Abstract
Background and aim
A 12 month, multicentre, randomised, double blind, placebo controlled, phase 3, dose‐response study was carried out. Exisulind inhibits tumour growth by induction of apoptosis. The aim of our study was to investigate if exisulind induces regression of sporadic colonic adenomas.
Patients and methods
A 12 month multicentre randomised double blind placebo controlled phase 3 dose response study was carried out. At baseline colonoscopy, left sided polyps (3–10 mm) were tattooed, measured, and left in place. Subjects received exisulind 200 or 400 mg, or placebo daily. Follow up sigmoidoscopy was performed after six months, and removal of any remaining polyps at the 12 month colonoscopy. The primary efficacy variable was change in polyp size from baseline.
Results
A total of 281 patients were enrolled and randomised; 155 (55%) fulfilled the criteria for the intention to treat (ITT) analysis and 114 (41%) fulfilled the criteria for the efficacy evaluation analysis (patients who underwent the 12 month colonoscopy). The decrease in median polyp size was significantly greater (p = 0.03) in patients who received exisulind 400 mg (−10 mm2) compared with those who received placebo (−4 mm2). Complete or partial response was significantly higher in the exisulind 400 mg group (54.6%) compared with the placebo group (30.2%), and disease progression was significantly lower (6.1% v 27.9%) (p = 0.04 and 0.02, respectively). Increased liver enzymes (8.4%) and abdominal pain (14.7%) were also reported at a greater frequency in the exisulind 400 mg group.
Conclusion
Exisulind caused significant regression of sporadic adenomatous polyps but was associated with more toxicity. This model of polyp regression, short in its term and involving a comparatively small patient sample size, may be the best available tool to assess a therapeutic regimen before launching into large preventive clinical studies.
Keywords: exisulind, sulindac sulfone, polyps, adenoma regression, randomised trial
Colorectal cancer (CRC) is a highly prevalent disease that is associated with considerable mortality and morbidity,1,2 with more than 945 000 new cases and 492 000 deaths expected worldwide in 2005.3 Adenomatous polyps are the putative precursor for most CRC.1,2,4,5,6,7,8,9,10,11
The past decade has witnessed the emergence of the new science of cancer chemoprevention, which refers to the use of specific natural or chemical compounds in order to prevent, inhibit, or reverse carcinogenesis prior to the development of clinical cancer.
Discovery of the potential chemopreventive activity of non‐steroidal anti‐inflammatory drugs (NSAIDs) in sporadic human CRC, almost 20 years ago, and their use to treat and prevent CRC represents an important example of this approach.12,13,14 Waddell and Loughry15 were the first to report that sulindac caused regression of rectal adenomatous polyps in familial adenomatous polyposis (FAP) patients. Since then, the effects of treatment with NSAIDs, in particular with sulindac, in FAP patients have been extensively investigated with promising results.7,16,17,18,19,20 In contrast, reports describing the effects of NSAIDs on regression of sporadic polyps have been limited.21,22,23,24,25
Unfortunately, consumption of NSAIDs is not problem free. Long term use of NSAIDs is limited due to the high incidence of side effects and the significant cost (of both the drugs and treatment of their side effects). Chronic intake of NSAIDs is associated with a high prevalence of gastroduodenal ulceration (in up to 20% of users) and with an estimated 2–5‐fold increase in the relative risk of ulcer complications and mortality.26 In 1997, in the US alone, there were 107 000 hospitalisations and 16 500 deaths due to NSAID consumption, equalling mortality from AIDS or leukaemia.26
Exisulind (Cell Pathway Inc. Horsham, PA, USA), the sulfone metabolite of sulindac, is the prototype of a new class of selective apoptotic antineoplastic drugs currently being investigated for the treatment of a variety of malignancies.27 In contrast with the parent sulindac, exisulind lacks antiprostaglandin synthetase activity.28 Despite its lack of effect on cyclooxygenases 1 and 2, exisulind has been shown both to inhibit cellular growth in vitro and to prevent chemically induced neoplasia in vivo.29 The antineoplastic effects of exisulind may be due to inhibition of cyclic guanosine monophosphate phosphodiesterase, with subsequent activation of protein kinase G, resulting in induction of programmed cell death (apoptosis).30
In a previous phase 1 clinical trial31,32,33 involving 18 FAP patients, daily administration of exisulind 600 mg, over a period of six months, produced 56% regression of exophytic polyps. Seventeen of the 18 patients were maintained on exisulind for 24 months with continued clinical response.33 In another trial of FAP patients with subtotal colectomy, exisulind (600 mg/day) significantly decreased new polyp formation by 25% over 12 months, and by an additional 54% over 24 months.34,35
Clinical trials remain the rate limiting step in the development of this agent. Novel trial designs are needed to hasten agent identification and testing for cancer prevention.
