Abstract
Background and aims
Achalasia is a disease of unknown aetiology. An immune mechanism has been suggested on the basis of previous morphological observations. The objective of this study was to test whether the serum of achalasia patients could reproduce the phenotype and functional changes that occur with disease progression in an ex vivo human model.
Methods
Specimens of normal human fundus were maintained in culture in the presence of serum from patients with achalasia, gastro‐oesophageal reflux disease (GORD), or healthy subjects (controls). Immunohistochemical detection of choline acetyltransferase (ChAT), neurone specific enolase (NSE), vasoactive intestinal polypeptide (VIP), nitric oxide synthase (NOS), and substance P was carried out in whole mounts of gastric fundus myenteric plexus. In addition, the effects of achalasia serum on electrical field stimulation (EFS) induced contractions were measured in circular muscle preparations.
Results
Serum from achalasia patients did not affect the number of myenteric neurones. Tissues incubated with serum from achalasia patients showed a decrease in the proportion of NOS (−26% of NSE positive neurones; p = 0.016) and VIP (−54%; p = 0.09) neurones, and a concomitant increase in ChAT neurones (+16%; p<0.001) compared with controls. In contrast, GORD serum did not modify the phenotype of myenteric neurones. Area under the curve of EFS induced relaxations (abolished by N‐nitro‐L‐arginine methyl ester) was significantly decreased following incubation with serum from achalasia patients compared with controls (−7.6 (2.6) v −14.5 (5.0); p = 0.036).
Conclusions
Serum from achalasia patients can induce phenotypic and functional changes which reproduce the characteristics of the disease. Further identification of putative seric factors and mechanisms involved could lead to the development of novel diagnostic and/or therapeutic strategies in achalasia.
Keywords: achalasia, myenteric plexus, neurochemical plasticity, NOS, human fundus
Achalasia is a primary motility disorder of the oesophagus characterised by the disappearance of oesophageal peristalsis and a poorly relaxing lower oesophageal sphincter (LOS) after swallowing, frequently associated with increased LOS pressure. Patients with achalasia exhibit loss of swallow induced oesophageal motor inhibitory activity1 as well as impaired function of the proximal stomach.2 The cause of achalasia remains unknown although available data suggest that hereditary, viral, neurodegenerative, or autoimmune factors may be involved.3
One of the hallmarks of achalasia is a phenotypic switch of myenteric neurones both in the oesophagus and proximal stomach. More specifically, a decrease in the number of nitric oxide (NO) synthase immunoreactive (IR) neurones has been reported in oesophageal tissues as well as in the proximal stomach of these patients.4,5 In addition, several observations have shown a decrease or even absence of myenteric neurones predominantly in the distal oesophagus of achalasia patients.6,7,8 Although the pathophysiological factors responsible for alterations of the enteric innervation remain unknown, various observations support a role for an immune/autoimmune component in achalasia: (a) activated T cell lymphocytic infiltrates within the myenteric plexus,9,10 (b) presence of antimyenteric neuronal antibodies in serum11,12,13,14,15 and finally, (c) association with antigens of class II major histocompatibility complex (MHC).14,16 Indeed, MHC II antigens (DR‐DQ) have been found to be associated with other autoimmune diseases such as multiple sclerosis, Sjögren's syndrome, Hashimoto's thyroiditis, and systemic lupus erythematosus.17,18 In addition, an important feature of some autoimmune neuropathies is that the serum as well as IgG of patients can alter neurone properties. These alterations range from changes in electrophysiological properties such as altered calcium flux,19,20,21 or blockade of nicotinic cholinergic receptors22 or voltage gated potassium channels,23 and can lead to neuronal cell death (paraneoplastic syndrome with anti‐Hu antibodies).24 The effects of serum of achalasia patients on the properties of myenteric neurones are however currently unknown. Therefore, demonstration of a direct effect of serum of achalasia patients on intact human tissues and reproduction of an achalasic phenotype as well as motility dysfunction could represent an important step in the understanding of the mechanisms of the disease with subsequent potential therapeutic applications.
The aim of this study was to test the hypothesis that serum from achalasia patients can affect the phenotype of human gastric myenteric neurones as well as neurally mediated motor response in an ex vivo model of normal human fundus. To test the specificity of serum from achalasia patients, we also characterised the effects of serum from patients with gastro‐oesophageal reflux disease (GORD) on the neurochemical phenotype of myenteric neurones.
Material and methods
Serum from achalasia patients, GORD patients, and controls
Sera from 18 achalasia patients obtained from our serum bank were used for either neurochemical coding and/or in vitro motility studies (11 males, seven females; mean age 47 (SD 18) years). Duration of disease was 7 (7) years, LOS pressure was 29 (23) cm H2O, and the percentage of LOS relaxation during swallowing was 27 (27)%. All but one had a non‐vigorous form of achalasia (that is, amplitude of contraction less than 50 cm H2O25). Twelve patients had never been treated for their achalasia prior to collection of serum samples. Three patients had myotomy and three others had pneumatic dilation.
