‐γ, TNF‐α, and IL‐12p40.
Discussion
DSS is a sulphated polymer that causes colitis by interfering with intestinal epithelial cell barrier function.34 Once the mucosal barrier is impaired, the submucosa is exposed to various luminal antigens, including foods and bacteria, and then the cells involved in innate immunity are activated, resulting in acute colitis characterised histologically by infiltration of inflammatory cells into the LP, focal crypt damage, and epithelial ulceration. These changes are thought to develop due to a toxic effect of DSS on the epithelium and production of proinflammatory cytokines, including TNF‐α and IFN‐γ.16,35,36 Innate immunity is largely mediated by polymorphonuclear leucocytes and macrophages that secrete proinflammatory cytokines. A notable finding in the present study is that IFN‐γ, TNF‐α, and IL‐12p40 production by LP cells was inhibited in DSS treated IL‐15 KO mice compared with that in DSS treated control mice. Although IFN‐γ deficient mice have been reported to normally develop acute colitis,37 IL‐12p35 deficient mice developed only mild diseases with smaller amounts of IFN‐γ and TNF‐α in LP cells.38 NO is an effector molecule for intestinal epithelia injury in humans and mice.39,40,41,42 A combination of TNF‐α and IFN‐γ is most potent for stimulation of inducible NO synthase induction.43,44 Thus impaired production of IL‐12p40, TNF‐α, and IFN‐γ in LP cells of IL‐15 KO mice may be mainly due to amelioration of DSS induced acute colitis in IL‐15 KO mice.
IL‐15 is known to stimulate NK cells and memory CD8+ T cells to produce IFN‐γ and enhance cytotoxic functions.45,46 Depletion of NK cells and CD8+ T cells by in vivo treatment with Abs attenuated the severity of acute colitis in DSS treated C57BL/6 mice, suggesting that these cells were partially responsible for the pathogenesis of acute DSS induced colitis. Moreover, IFN‐γ, TNF‐α, or IL‐12 production from LP cells was significantly decreased in both anti‐CD8 mAb and asialo GM1 Ab treated mice on day 5 after DSS administration. We have reported that memory CD8+ T cells play an important role in innate immunity through IFN‐γ production in a bystander manner in response to IL‐12 plus IL‐15.47 Apart from NK cells, effector/memory CD8+ T cells may play an important role in acute colitis induced by DSS. IL‐15 has also been reported to stimulate macrophages or DCs to produce IL‐12, IFN‐γ, and other proinflammatory cytokines.48 Macrophages migrating into the LP of the colon play a critical role in the pathogenesis of acute colitis.16 We found that there were no significant differences between the proportions of macrophages or DCs in LP cells of DSS treated IL‐15 KO mice and of DSS treated control mice. However, we found that depletion of DCs reduced the level of IFN‐γ in culture supernatants from LP cells (data not shown). We have also found that BM derived DCs from non‐treated IL‐15 KO mice produced smaller amounts of IFN‐γ and IL‐12p40 in response to lipopolysaccharide (our unpublished data). Therefore, it is also possible that inhibition of IFN‐γ and IL‐12p40 production in DSS treated IL‐15 KO mice is caused, at least partially, by impaired function of DCs in IL‐15 KO mice to produce IFN‐γ and IL‐12p40 on stimulation with bacterial products via pattern recognition receptors.
There are several lines of evidence showing that proinflammatory cytokines are involved in the pathogenesis of colitis. IL‐6‐deficient mice developed less severe colitis induced by DSS,49 suggesting that IL‐6 plays a promotive role in T cell independent acute colitis. IL‐1β converting enzyme (caspase‐1) deficient mice exhibited a significant reduction in colitis severity,50 suggesting that IL‐1β plays a promotive role in T cell independent acute colitis. We found that IL‐6 and IL‐1β from LP cells in DSS treated IL‐15 KO mice were produced to about the same extent as those in DSS treated control mice. Therefore, our results indicate that IL‐15 may not affect the ability of inflammatory cells to produce IL‐6 and IL‐1β. It is possible that IL‐15 KO mice could not be completely protected against induction of acute colitis by DSS. Low dose TNF‐α mAb treatment protects against chronic colitis51 although TNF‐α‐deficient mice were susceptible to induction of acute colitis by DSS.52 These results suggest that TNF‐α plays dichotomous roles in the protection of DSS induced acute colitis and the promotion of DSS induced chronic colitis. Our results revealed that TNF‐α production by LP cells in IL‐15 KO mice was partially inhibited in DSS induced acute and chronic colitis. We speculate that TNF‐α levels in IL‐15 KO mice are sufficient for protection against inflammation through antiapoptotic actions but insufficient for induction of acute colitis.