These statistics are sobering, considering that in most instances CRC can and should be preventable by well established screening and surgical techniques. Nevertheless, CRC death rates remain unacceptably high. One promising strategy is cancer chemoprevention that strives to block reserve or delay carcinogenesis before the development of invasive disease by targeting key molecular derangements using pharmacological or nutritional agents.
The objective of the present study was to determine the efficacy and safety of exisulind in causing regression of sporadic adenomatous polyps of the colon. The study also provides insight into the complexity of conducting polyp regression studies. This new model of polyp regression, short in its term and involving a comparatively small patient sample size, may be a promising tool to assess a new therapeutic regimen before launching into large preventive clinical studies.
Patients and methods
Patient enrolment
Male and female patients, 18 years of age or older, diagnosed with at least one sporadic adenomatous polyp in the left colon and with no familial syndromes, were eligible for inclusion. Polyp size was limited to 3–10 mm in diameter and polyps had to be easily accessible using a flexible sigmoidoscope. Women of childbearing potential who were pregnant, breast feeding, or were not using an acceptable method of contraception were excluded from the study. Patients who had taken: (a) an NSAID or aspirin for two weeks prior to the study (with the exception of low dose aspirin for cardiovascular prophylaxis), (b) sulindac on a regular basis for any indication for three months prior to enrolment, or (c) an investigational drug within one month before enrolment, were also excluded from the study. Patients with known hypersensitivity to sulindac, active peptic ulcer disease, any gastrointestinal problem that might affect absorption of the study drug, known hepatic, biliary tract, renal, or haematological dysfunction that might obscure laboratory analysis, cancer during the last 10 years (excluding non‐melanoma skin cancers), or a history of alcohol or drug abuse within the last three years were also excluded. All subjects were willing to abstain from chronic use of all NSAIDs or COX‐2 inhibitors, excluding aspirin, at cardioprotective doses of ⩽100 mg/day for the duration of the study. Subjects agreed to participate in the study and signed informed consent forms before undergoing routine colonoscopy. Overall, one in 10 candidates was recruited to the study.
Study design
This randomised, double blind, placebo controlled, parallel group, dose‐response, multicentre study was conducted in the USA, Sweden, and Israel, to determine the efficacy and safety of exisulind in producing regression of sporadic adenomatous polyps of the colon. After giving written informed consent, patients were randomised using a computer generated randomisation list stratified by investigative site. A blocking size of six was used. Patients received one of the following three treatments administered orally twice daily for 12 months: exisulind 100 mg (200 mg/day total dose), exisulind 200 mg (400 mg/day total dose), or placebo. At the screening visit, demographic data and medical history were obtained, complete physical examination (including weight and vital sign measurements) was performed, concomitant medication was reviewed, and blood samples were obtained for clinical laboratory testing (haemoglobin, red blood cells, haematocrit, white blood cells, platelets, mean corpuscular volume, mean corpuscular haemoglobin, electrolytes, transaminases, albumin, globulin, alkaline phosphatase, bilirubin, urea, creatinine, sodium, potassium, and calcium).
Colonoscopy was performed at baseline. All easily visible adenomatous colonic polyps (⩽1 cm) that could be followed using a flexible sigmoidoscope were identified, measured, photographed, and tattooed using sterilised India ink. To validate the accuracy and reproducibility of colonoscopic size measurements, the polyp was photographed with open forceps (with calibrated scale) that were placed on the surface of the polyp. The location of the polyp was determined by centimetres of insertion depth from the anal verge. All polyps beyond the reach of the flexible sigmoidoscope, or those larger than 1 cm, were removed using standard techniques (cold or hot biopsies in small polyps and snare cutery technique in large polyps). After six months of treatment with the study drug, polyps that had been left in place and tattooed at baseline were again measured as described above using a flexible sigmoidoscope. At the end of the study, colonoscopy was performed again. All tattooed polyps were clearly identified after 12 months and measured in situ. This time they were removed and processed routinely. There were no adverse effects related to tattooing. In each patient, the same examiner performed all of the procedures. Diagnosis of the tissue was done by a well trained gastrointestinal pathologist, and histology was confirmed by central pathology. While all of the patients with polyps entered the study, only those with adenomas who underwent colonoscopy at 12 months (or colonoscopy at a premature discontinuation visit) were included in the analysis.