Sera of 11 healthy volunteers (nine males, two females; mean age 31 (SD 8) years) and five GORD patients (three males, two females; mean age 57 (SD 13) years) were used as separate controls. All GORD patients had reflux symptoms requiring continuous proton pump inhibitor treatment.
Blood was collected and centrifuged (7000 g, 15 minutes). Sera were stored at −80°C.
Ex vivo model of normal human fundus
Specimens were obtained from the intact fundus of patients not previously treated with radiotherapy undergoing surgery for adenocarcinoma of the oesophagus. According to the guidelines of the French Ethics Committee for Research on Human Tissues, these specimens were considered as waste and cannot be used for pathological diagnosis. Neurochemical coding was studied in 10 specimens (seven from male and three from female patients; mean age 66 (11) years). In vitro motility studies were conducted in six specimens (two from male and four from female patients; mean age 71 (SD 12) years).
Specimens from the large curvature (approximately 5 cm away from the LOS), at a distance from the tumour, were resected and immediately processed in the pathology department. Tissue was placed in 4°C oxygenated sterile Krebs solution containing (in mM) 117 NaCl, 4.7 KCl, 1.2 MgCl2 6.0 H2O, 1.2 NaH2PO4, 25.0 NaHCO3, 2.5 CaCl2 2H2O, and 11.0 glucose. It was then rapidly transported to the laboratory for dissection.
The piece was pinned flat, mucosa up, in a dissection dish containing ice cold sterile oxygenated Krebs solution that was changed every 10–15 minutes. Preparations were 4–7×4–5 cm in size. The mucosa was then carefully removed under a dissection microscope. Tissue was washed four times with sterile Krebs solution and pinned back in a sterile Sylgard coated Petri dish before addition of sterile culture medium (30 ml). Culture medium (Dulbecco's modified Eagle's medium/F12; Sigma, Saint Quentin, France) was supplemented with 10% heat inactivated serum from control, achalasia, or GORD patients, 100 IU/ml penicillin, 100 μg/ml streptomycin, 1.1 μg/ml amphotericin B, 20 μg/ml gentamicin (Sigma), 6 mM glutamine, and 2.1 g/l NaHCO3. Colchicine (40 μM) was added to the medium to enhance the immunoreactivity of nerves to peptides. Tissue was then maintained at 37°C overnight (16–18 hours) in a humidified incubator containing 5% CO2 and placed on a rocking tray. This time of incubation was chosen because we observed in preliminary experiments that changes in neuronal phenotypes induced by the serum of achalasia patients were similar after incubation times of 16–18 hours and 72 hours.
Immunohistochemistry
The effects of 18 sera from achalasia patients, 11 healthy controls, and five patients with GORD were tested. An average of three (range 2–6) different sera among which at least one control were tested, on each fundic specimen (n = 10).
After culture, tissue was stretched and fixed for 4–6 hours in 0.1 M phosphate buffered saline (PBS) containing 4% paraformaldehyde at room temperature. Following several washes in PBS, the tissue was pinned in a dissection dish. Under a dissection microscope, the circular muscle, oblique layer, and part of the longitudinal muscle were removed to expose the myenteric plexus.
Myenteric plexus/longitudinal muscle was then permeabilised for 1–2 hours in PBS/NaN3 containing 0.5% Triton X‐100 and 4% horse serum. Tissue was incubated with the following primary antibodies diluted in PBS/NaN3, 4% horse serum, and 0.5% Triton‐X for 18–20 hours at room temperature: goat anti‐choline acetyltransferase (ChAT) (1:200; AB144P, Chemicon, USA), rat anti‐substance P (SP) (1:1000; 10‐SO15; Fitzgerald, Concord, California, USA), mouse anti‐vasoactive intestinal polypeptide (VIP) (1:1000; Biogenesis, UK), and rabbit anti‐nitric oxide synthase (NOS) (1:2000; R025; Alexis, USA). Following incubation with primary antisera, the tissue was washed with PBS and incubated for 2–3 hours with donkey antigoat IgG conjugated to carboxymethylindocyanine (1:500), donkey antirat IgG conjugated to 7‐amino‐4‐indodicarbocyanin (1:500), donkey antimouse IgG conjugated to 7‐amino‐4‐methyl‐coumarin‐3‐acetate (1:50), and donkey antirabbit IgG conjugated to fluorescein isothiocyanate (FITC) (1:200).
After the population of neurones IR for ChAT, SP, VIP, and NOS was identified, some tissues were washed in PBS (four times, 10 minutes) and labelled with rabbit anti‐neurone specific enolase (NSE) (1:2000; 17437; Polysciences, Germany) for 18–20 hours. Tissue was then washed with PBS and incubated for 2–3 hours with donkey antirabbit IgG conjugated to FITC.
Specimens were viewed under an Olympus IX 50 fluorescence microscope fitted with adequate filter cubes. Pictures were acquired with a black and white video camera (model 4910; Cohu Inc., SL Microtest, Jena, Germany) connected to a Macintosh computer through a frame grabber card (Scion Image; SL Microtest).