In chronic colitis induced by multiple cycles of DSS or in the recovery phase of exaggerated colitis induced by DSS, acquired immunity plays an important role in worsening the diseases.17,18 In the present study, clinical activities of diseases were also improved in IL‐15 KO mice in the first recovery phase. Proportions of CD44high CD8+ T cells and NK cells in LP cells, and levels of IFN‐γ, TNF‐α, and IL‐12p40 in culture supernatants of LP cells were also reduced in IL‐15 KO mice on day 30. CD4+ T cells initially stimulated in the presence of IL‐12 and IFN‐γ tend to develop into CD4+ Th1 cells. Early cytokine production by IL‐15 dependent NK cells and memory CD8+ T cells have important roles in determining whether a naive CD4+ T cells will differentiate into Th1 cells. Thus early IFN‐γ production by these cells may account for inflammation during the period between innate immunity covered mainly by phagocytes and acquired immunity covered by Th1 cells in terms of the time sequence. Taken together, IL‐15 plays important roles not only in the pathogenesis of DSS induced acute colitis as innate immunity but also in the pathogenesis of DSS induced chronic colitis by linking innate immunity with adaptive immunity via activation of NK and memory‐phenotype CD8+ T cells. However, IL‐15 KO mice did not show body weight variation, particularly during the second and third periods of DSS administration. Therefore, IL‐15 may be more effective for prevention, rather than treatment, of DSS induced chronic colitis. Further investigation is needed to elucidate these possibilities.
In summary, we have shown that the severity of both acute and chronic colitis was improved in DSS treated IL‐15 KO mice. Accumulation of CD44high CD8+ T cells and NK1.1+ cells and production of TNF‐α, IL‐12, and IFN‐γ were inhibited in LP cells of DSS treated IL‐15 KO mice. IL‐15 plays a critical role in the pathogenesis of colitis induced by DSS. IL‐15 may be one of the target molecules for prevention and treatment of IBD.
Acknowledgements
We thank Yohko Kobayashi and Kazue Kaneda for their excellent technical assistance. This work was supported in part by a Grant‐in‐Aid for Scientific Research on Priority Areas and Young Scientists (B), Japan Society for Promotion of Science, and grants from the Japanese Ministry of Education, Science, and Culture (YY), Yakult Bioscience Foundation (YY), Uehara Memorial Foundation (YY), and Nakamura Jishirou Foundation (TY).