Safety
To monitor for safety, study site personnel contacted patients by telephone every two weeks throughout the study. A comprehensive symptom questionnaire, designed to elicit information on adverse events, concomitant medications, and to encourage compliance with study medication, was administered by telephone at two week intervals. Adverse events were coded according to the World Health Organisation Adverse Reaction Terminology and graded for severity with the National Cancer Institute Common Toxicity Criteria (Cancer Therapy Evaluation Program: Common toxicity criteria. Bethesda, Maryland: National Cancer Institute, March 1998). Laboratory analyses were performed every two weeks for the first three months and monthly thereafter. Serum pregnancy tests were performed on all fertile women at monthly intervals for 12 months.
Study medications
Study medications (exisulind and placebo) were supplied in the form of gelatine capsules, identical in appearance, containing either 100 mg or 200 mg of exisulind or placebo. Patients were instructed to take two capsules of the study drug twice daily at approximately the same time each day (at breakfast and at dinner), yielding exisulind dosages of 0 mg/day, 200 mg/day, or 400 mg/day. Compliance was monitored by the study coordinator using pill counts, review of diaries completed by patients, and monthly telephone contact.
Statistical analyses
The outcome of interest was regression of sporadic adenomas. The primary efficacy variable was change in polyp size (length × width) from baseline to final evaluation after 12 months of daily treatment with exisulind 200 mg, 400 mg, or placebo. Prior to breaking blinding of the study, examination of the distribution of changes in polyp size from baseline indicated that a non‐parametric approach should be used. Therefore, the Wilcoxon rank sum test was used to compare the change in polyp size between each exisulind treatment group and placebo. No comparisons between the two active treatment groups were planned for any of the primary or secondary efficacy assessments.
Secondary efficacy analyses included comparison of the number of patients with a therapeutic response (defined as complete response (CR) and partial response (PR)), stable disease (STD), and progressive disease (PD) among each exisulind treatment group and the placebo group. Response was based on per cent change in polyp size. CR was defined as complete resolution of the polyp, PR was defined as ⩾50% reduction in polyp size, STD was <50% reduction and <25% increase, and PD was defined as ⩾25% increase in polyp size. The Cochran Mantel Haentszel procedure, with site as strata, was used to compare the percentage of patients meeting the criteria for CR, PR, STD, and PD. Pairwise comparisons between each exisulind dose group and placebo were performed.
Patients with histologically confirmed hyperplasic polyps without any adenoma (using tissue obtained during the end of study colonoscopy) were excluded from the primary statistical analysis. Consequently, only adenomatous polyps were included in the analysis. Any patient who received at least one dose of study medication and had at least one adenomatous polyp was included in the intention to treat (ITT) analysis. The efficacy evaluable (per protocol) population included all ITT patients who underwent colonoscopy at 12 months (or colonoscopy at a premature discontinuation visit). The baseline value was carried forward for patients who dropped out. This is the most appropriate method when longitudinal data are available only before the missing value.36
The study was sized with the assumption that 40–50% of patients would be excluded from the primary statistical analysis due to histological diagnosis of hyperplastic polyps. It was also predicted that a change in polyp size of 5 mm2 and 8 mm2 with 200 mg and 400 mg exisulind, respectively, would be achieved over a 12 month period. In order to achieve a power of 90%, a sample size of 90 for each group was calculated.
Descriptive statistics were used to summarise demographic characteristics at baseline and the percentage of patients who experienced drug related adverse events. Comparability of demographic and baseline clinical variables between treatment groups was assessed by analysis of variance (ANOVA) for continuous variables (for example, age, height, weight, body mass index (BMI)) and by Fisher's exact test for dichotomous variables (race and sex). Safety analysis included all patients who received at least one dose of the study medication. Adverse events were summarised according to treatment group. Differences between treatment groups were tested using Fisher's exact procedure only if the number of events was sufficient to permit a meaningful assessment. Mean changes in laboratory variables and vital signs from baseline to the final visit were compared between treatment groups using ANOVA. In addition, the significance of the mean change in laboratory or vital sign variable from baseline to the final visit, in each treatment arm, was assessed using a paired t test. An arbitrary level of 5% statistical significance (two tailed) was assumed.
Ethics
The study protocol and investigator informed consent documents were either approved by an IRB or accepted by an ethics committee. When it was needed, it received approval of the ministry of health. The study was conducted in accordance with US FDA regulations and ICH guidance on Good Clinical Practice Consolidated Guidance. In sites outside the USA, the study was also conducted in accordance with the current version of the Declaration of Helsinki and the laws and regulations of the countries in which the investigation was conducted.
Results
Patient characteristics
Patient distribution is summarised in table 1. A total of 281 patients, from 25 sites, were enrolled in the study between December 1997 and May 1999. Of the 281 enrolled patients, 91 and 95 patients were randomised to the exisulind 200 and 400 mg/day treatment groups, respectively, and 95 were randomised to the placebo group. A total of 205 patients (73.0%) completed the study, and 76 patients were discontinued prematurely. There was no statistically significant differences among the different treatment groups in percentage of patients completing the 12 month study (p = 0.49). Low dose aspirin usage was similar in the three treatment arms. Of the 281 enrolled patients, 126 (44.8%) were found to have hyperplastic polyps at the end of the study colonoscopy, and hence were excluded from the per study protocol.