Identification of neuronal cell populations and phenotypic analysis
To determine the general coding of the myenteric plexus, an average of 300 (23) neurones were analysed for each condition. The number of ChAT, VIP, NOS, and SP IR myenteric neurones was determined by quadruple immunohistochemistry. In 18 tissues, an anti‐NSE antibody was used as a general neuronal marker to ascertain the total number of cell bodies in the same previously stained ganglia. The proportion of neurochemically identified populations was then expressed relative to the number of NSE IR neurones, which was assumed to represent the total neurone population.26
Immunohistochemical identification of antineuronal antibodies
Frozen and unfixed sections of primate gut (Euroimmun, Lübeck, Germany) were incubated with individual serum samples (five healthy controls and 16 patients with achalasia; dilution 1:100) for one hour at room temperature. After washing in PBS‐Tween (0.2%), sections were exposed to FITC labelled goat‐antihuman IgG (Euroimmun) for 30 minutes. Slides were then evaluated for specific staining of neuronal structure under an Olympus IX‐50 fluorescence microscope using a semiquantitative rating scale: 0, no staining above background; +, moderate staining; and ++, intense staining of neurones.
In vitro motility studies
The effects of 10 sera from achalasia patients and seven sera from healthy controls were tested. An average of three (range 2–4) different sera were evaluated, of which at least one control serum was tested on each fundic specimen (n = 6).
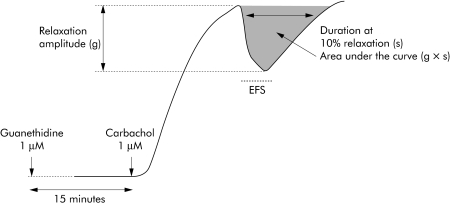
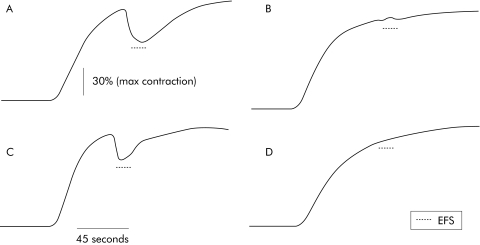
Following a 16 hour incubation with serum, tissue was transferred to a sterile dish and the submucosa was removed under a microscope. Small strips of circular muscle (approximately 5×10 mm) were dissected along the circular axis. These muscle strips were then placed in an organ bath filled with 10 ml of Krebs solution at 37°C, continuously bubbled with 95% O2 and 5% CO2. Strips were initially stretched with a preload of 10 mN which was maintained during an equilibration period of at least 90 minutes. After 3–4 washes every 10 minutes, 10 μM guanethidine were added to block sympathetic influence. After 10 minutes the preparation was contracted through exposure to carbachol (1 μM). At peak contraction, enteric neurones were electrically field stimulated (EFS) using the following parameters: train duration 15 seconds, stimulation frequency 20 Hz, pulse duration 400 μs, and pulse amplitude 15 V. This procedure was repeated 2–3 times with a 30 minute washout period between each stimulation. In order to characterise NO dependant relaxation, the same protocol was used in the presence of N‐nitro‐L‐arginine methyl ester (L‐NAME 50 μM). Duration of the relaxation period, which corresponded to 10% of the maximal relaxation period, and area under the curve, were measured for each EFS induced response (fig 1). The area under the curve was normalised to carbachol induced maximum contractile amplitude.
Figure 1 Illustration of relaxation of human fundic circular muscle preparation induced by electrical field stimulation (EFS). Following carbachol induced contraction in the presence of guanethidine, myenteric neurones were electrically field stimulated.
The contractile response of circular muscle was continuously recorded using isometric force transducers (Basile No 7005; Comerio, VA, Italy), and data transferred onto a PowerMac Performa 7100/80 computer equipped with the MacLAb/4s System (ADI, Spechbach, Germany).
Statistical analysis
Data are expressed as mean (SEM) or median (quartile), where appropriate. Results were compared using a paired or unpaired t test. For non‐normally distributed data, comparisons were performed using the Mann‐Whitney non‐parametric test. Correlations between patient parameters and observed changes were calculated using linear regression analysis. Differences were considered significant at p<0.05.
Results
Effects of serum from achalasia patients on neuronal phenotype
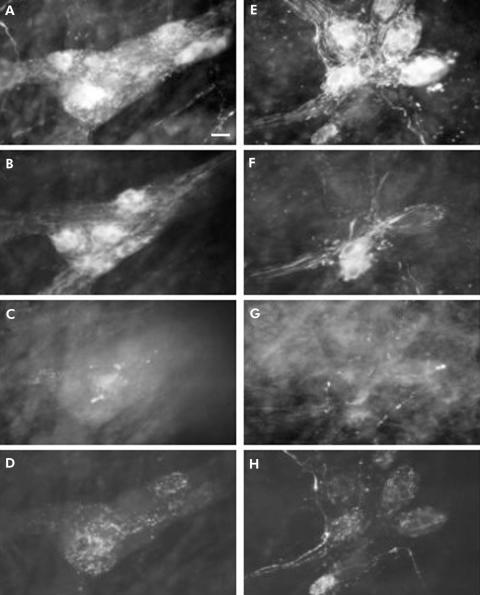
Quadruple immunohistochemical staining was performed on fundic specimens incubated with sera from healthy controls (fig 2A‐D) and with sera from achalasia patients (fig 2E‐H).