Abbreviations
IL - interleukin
NK - natural killer
DSS - dextran sulphate sodium
KO - knockout
LP - lamina propria
IFN - interferon
TNF - tumour necrosis factor, IBD, inflammatory bowel disease
CD - Crohn's disease
UC - ulcerative colitis
Th1 - T helper type 1
DC - dendritic cell
FCM - flow cytometry
FITC - fluorescein isothiocyanate
mAb - monoclonal antibody
PE - phycoerythrin
APC - allophycocyanin
ELISA - enzyme linked immunosorbent assay
TCR - T cell receptor
WT - wild‐type
FBS - fetal bovine serum
Footnotes
Conflict of interest: None declared.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998115182–205. [DOI] [PubMed] [Google Scholar]
- 2.McKay D M. Intestinal inflammation and the gut microflora. Can J Gastroenterol 199913509–516. [DOI] [PubMed] [Google Scholar]
- 3.Sartor R B. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res Immunol 1997148567–576. [DOI] [PubMed] [Google Scholar]
- 4.Cong Y, Brandwein S L, McCabe R P.et al CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med 1998187855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maaser C, Kagnoff M F. Role of the intestinal epithelium in orchestrating innate and adaptive mucosal immunity. Z Gastroenterol 200240525–529. [DOI] [PubMed] [Google Scholar]
- 6.Reinecker H C, Steffen M, Witthoeft T.et al Enhanced secretion of tumour necrosis factor‐alpha, IL‐6, and IL‐1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 199394174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuss I J, Neurath M, Boirivant M.et al Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN‐gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL‐5. J Immunol 19961571261–1270. [PubMed] [Google Scholar]
- 8.Braegger C P, MacDonald T T. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy 199472135–141. [PubMed] [Google Scholar]
- 9.Monteleone G, Biancone L, Marasco R.et al Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 19971121169–1178. [DOI] [PubMed] [Google Scholar]
- 10.Parronchi P, Romagnani P, Annunziato F.et al Type 1 T‐helper cell predominance and interleukin‐12 expression in the gut of patients with Crohn's disease. Am J Pathol 1997150823–832. [PMC free article] [PubMed] [Google Scholar]
- 11.Morris G P, Beck P L, Herridge M S.et al Hapten‐induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 198996795–803. [PubMed] [Google Scholar]
- 12.Sadlack B, Merz H, Schorle H.et al Ulcerative colitis‐like disease in mice with a disrupted interleukin‐2 gene. Cell 199375253–261. [DOI] [PubMed] [Google Scholar]
- 13.Powrie F, Leach M W, Mauze S.et al Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 19941553–562. [DOI] [PubMed] [Google Scholar]
- 14.Boirivant M, Fuss I J, Chu A.et al Oxazalone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med 1998188129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller F, Fuss I J, Nieuwenhuis E E.et al Oxazolone colitis, a TH2 colitis model resembling ulcerative colitis, is mediated by IL‐13‐producing NK‐T cells. Immunity 200217629–638. [DOI] [PubMed] [Google Scholar]
- 16.Dieleman L A, Ridwan B U, Tennyson G S.et al Dextran sulfate sodium‐induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 19941071643–1652. [DOI] [PubMed] [Google Scholar]
- 17.Dieleman L A, Palmen M J, Akol H.et al Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998114385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabashima K, Saji T, Murata T.et al The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest 2002109883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabstein K H, Eisenman J, Shanebeck K.et al Cloning of a T cell growth factor that interacts with the beta chain of the interleukin‐2 receptor. Science 1994264965–968. [DOI] [PubMed] [Google Scholar]
- 20.Giri J G, Ahdieh M, Eisenman J.et al Utilization of the beta and gamma chains of the IL‐2 receptor by the novel cytokine IL‐15. EMBO J 1994132822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagaya Y, Bamford R N, DeFilippis A P.et al IL‐15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 19964329–336. [DOI] [PubMed] [Google Scholar]
- 22.Bamford R N, Grant A J, Burton J D.et al The interleukin (IL) 2 receptor beta chain is shared by IL‐2 and a cytokine, provisionally designated IL‐T, that stimulates T‐cell proliferation and the induction of lymphokine‐activated killer cells. Proc Natl Acad Sci U S A 1994914940–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinecker H C, MacDermott R P, Mirau S.et al Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 19961111706–1713. [DOI] [PubMed] [Google Scholar]
- 24.Carson W E, Giri J G, Lindemann M J.et al Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL‐2 receptor. J Exp Med 19941801395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armitage R J, Macduff B M, Eisenman J.et al IL‐15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol 1995154483–490. [PubMed] [Google Scholar]