Table 1 Patient disposition by treatment group.
| Placebo (n (%)) | Exisulind 200 mg (n (%)) | Exisulind 400 mg (n (%)) | Total | |
|---|---|---|---|---|
| Total No of patients enrolled | 95 | 91 | 95 | 281 |
| Hyperplastic polyps* | 41 | 43 | 42 | 126 |
| ITT population (adenomatous polyps only) | 54 | 48 | 53 | 155 |
| Discontinued prematurely† | 11 (20.4) | 10 (20.8) | 20 (37.7) | 41 |
| Efficacy evaluable population | 43 (79.6) | 38 (79.2) | 33 (62.3) | 114 |
*Patients with histologically confirmed hyperplastic polyps were excluded from the per study protocol.
†Reasons for discontinuation included adverse events, consent withdrawal, protocol violation, and other (among 155 patients with adenoma).
ITT, intention to treat.
Baseline demographics and disease characteristics
As can be seen in table 2, baseline characteristics were comparable in all groups. Approximately two thirds of patients (66.5%) were men, and most were Caucasian (90.7%). Mean age was 59.9 (10.8) years (range 22–85).
Table 2 Baseline demographic characteristics*.
| Placebo (n = 95) | Exisulind 200 mg (n = 91) | Exisulind 400 mg (n = 95) | |
|---|---|---|---|
| Sex (n (%)) | |||
| Male | 68 (72%) | 59 (65%) | 60 (63%) |
| Female | 27 (28%) | 32 (35%) | 35 (37%) |
| Age (y) | |||
| Mean (SD) | 61.1 (10.7) | 60.2 (10.4) | 58.3 (11.2) |
| Range | 22–85 | 37–85 | 33–82 |
| Race (n (%)) | |||
| White | 85 (90%) | 82 (90%) | 88 (93%) |
| Black | 8 (8%) | 8 (9%) | 6 (6%) |
| Asian | 1 (1%) | 0 (0%) | 0 (0%) |
| Other | 1 (1%) | 1 (1%) | 1 (1%) |
| Weight (kg) | |||
| Mean (SD) | 85.1 (16.1) | 86.4 (15.7) | 84.6 (16.7) |
| Range | 49.5–139.5 | 55.8–124.0 | 52.5–126.8 |
| Mean (SD) body mass index | 28.5 (4.5) | 29.1 (4.8) | 28.8 (5.4) |
*There were no significant differences between treatment groups for sex, age, race, weight, or body mass index for all patients enrolled (n = 281), the intention to treat population (n = 155), or the efficacy evaluable population (n = 114).
Efficacy
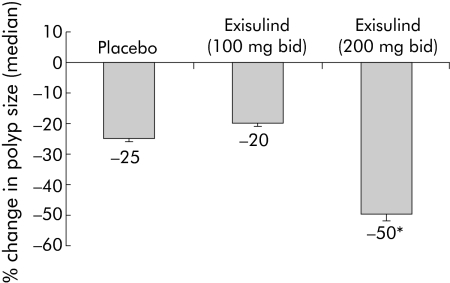
Of the 281 patients enrolled in the study, 155 fulfilled the criteria for the ITT analysis and 114 fulfilled the criteria for the efficacy evaluable analysis (table 1). The focus of the efficacy data presentation below is the group of patients with biopsy results at 12 months (efficacy evaluable population). Table 3 shows median changes in polyp size from baseline to 12 months. Figure 1 shows the per cent change in median polyp size by treatment group. Median change from baseline was significantly greater in the exisulind 400 mg treatment group compared with the placebo group (p = 0.03). No significant difference was found between the placebo and exisulind 200 mg treatment groups. In patients with hyperplastic polyps, there was no difference in median change in polyp size between the placebo (−2.5 mm2) and exisulind 400 mg (−4 mm2) treatment groups (p = 0.64).
Table 3 Change in polyp size (length × width) from baseline to final evaluation.
| Placebo | Exisulind 200 mg | Exisulind 400 mg | |
|---|---|---|---|
| Intent to treat population (n = 155) | |||
| Total No | 54 | 48 | 53 |
| Median (mm2) | −2 | −4 | −4 |
| p Value (v placebo) | 0.7* | 0.3† | |
| Efficacy evaluable population (n = 114) | |||
| Total No | 43 | 38 | 33 |
| Median (mm2) | −4 | −4 | −10 |
| p Value (v placebo) | 0.9* | 0.03† | |
*Comparison between exisulind 200 mg and placebo.