Figure 2 Immunohistochemical detection of transmitter coding in human fundic myenteric plexus incubated with sera from achalasia patients and healthy subjects. Quadruple labelling with antibodies against choline acetyltransferase (ChAT) (A), nitric oxide synthase (NOS) (B), vasoactive intestinal polypeptide (VIP) (C), and substance P (SP) (D) performed on a myenteric ganglion following incubation with serum from a control patient. Quadruple labelling with antibodies against ChAT (E), NOS (F), VIP (G), and SP (H) performed on a myenteric ganglion following incubation with serum from a achalasia patient. Two fragments of gastric fundus from the same patient were incubated with serum from a control subject or from an achalasia patient. Scale bar 25 μm.
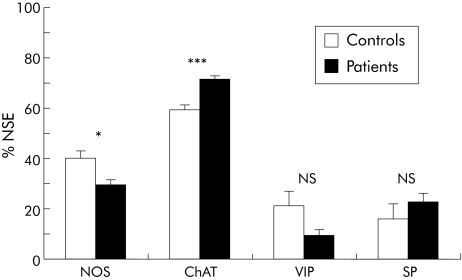
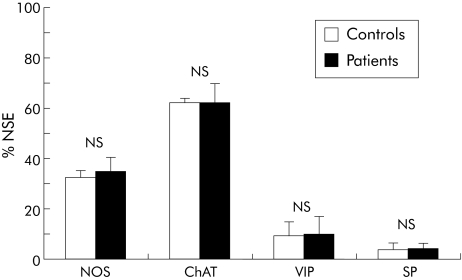
Serum from patients with achalasia induced significant changes in two of the four IR populations (fig 3). The proportion of NOS‐IR neurones was decreased by 26% on average in the presence of achalasia serum compared with controls (p = 0.016). All sera from achalasia patients induced a reduction in the proportion of NOS‐IR neurones, ranging from −11% to −43%. Conversely, the proportion of ChAT‐IR neurones was increased by 16% in the presence of achalasia serum compared with controls (p<0.001). All sera from achalasia patients induced an increase in the proportion of ChAT‐IR neurones, ranging from 4% to 46%. The proportion of VIP‐IR neurones was decreased by 54% compared with controls (p = 0.09). Although the difference did not reach statistical significance, the proportion of SP‐IR neurones tended to increase (fig 3).
Figure 3 Proportion of nitric oxide synthase (NOS), choline acetyltransferase (ChAT), vasoactive intestinal polypeptide (VIP), and substance P (SP) immunoreactive myenteric neurones in human fundus after incubation with serum from healthy controls and patients with achalasia. Results are presented as percentages of the total neuronal population, as determined by neurone specific enolase (NSE) immunostaining. *p<0.05, ***p<0.001.
Following incubation of fundic tissue with serum from patients with achalasia, no significant change in the number of NSE positive cells per ganglion was observed compared with controls (serum from healthy subjects) (median 34 (quartiles 22–60) v 33 (16–72) respectively; p = 0.70).
There was no statistically significant correlation between clinical characteristics of the patients (age, duration of symptoms, LOS pressure, percentage of LOS relaxation in response to swallowing) and changes in the neurochemical coding of myenteric neurones.
Effects of serum from GORD patients on neuronal phenotype
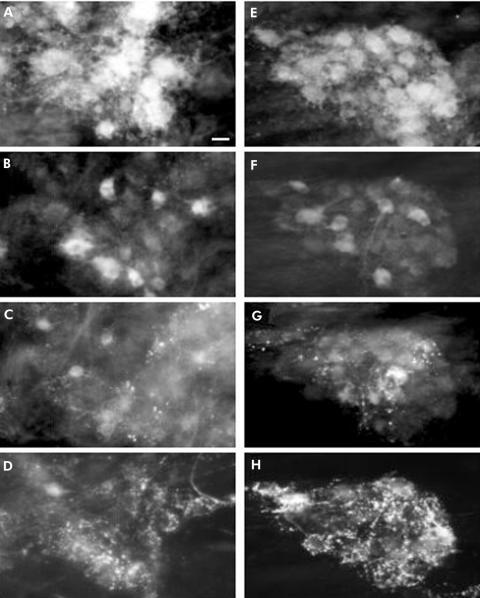
Quadruple immunohistochemical staining was performed on fundic samples incubated with sera from healthy controls (fig 4A–D) and GORD patients (fig 4E–H). Serum from patients with GORD did not induce significant changes in ChAT‐, NOS‐, VIP‐, or SP‐IR populations compared with controls (fig 5).