- 26.Hiromatsu K, Yoshikai Y, Matsuzaki G.et al A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med 199217549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura H, Hiromatsu K, Kobayashi N.et al IL‐15 is a novel growth factor for murine gamma delta T cells induced by Salmonella infection. J Immunol 1996156663–669. [PubMed] [Google Scholar]
- 28.Kirman I, Nielsen O H. Increased numbers of interleukin‐15‐expressing cells in active ulcerative colitis. Am J Gastroenterol 1996911789–1794. [PubMed] [Google Scholar]
- 29.Sakai T, Kusugami K, Nishimura H.et al Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology 19981141237–1243. [DOI] [PubMed] [Google Scholar]
- 30.Ohta N, Hiroi T, Kweon M N.et al IL‐15‐dependent activation‐induced cell death‐resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. J Immunol 2002169460–468. [DOI] [PubMed] [Google Scholar]
- 31.Cooper H S, Murthy S N S, Shah R S.et al Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 199369238–249. [PubMed] [Google Scholar]
- 32.Obermeier F, Dunger N, Deml L.et al CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium‐treated mice. Eur J Immunol 2002322084–2092. [DOI] [PubMed] [Google Scholar]
- 33.Arstila T, Arstila T P, Calbo S.et al Identical T cell clones are located within the mouse gut epithelium and lamina propria and circulate in the thoracic duct lymph. J Exp Med 2000191823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 20033521–533. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim 199948137–143. [DOI] [PubMed] [Google Scholar]
- 36.Egger B, Bajaj‐Elliott M, MacDonald T T.et al Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 200062240–248. [DOI] [PubMed] [Google Scholar]
- 37.Dohi T, Fujihashi K, Kiyono H.et al Mice deficient in Th1‐ and Th2‐type cytokines develop distinct forms of hapten‐induced colitis. Gastroenterology 2000119724–733. [DOI] [PubMed] [Google Scholar]
- 38.Takagi H, Kanai T, Okazawa A.et al Contrasting action of IL‐12 and IL‐18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol 200338837–844. [DOI] [PubMed] [Google Scholar]
- 39.Iwashita E, Miyahara T, Hino K.et al High nitric oxide synthase activity in endothelial cells in ulcerative colitis. J Gastroenterol 199530551–554. [DOI] [PubMed] [Google Scholar]
- 40.Rachmilewitz D, Stamler J S, Bachwich D.et al Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut 199536718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenzie S J, Baker M S, Buffinton G D.et al Evidence of oxidant‐induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest 199698136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rachmilewitz D, Karmeli F, Okon E.et al Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut 199537247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermeier F, Kojouharoff G, Hans W.et al Interferon‐gamma (IFN‐gamma)‐ and tumour necrosis factor (TNF)‐induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)‐induced colitis in mice. Clin Exp Immunol 1999116238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolios G, Brown Z, Robson R L.et al Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT‐29. Br J Pharmacol 19951162866–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehniger T A, Caligiuri M A. Interleukin 15: biology and relevance to human disease. Blood 20019714–32. [DOI] [PubMed] [Google Scholar]
- 46.Mastroianni C M, d'Ettorre G, Forcina G.et al Teaching tired T cells to fight HIV: time to test IL‐15 for immunotherapy? Trends Immunol 200425121–125. [DOI] [PubMed] [Google Scholar]
- 47.Tsunobuchi H, Nishimura H, Goshima F. Memory‐type CD8+ T cells protect IL‐2 receptor alpha‐deficient mice from systemic infection with herpes simplex virus type 2. J Immunol 20001654552–4560. [DOI] [PubMed] [Google Scholar]
- 48.Ohteki T, Suzue K, Maki C.et al Critical role of IL‐15‐IL‐15R for antigen‐presenting cell functions in the innate immune response. Nat Immunol 200121138–1143. [DOI] [PubMed] [Google Scholar]
- 49.Naito Y, Takagi T, Uchiyama K.et al Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin‐6‐deficient mice. Int J Mol Med 200414191–196. [PubMed] [Google Scholar]
- 50.Siegmund B, Lehr H A, Fantuzzi G.et al IL‐1 beta ‐converting enzyme (caspase‐1) in intestinal inflammation. Proc Natl Acad Sci U S A 20019813249–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kojouharoff G, Hans W, Obermeier F.et al Neutralization of tumor necrosis factor (TNF) but not of IL‐1 reduces inflammation in chronic dextran sulphate sodium‐induced colitis in mice. Clin Exp Immunol 1997107353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naito Y, Takagi T, Handa O.et al Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor‐alpha deficient mice. J Gastroenterol Hepatol 200318560–569. [DOI] [PubMed] [Google Scholar]