†Comparison between exisulind 400 mg and placebo.
Figure 1 Per cent change in median polyp size in the three treatment groups. *p = 0.03 (exisulind 200 mg bid v placebo).
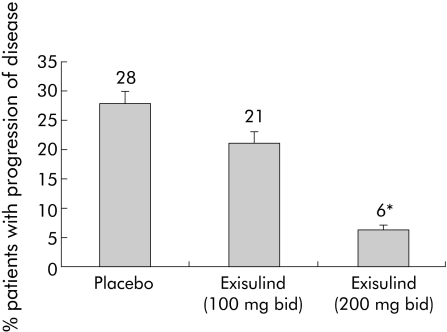
Therapeutic response (CR+PR) was significantly higher in the exisulind 400 mg treatment group (54.6%) compared with the placebo group (30.2%) (p = 0.04), as shown in table 4. Moreover, only two of the 33 patients (6.1%) in this group experienced PD compared with 12 of 43 patients (27.9%) treated with placebo (p = 0.02) (table 4, fig 2).
Table 4 Therapeutic response in the efficacy evaluable population (n = 114).
| Response | Placebo (n = 43) | Exisulind 200 mg (n = 38) | Exisulind 400 mg (n = 33) |
|---|---|---|---|
| Therapeutic response* | 13 (30.2%) | 13 (34.2%)† | 18 (54.6%)†† |
| Complete response | 8 (18.6%) | 4 (10.5%) | 9 (27.3%) |
| Partial response | 5 (11.6%) | 9 (23.7%) | 9 (27.3%) |
| Stable disease | 18 (41.9%) | 17 (44.7%) | 13 (39.4%) |
| Progressive disease | 12 (27.9%) | 8 (21.1%) | 2 (6.1%)††† |
*Therapeutic response = complete response+partial response.
†p = 0.8, ††p = 0.04, †††p = 0.02 compared with placebo.
Figure 2 Disease progression in the three treatment groups. *p = 0.02 (exisulind 200 mg bid v placebo).
No new lesions were identified at the six or 12 month time points in all three groups. Age, BMI, weight, or any other factors did not influence change in polyp size.
Safety and tolerability
A total of 251 of 281 patients (89.3%) reported at least one adverse event during the 12 months of treatment. Percentage of adverse events was similar across the placebo (86.3%), exisulind 200 mg/day (89.0%), and exisulind 400 mg/day (92.6%) treatment groups. Of the 281 patients, 122 (43.4%) reported at least one treatment related adverse event (as judged by the investigator to be possibly or probably related to the study drug). The most common events (⩾5%) are summarised in table 5. Elevated liver enzymes (aspartate aminotransferase, alanine aminotransferase) and abdominal pain were reported at a significantly greater frequency in patients treated with exisulind 400 mg compared with the two other groups (p<0.05). The incidence of treatment related adverse events was similar in the placebo and exisulind 200 mg groups. One patient died of a myocardial infarction 20 days after starting exisulind 400 mg/day; the event was considered unrelated to study medication. Other serious adverse events occurred in 36 of 281 patients (12.8%) and were similar across treatment groups; placebo 13/95 (13.7%); 200 mg/day 12/91 (13.2%); 400 mg/day 11/95 (12.5%). The most common serious events were carcinoma (n = 4), accidental injury (n = 4), chest pain (n = 4), and pancreatitis (n = 3). Twenty seven patients withdrew from the study prematurely as a result of adverse events; placebo (six patients, 6.3%), exisulind 200 mg (nine patients, 10.0%), exisulind 400 mg (12 patients, 12.6%). The most common adverse events that resulted in discontinuation were abdominal pain and nausea. Exisulind did not cause any clinically significant changes in blood chemistry, haematology, or urinalysis results, with the exception of elevated liver enzymes. Most (87–94%) of the liver test abnormalities were mild (grade 1) and reversible following discontinuation or temporary cessation of therapy. Of the five patients with grade 3 elevations, two were on placebo, one was on exisulind 200 mg, and two were on exisulind 400 mg at the time. Biliary events (that is, cholecystitis and/or cholelithiasis) occurred in one patient in the placebo group and in two patients in each of the 200 mg and 400 mg exisulind groups.