Figure 4 Immunohistochemical detection of transmitter coding in human fundic myenteric plexus incubated with sera from patients with gastro‐oesophageal reflux disease (GORD) and healthy subjects. Quadruple labelling with antibodies against choline acetyltransferase (ChAT) (A), nitric oxide synthase (NOS) (B), vasoactive intestinal polypeptide (VIP) (C), and substance P (SP) (D) performed on a myenteric ganglion following incubation with serum form a control patient. Quadruple labelling with antibodies against ChAT (E), NOS (F), VIP (G), and SP (H) performed on a myenteric ganglion following incubation with serum from a GORD patient. Two fragments of gastric fundus from the same patient were incubated with serum from a control subject or an achalasia patient. Scale bar 25 μm.
Figure 5 Proportion of nitric oxide synthase (NOS), choline acetyltransferase (ChAT), vasoactive intestinal polypeptide (VIP), and substance P (SP) immunoreactive myenteric neurones in human fundus after incubation with serum from healthy controls and patients with gastro‐oesophageal reflux disease. Results are presented as a percentage of the total neuronal population, as determined by neurone specific enolase (NSE) immunostaining.
Effects of serum from achalasia patients and healthy controls on immunolabelling of enteric ganglia
In the achalasia group, 12% of serum samples (2/16 samples) labelled enteric neurones while in the control group no neurones were labelled (five samples).
Effects of achalasia serum on EFS induced human fundic motor response
In presence of guanethidine, carbachol induced contraction of the fundic circular muscle. The amplitude of this contractile response was not significantly different between tissues incubated with serum from achalasia patients and controls (1.6 (0.6) v 1.5 (0.8) N/g, respectively; paired t test).
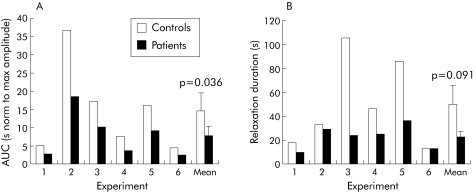
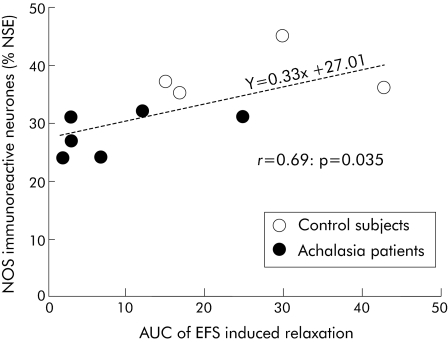
EFS induced relaxation of the preparation was significantly altered by serum from achalasia patients compared with controls (fig 6). The normalised area under the curve was significantly decreased in tissues incubated with serum from achalasia patients compared with controls (7.6 (2.6) v 14.5 (5.0); p = 0.036, paired t test) (fig 7A). In addition, duration of relaxation at 10% of maximal relaxation values was reduced by serum from achalasia patients compared with controls, but this fell short of statistical significance (23 (4) v 50 (15) seconds; p = 0.09, paired t test) (fig 7B). The normalised amplitude of EFS induced relaxation was however not altered by serum from achalasia patients compared with controls (33 (10) v 37 (5)%, respectively; p = 0.71, paired t test). For the 10 sera tested for both immunochemistry and motility (six achalasia patients and four controls), there was a statistically significant correlation between the area under the curve of EFS induced relaxation and the proportion of NOS‐IR neurones (fig 8).
Figure 6 Typical traces illustrating the decrease in electrical field stimulation (EFS) induced nitric oxide (NO) dependent relaxation in human fundic muscle strips incubated with serum from a healthy control subject and from a patient with achalasia. Serum from the achalasia patient was found to decrease EFS induced NO dependent relaxation in human fundic circular muscle strips. Following carbachol induced contraction, EFS induced relaxation in tissue preincubated with control serum (A). In the presence of N‐nitro‐L‐arginine methyl ester (L‐NAME 100 μM), EFS induced relaxation was precluded (B). Following carbachol induced contraction, EFS also induced relaxation in tissue preincubated with serum from achalasia patients (C). Relaxation was however reduced compared with controls. In the presence of L‐NAME (100 μM), EFS induced relaxation was also precluded (D). Effects of L‐NAME were studied on the same muscle strips. Data were normalised to the amplitude of the contraction prior to EFS.
Figure 7 Effect of preincubation with control serum and serum from achalasia patients on area under the curve (AUC) (A) and duration (determined at 10% of maximal relaxation amplitude) (B) of electrical field stimulation (EFS) induced relaxation of human fundic circular muscle strips (individual results of six separate experiments and mean of the experiments are shown).
Figure 8 Correlation between the area under the curve (AUC) of electrical field stimulation (EFS) induced relaxation of human fundic muscular strips and the proportion of nitric oxide synthase (NOS) immunoreactive neurones. The correlation was calculated in the four control sera and six achalasia patient sera, for which both neurochemical coding and the ex vivo motility study were performed. NSE, neurone specific enolase.
In the presence of L‐NAME, carbachol induced contractions were not altered in tissues incubated with serum from control or achalasia patients (data not shown). EFS induced relaxation was however significantly reduced and almost abolished in all of the preparations (fig 6) (p<0.0004 for serum of controls and p<0.025 for serum of achalasia patients; paired t test).
Discussion
We have shown that serum from achalasia patients induces changes in the neurochemical phenotype of myenteric neurones (which are reminiscent of those observed in achalasia) in an ex vivo model of human healthy fundus. These effects seem to be specific for achalasia as serum from GORD patients did not change the neurochemical phenotype of fundic myenteric neurones. Furthermore, ex vivo motility experiments performed on human fundic strips revealed that EFS induced NO dependent relaxation that was significantly inhibited in tissues incubated with serum from achalasia patients compared with controls.
This study documents for the first time the effects of serum from achalasia patients on both the neurochemical coding of human myenteric neurones and on one of its functional correlates (that is, neurally mediated motor activity). One of the strengths of this study is the fact that it was performed on human tissues thereby avoiding interspecies differences. This is particularly important as high interspecies variability in the organisation and neurochemical coding of the enteric nervous system (ENS) as well as in the functions regulated by the ENS have been reported.27 Drawbacks of using human tissues include relative paucity of tissues and the fact that specimens are usually collected from non‐healthy older subjects. We tried to minimise this latter point as tissues were taken at a distance from tumours in a macroscopically and histologically normal fundic area. Although control patients were younger than achalasia patients, age was probably not a factor in serum induced alterations as: (1) no correlation between age and changes in phenotype was observed in achalasia patients and (2) GORD patients were of similar age as achalasia patients. In addition, serum from achalasia patients of similar age as control patients also induced alterations in the neurochemical phenotype.
Incubating human fundic tissues with serum from achalasia patients induced a significant decrease in the proportion of NOS‐IR and a decrease (although not significant) in the proportion of VIP‐IR myenteric neurones. This observation is consistent with previous studies showing both a decrease in the proportion of NOS‐IR myenteric neurones in the human fundus5 as well as a decrease in VIPergic innervation in achalasia patients.28 Furthermore, our study revealed an increase in the population of ChAT‐IR neurones. Whether the latter phenomenon occurs in achalasia is unknown as previous studies have not thoroughly evaluated the impact of achalasia on cholinergic innervation. However, the cholinergic component of LOS pressure (studied using acetylcholinesterase inhibitors) was found to increase in achalasia patients compared with healthy controls.29
However, in our study, we did not find clear signs of NOS‐IR neuronal cell degeneration, as previously reported in achalasia.4,7 Indeed, no change in the total number of neurones (identified with NSE) was observed after incubation with serum from achalasia patients. However, the method used may not have been sensitive enough to detect small changes in neuronal populations, in particular under conditions where the variability in the number of neurones per ganglia is large. Consequently, future studies using specific and early markers of cell death are needed to clarify the putative involvement of neurodegeneration. The decrease in NOS‐IR neurones would rather be due to a decrease in expression of nNOS. The rapid occurrence of these changes (16–18 hours) is consistent with an effect of serum on regulation of nNOS expression at the mRNA and/or protein level. Indeed, in the rat stomach, nNOS expression can be significantly decreased as early as four hours following intraperitoneal injection of endotoxin.30 Furthermore, IgG of patients with Sjögren's syndrome are able to increase nNOS mRNA expression as rapidly as one hour after incubation in rat cerebral cortex preparations.31
These alterations in the neurochemical phenotype could be the basis for the altered motor response to EFS observed in our study. Indeed, and as suggested by the correlation between the proportion of NOS‐IR neurones and amplitude of EFS induced relaxation (fig 8), the decrease in NOS‐IR myenteric neurones could explain the decrease in EFS induced relaxation following incubation with serum from patients with achalasia. This EFS induced relaxation was NO dependent as it was blocked by L‐NAME. These results are consistent with previous studies showing that in the human fundus, EFS induced relaxation has an important nitrergic component.32 This decrease in NO mediated relaxation could be involved in various gastric and oesophageal altered functions observed in achalasia.2,33,34 Alteration in expression of NOS in fundic myenteric neurones could also partly explain the increased LOS tone in achalasia as NOS‐IR fundic myenteric neurones were shown to innervate and regulate relaxation of the LOS.35
Both the mechanisms and mediators involved in the changes in phenotype of myenteric neurones induced by serum from achalasia patients are currently unknown. The putative mediators responsible for the changes in neurochemical phenotype (as observed in our study) include inflammatory mediators. Involvement of cytokines is suggested by the following observations: the increase in interleukin 8 levels in the serum of achalasia patients compared with controls (unpublished personal results); the ability of cytokines, such as interleukin 1β, to regulate gene expression in enteric neurones36,37; and the presence of inflammatory cells in the myenteric plexus in patients with achalasia.8,38 Apart from cytokines, various neurotrophic factors have been shown to differentially regulate expression of some neuromediators in the ENS. In particular, NT‐3 increases the VIP and SP content of enteric or central neurones.39 Reinforcing the concept that neurotrophic factors could be involved in achalasia is the preliminary observation that a decrease in neurotrophin expression occurs in syndromic forms of achalasia.40
Changes in neurochemical phenotype could also be associated with the presence of antineuronal autoantibodies in the serum of achalasia patients.12,15 However, in our study, a minority of serum from achalasia patients (12% of the sera tested) labelled enteric neurones, further suggesting that the presence of neuronal antibodies is rather an epiphenomenon than a causative factor in achalasia, as first proposed by Moses and colleagues.15 In their study, they also observed that a large proportion of achalasia sera (49%) did not label enteric neurones.15 In addition, in our study, serum from GORD patients (which also contains antineuronal antibodies15) did not change the neurochemical phenotype of human myenteric neurones.