Table 5 Most common (⩾5%) treatment related adverse events.
| Adverse event | Placebo (n = 95) | Exisulind 200 mg (n = 91) | Exisulind 400 mg (n = 95) | p Value* |
|---|---|---|---|---|
| Abdominal pain | 3 (3.2%) | 3 (3. 3%) | 14 (14.7%) | 0.005 |
| ALT increased | 1 (1.1%) | 1 (1.1%) | 8 (8.4%) | 0.017 |
| AST increased | 1 (1.1%) | 1 (1.1%) | 8 (8.4%) | 0.017 |
| Alkaline phosphatase | 2 (2.1%) | 2 (2.2%) | 7 (7.4%) | 0.085 |
| Dyspepsia | 4 (4.2%) | 4 (4.4%) | 5 (5.3%) | 0.5 |
| Diarrhoea | 3 (3.2%) | 3 (3.3%) | 5 (5.3%) | 0.36 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*Exisulind 400 mg versus placebo.
Forty one patients discontinued therapy prematurely: 11 (20.4%), 10 (20.8%), and 20 (37.7%) in the placebo, and 200 and 400 mg exisulind groups, respectively. Reasons for discontinuation mostly included adverse events, consent withdrawal, and protocol violation.
Discussion
In the present randomised, double blind, multicentre study, the effects of daily treatment with exisulind 200 mg or 400 mg on the growth of sporadic adenomatous colorectal polyps were compared with placebo in 281 patients. In the study population available for efficacy evaluation, adenomatous polyp size decreased significantly and polyps were less likely to grow in patients who received exisulind 400 mg/day compared with placebo. In the ITT analysis, median change in polyp size from baseline between the exisulind 400 mg and placebo treatment groups was not statistically significant (p = 0.3). One factor contributing to this lack of statistical significance is the fact that a 12 month polyp assessment could not be obtained from 17 of the 53 ITT patients treated with exisulind 400 mg. When no follow up evaluation was available, it was assumed, per study protocol, that no change occurred. With regard to the exisulind 200 mg treatment group, no significant differences in polyp size were found compared with placebo in either analysis. The efficacy evaluable analysis was a more useful marker in our model, as it better represented the appropriate regimen to be used in future studies. This is because the efficacy evaluable analysis only included patients who underwent a 12 month colonoscopy (or colonoscopy at a premature discontinuation visit).
Study of the growth rate of adenomas has been limited since the advent of colonoscopy because most polyps are removed following discovery. In a small epidemiology study, Bersentes and colleagues37 followed in situ adenomatous and hyperplastic colorectal polyps (initially measuring 3–10 mm in diameter) over a two year period. While the size of hyperplastic polyps remained constant, adenomatous polyps increased in size. The apoptotic effects of exisulind appear to be tissue selective as regression of the adenoma is accompanied by increased apoptosis in the adenoma but not in the surrounding normal mucosa.29,31 This selectivity may also explain the lack of effect of exisulind on hyperplastic polyps observed in this study.
Exisulind 400 mg/day significantly decreased median polyp size, increased therapeutic response, and decreased progressive disease compared with placebo. It should be noted, however, that a high placebo response occurred in this study. Placebo patients experienced a −4 mm2 decrease in median polyp size and a therapeutic response of 30% (that is, CR+PR). One plausible explanation is that the endoscopists were hoping that the drug was effective and hence may have inaccurately under measured the size of the polyp during the last colonoscopy. Interestingly, a similar placebo response was observed in the sulindac study by DiSario and colleagues21 and the national polyp regression study.38 In addition, some of the polyps that completely disappeared may represent overuse of suction creating suction polyps, or a natural complete resolution of polyps.
Placebo and exisulind 200 mg/day dosage regimens were well tolerated in this study. The higher dosage of exisulind was less tolerated (table 5). Abdominal pain, reversible hepatic transaminase elevations, and biliary events have been previously reported in FAP patients and prostate cancer patients treated with exisulind.27,31 Similarly, in the current study, transaminase elevations typically occurred early in therapy, were mild or moderate in intensity, and resolved in many cases despite continued treatment.
Exisulind has been shown to be choleretic as it may increase bile flow that could precipitate gall stone movement into the cystic or bile duct, resulting in cholecystitis and/or pancreatitis. In this study, three of the four patients who experienced biliary events while on exisulind had evidence of underlying gall bladder disease. All four patients recovered uneventfully and completed the study.
The percentage of hyperplastic polyps (44.8%) was consistent with our prediction. This percentage would normally be expected in a population of small polyps (3–10 mm) in the rectosigmoid colon.
Our study suggests that a short term study design, employing two orthogonal measurements of the polyps, and a comparatively small patient sample size, enables the assessment of the activity of potential chemoprevention agents prior to embarking on significantly larger, costly, and longer term polyp prevention trials.
A dropout rate of approximately 20% in the placebo and low dose exisulind groups was expected and was similar to the dropout rate in other trials. However, a dropout rate of 38%, in the high dose exisulind group, was larger than expected.
The results of the present study also suggest that the model used in the study to evaluate polyp size regression should be employed in assessing potential therapeutic regimens before launching the larger studies needed to prove the clinical efficacy and safety of a chemopreventive agent.