In conclusion, our study demonstrates that serum from patients with achalasia can change the phenotype of human fundic myenteric neurones and modulate EFS induced NO dependent relaxation of the human fundus. Identification of this (these) seric component(s) is of pivotal importance to enable the development of novel diagnostic and/or therapeutic strategies. In addition, similar experimental approaches could be used to test the effects of seric components on ENS and gastrointestinal functions in other enteric neuropathies.38
Acknowledgements
The authors are grateful to the hospital surgery and pathology teams for valuable contributions to this work.
Abbreviations
ChAT - choline acetyltransferase
ENS - enteric nervous system
IR - immunoreactive
LOS - lower oesophageal sphincter
NSE - neurone specific enolase
NOS - nitric oxide synthase
SP - substance P
VIP - vasoactive intestinal polypeptide
L‐NAME - N‐nitro‐L‐arginine methyl ester
EFS - electrical field stimulation
GORD - gastro‐oesophageal reflux disease
MHC - major histocompatibility complex
PBS - phosphate buffered saline
FITC - fluorescein isothiocyanate
Footnotes
Conflict of interest: None declared.
Presented in part at the Annual Meeting of the American Gastroenterological Association (Digestive Disease Week), Orlando, Florida, 17–22 May 2003
References
- 1.Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary oesophageal motility disorders. Gastroenterology 1994106875–882. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Papo M, Malagelada J R. Impaired gastric relaxation in patients with achalasia. Gut 199536363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson C S, Martin B A B, Atkinson M. Varicella‐zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut 199334299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mearin F, Mourelle M, Guarner F.et al Patients with achalasia lack nitric oxide synthase in the gastro‐oesophageal junction. Eur J Clin Invest 199323724–728. [DOI] [PubMed] [Google Scholar]
- 5.De Giorgio R, Di Simone M P, Stanghellini V.et al Oesophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am J Gastroenterol 1999942357–2362. [DOI] [PubMed] [Google Scholar]
- 6.Lendrum F C. Anatomic features of the cardiac orifice of the stomach with special reference to cardiospasm. Arch Intern Med 193759474–511. [Google Scholar]
- 7.Goldblum J R, Whyte R I, Orringer M B.et al Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol 199418327–337. [PubMed] [Google Scholar]
- 8.Goldblum J R, Rice T W, Richter J E. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology 1996111648–654. [DOI] [PubMed] [Google Scholar]
- 9.Raymond L, Lach B, Shamji F M. Inflammatory aetiology of primary oesophageal achalasia: an immunohistochemical and ultrastructural study of Auerbach's plexus. Histopathology 199935445–453. [DOI] [PubMed] [Google Scholar]
- 10.Clark S B, Rice T W, Tubbs R R.et al The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am J Surg Pathol 2000241153–1158. [DOI] [PubMed] [Google Scholar]
- 11.Storch W B, Eckardt V F, Wienbeck M.et al Autoantibodies to Auerbach's plexus in achalasia. Cell Mol Biol 1995411033–1038. [PubMed] [Google Scholar]
- 12.Verne G N, Sallustio J E, Eaker J Y. Anti‐myenteric neuronal antibodies in patients with achalasia: a prospective study. Dig Dis Sci 199742307–313. [DOI] [PubMed] [Google Scholar]
- 13.Smith V V, Gregson N, Foggensteiner L.et al Acquired intestinal aganglionosis and circulating autoantibodies without neoplasia or other neural involvement. Gastroenterology 19971121366–1371. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz de Leon A, Mendoza J, Sevilla‐Mantilla C.et al Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci 20024715–19. [DOI] [PubMed] [Google Scholar]
- 15.Moses P L, Ellis L M, Anees M R.et al Antineuronal antibodies in idiopathic achalasia and gastro‐oesophageal reflux disease. Gut 200352629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verne G N, Hahn A B, Pineau B C.et al Association of HLA‐DR and ‐DQ alleles with idiopathic achalasia. Gastroenterology 199911726–31. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein R, Sengar D P. Comparative studies of the major histocompatibility complex in French Canadian and non‐French Canadian Caucasians with systematic lupus erythematosus. Arthritis Rheum 1993361121–1127. [DOI] [PubMed] [Google Scholar]
- 18.Harley J B, Reichlin M, Arnett F C.et al Gene interaction at HLA‐DQ enhances autoantibody production in primary Sjogren's syndrome. Science 19862321145–1147. [DOI] [PubMed] [Google Scholar]
- 19.Ristic H, Srinivasan S, Hall K E.et al Serum from diabetic BB/W rats enhances calcium currents in primary sensory neurones. J Neurophysiol 1998801236–1244. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan S, Stevens M J, Sheng H.et al Serum from patients with type 2 diabetes with neuropathy induces complement‐independent, calcium‐dependent apoptosis in cultured neuronal cells. J Clin Invest 19981021454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson M W, Gordon T P, Waterman S A. Disruption of intestinal motility by a calcium channel‐stimulating autoantiobody in type 1 diabetes. Gastroenterology 2004126819–828. [DOI] [PubMed] [Google Scholar]
- 22.Vernino S, Lennon V A. Neuronal ganglionic acetylcholine receptor autoimmunity. Ann N Y Acad Sci 2003998211–214. [DOI] [PubMed] [Google Scholar]
- 23.Wulff H, Beeton C, Chandy K G. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel 20036640–647. [PubMed] [Google Scholar]
- 24.Schafer K H, Klotz M, Mergner D.et al IgG‐mediated cytotoxicity to myenteric plexus cultures in patients with paraneoplastic neurological syndromes. J Autoimmun 200015479–484. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg S P, Burrell M, Fette G G.et al Classic and vigorous achalasia: a comparison of manometric, radiographic and clinical findings. Gastroenterology 1991101743–748. [DOI] [PubMed] [Google Scholar]
- 26.Pimont S, Bruley des Varannes S, Le Neel J C.et al Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol Motil 200315655–662. [DOI] [PubMed] [Google Scholar]
- 27.Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil 200416(suppl 1)55–59. [DOI] [PubMed] [Google Scholar]
- 28.Wattchow D A, Costa M. Distribution of peptide‐containing nerve fibres in achalasia of the oesophagus. J Gastroenterol Hepatol 199611478–485. [DOI] [PubMed] [Google Scholar]
- 29.Holloway R H, Dodds W J, Helm J F.et al Integrity of cholinergic innervation to the lower oesophageal sphincter in achalasia. Gastroenterology 198690924–929. [DOI] [PubMed] [Google Scholar]
- 30.Calatayud S, Garcia‐Zaragoza E, Hernandez C.et al Downregulation of nNOS and synthesis of PGs associated with endotoxin‐induced delay in gastric emptying. Am J Physiol 2002283G1360–G1367. [DOI] [PubMed] [Google Scholar]
- 31.Reina S, Sterin‐Borda L, Orman B.et al Human mAChR antibodies from Sjogren syndrome sera increase cerebral nitric oxide synthase activity and nitric oxide synthase mRNA level. Clin Immunol 2004113193–202. [DOI] [PubMed] [Google Scholar]
- 32.Tonini M, De Giorgio R, De Ponti F.et al Role of nitric oxide‐ and vasoactive intestinal polypeptide‐containing neurones in human gastric fundus strip relaxations. Br J Pharmacol 200012912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittinger M, Barnert J, Eberl T.et al Postprandial gastric relaxation in achalasia. Eur J Gastroenterol Hepatol 199810741–744. [DOI] [PubMed] [Google Scholar]
- 34.Yaghoobi M, Mikaeli J, Montazeri G.et al Correlation between clinical severity score and the lower oesophageal sphincter relaxation pressure in idiopathic achalasia. Am J Gastroenterol 200398278–283. [DOI] [PubMed] [Google Scholar]
- 35.Yuan S, Costa M, Brookes S J. Neuronal pathways and transmission to the lower oesophageal sphincter of the guinea pig. Gastroenterology 1998115661–671. [DOI] [PubMed] [Google Scholar]
- 36.Hurst S M, Stanisz A M, Sharkey K A.et al Interleukin 1 beta‐induced increase in substance P in rat myenteric plexus. Gastroenterology 19931051754–1760. [DOI] [PubMed] [Google Scholar]
- 37.Neunlist M, Barouk J, Just I.et al Modification of human submucosal neurones by toxin B of clostridium difficile: role of interleukin 1β and cyclooxygenase 2. Gastroenterology 2002122A38 [Google Scholar]
- 38.De Giorgio R, Guerrini S, Barbara G.et al Inflammatory neuropathies of the enteric nervous system. Gastroenterology 20041261872–1883. [DOI] [PubMed] [Google Scholar]
- 39.Sterne G D, Brown R A, Green C J.et al NT‐3 modulates NPY expression in primary sensory neurones following peripheral nerve injury. J Anat 1998193273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milla P J, Smith V V, Spitz L.et al The role of neurotrophins in achalasia of the oesophagus. Neurogastroenterol Motil 200315231 [Google Scholar]