In summary, patients treated with exisulind 400 mg daily over a 12 month period had significant regression of sporadic adenomatous polyps and were less likely to have further growth of their polyps in comparison with placebo. Unfortunately, the clinical potential of this higher dose may be hampered due to the significantly greater toxicity associated with it. As a chemopreventive agent must have a very low profile of side effects, the current model suggests that a dose of exisulind (400 mg twice daily) is not suitable for long term usage and that a lower dose (but not as low as 200 mg twice daily) should be tried in the next clinical trial.
Acknowledgements
We are indebted to the patients and their families for their patience and willingness to participate in and contribute to such a demanding study.
Abbreviations
CRC - colorectal cancer
NSAIDs - non‐steroidal anti‐inflammatory drugs
ITT - intention to treat
FAP - familial adenomatous polyposis
CR - complete response
PR - partial response
STD - stable disease
PD - progressive disease
ANOVA - analysis of variance
BMI - body mass index
Appendix
In addition to the authors, the exisulind study group included the following investigators: R Marks (Alabama Digestive Research Center), R Guinan (Gastroenterology Associates of North Texas), R Kotfila (Mississippi Center of Clinical Research), C Schmitt (Southeastern Clinical Research, Chattanooga), J Pressman (Digestive Disease Consultants), R Foley (Regional Gastroenterology Associates), J Johanson (Rockford Gastroenterology Associates), R Pruitt (Nashville Medical Research Institute), J Geenen (Wisconsin Center for Advanced Research), R Hardi (Metropolitan Gastroenterology Group), L Wrubel (University of Utah Health Sciences Center), M Murphy (Savannah Center for Digestive and Liver Disease), E Morris (Rocky Mountain Clinical Research), D Weinberg (Jefferson Medical College), T Werth (Charlotte Gastroenterology and Hepatology), M Kurtz (Progressive Clinical Research), R Baerg (Tacoma Digestive Disease Center), S Bank (Long Island Jewish Medical Center), D Ruff (Healthcare Discoveries), D Abrahm (Newport Beach Orange Coast Endoscopy Center).
Footnotes
Cell Pathways, Inc., Horsham, PA, supported this study.
Conflict of interest: None declared.
References
- 1.Bond J H. Polyp guideline: diagnosis, treatment, and surveillance for patients with non‐familial colorectal polyps: the Practice Parameters Committee of the American College of Gastroenterology. Ann Intern Med 1993119836–843. [DOI] [PubMed] [Google Scholar]
- 2.Burt R W, Bishop D T, Lynch H T.et al Risk and surveillance of individuals with colorectal polyps: WHO Collaborative Centre for the Prevention of Colorectal Cancer. Bull World Health Organ 199068789–795. [PMC free article] [PubMed] [Google Scholar]
- 3.Colorectal cancer In: Steward BW, Kleihues P, eds. World cancer report. Lyon: IARC Press, 2003198–202.
- 4.Stryker S J, Wolff B G, Culp C E.et al Natural history of untreated colonic polyps. Gastroenterology 1987931009–1013. [DOI] [PubMed] [Google Scholar]
- 5.Fearon E R. Molecular genetic studies of the adenoma‐carcinoma sequence. Adv Intern Med 199439123–130. [PubMed] [Google Scholar]
- 6.Chung D C. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000119854–865. [DOI] [PubMed] [Google Scholar]
- 7.Itzkowitz S H, Kim Y S. Colonic polyps and polyposis syndromes. In: Feldman M, Sleisenger MH, Scharschmidt BF, eds. Sleisenger & Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, 6th edn. Philadelphia: Saunders, 1998;1865–912,
- 8.Anwar S, White J, Hall C.et al Sporadic colorectal polyps: management options and guidelines. Scand J Gastroenterol 1999344–11. [DOI] [PubMed] [Google Scholar]
- 9.Bond J H. Colorectal cancer update. Prevention, screening, treatment, and surveillance for high‐risk groups. Med Clin North Am 2000841163–1182. [DOI] [PubMed] [Google Scholar]
- 10.Burt R W. Colon cancer screening. Gastroenterology 2000119837–853. [DOI] [PubMed] [Google Scholar]
- 11.Winawer S J, Zauber A G, Ho M N.et al Prevention of colorectal cancer in colonoscopic polypectomy. N Engl J Med 19933291977–1981. [DOI] [PubMed] [Google Scholar]
- 12.Williams C S, Smalley W, DuBois R N. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest 19971001325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahnen D J. Colon cancer prevention by NSAIDs: What is the mechanism of action? Eur J Surg 1998582(suppl)111–114. [DOI] [PubMed] [Google Scholar]
- 14.Arber N. NSAIDs prevent colorectal cancer. Can J Gastroenterol 200014299–307. [DOI] [PubMed] [Google Scholar]
- 15.Waddell W R, Loughry R W. Sulindac for polyposis of the colon. J Surg Oncol 19832483–87. [DOI] [PubMed] [Google Scholar]
- 16.Waddell W R, Ganser G F, Cerise E J.et al Sulindac for polyposis of the colon. Am J Surg 1989157175–179. [DOI] [PubMed] [Google Scholar]
- 17.Rigau J, Pique J M, Rubio E.et al Effects of long‐term sulindac therapy on colonic polyposis. Ann Intern Med 1991115952–954. [DOI] [PubMed] [Google Scholar]
- 18.Labayle D, Fischer D, Vielh P.et al Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 1991101635–639. [DOI] [PubMed] [Google Scholar]
- 19.Giardiello F M, Hamilton S R, Krush A J.et al Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 19933281313–1316. [DOI] [PubMed] [Google Scholar]
- 20.Hurlimann R, Muller A, Meyenberger C.et al Regression of adenomas in familial adenomatous polyposis (FAP) is enhanced in the proximal colon by sulindac. Gastroenterology 1994106A394 [Google Scholar]
- 21.DiSario J A, Alberts D S, Tietze C C.et al Sulindac induces regression and prevents progression of sporadic colorectal adenomas. Gastrointestinal Oncol 1997112A555 [Google Scholar]
- 22.Matsuhashi N, Nakajima A, Fukushima Y.et al Effects of sulindac on sporadic colorectal adenomatous polyps. Gut 199740344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladenheim J, Garcia G, Titzer D.et al Effect of sulindac on sporadic colonic polyps. Gastroenterology 19951081083–1087. [DOI] [PubMed] [Google Scholar]
- 24.Hixson L J, Earnest D L, Fennerty M B.et al NSAID effect on sporadic colon polyps. Am J Gastroenterol 1993881652–1656. [PubMed] [Google Scholar]
- 25.Hawk E, Prindiville S, Kelloff G. NSAID trials, sporadic adenomas, and conservative inferences. Gastroenterology 1996110654. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe M M, Lichtenstein D R, Singh G. Gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs. N Engl J Med 19993401888–1899. [DOI] [PubMed] [Google Scholar]
- 27.Goluboff E T. Exisulind, a selective apoptotic antineoplastic drug. Expert Opin Invest Drugs 2001101875–1882. [DOI] [PubMed] [Google Scholar]
- 28.Piazza G A, Rahm A K, Finn T S. Apoptosis primarily accounts for the growth‐inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res 1997572452–2459. [PubMed] [Google Scholar]
- 29.Piazza G A, Alberts D S, Hixson L J.et al Sulindac sulfone inhibits azoxymethane‐induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res 1997572909–2915. [PubMed] [Google Scholar]
- 30.Thompson W J, Piazza G A, Li H.et al Exisulind induction of apoptosis involves guanosine 3′,5′‐cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β‐catenin. Cancer Res 2000603338–3342. [PubMed] [Google Scholar]
- 31.Van Stolk R, Stoner G, Hayton W L.et al Phase I trial of exisulind (sulindac sulfone, FGN‐1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res 2000678–89. [PubMed] [Google Scholar]
- 32.Piazza G A, Xu S, Klein‐Szanto et al Overexpression of cGMP phosphodiesterase (cG‐PDE) in colonic neoplasias compared to normal mucosa. Gastroenterology 20001181590 [Google Scholar]
- 33.Burke C. The effect of exisulind on rectal adenomas in adults with familial adenomatous polyposis. Cancer Invest 2000191 [Google Scholar]
- 34.Burke C, Van Stolk R, Arber N.et al Phillips. Exisulind prevents adenoma formation in familial adenomatous polyposis (FAP). Gastroenterology 2000118A657 [Google Scholar]
- 35.Phillips R, Hultcrantz R, Bjork J.et al Exisulind, a pro‐apoptotic drug, prevents new adenoma formation in patients with familial adenomatous polyposis. Gut 200047A2–A3. [Google Scholar]
- 36.Engels J M, Diehr P. Imputation of missing longitudinal data: a comparison of methods. J Clin Epidemiol 200356968–976. [DOI] [PubMed] [Google Scholar]
- 37.Bersentes K, Fennerty M B, Sampliner R E.et al Lack of spontaneous regression of tubular adenomas in two years of follow‐up. Am J Gastroenterol 1997921117–1120. [PubMed] [Google Scholar]
- 38.Loeve F, Boer R, Zauber A G.et al National Polyp Study data: evidence for regression of adenomas. Int J Cancer 2004111633–639. [DOI] [PubMed] [Google Scholar]