Abstract
We evaluated the effects of the lipophilic nonpeptide corticotropin-releasing hormone (CRH) type 1 receptor antagonist antalarmin on the behavioral, neuroendocrine, and autonomic components of the stress response in adult male rhesus macaques. After oral administration, significant antalarmin concentrations were detected in the systemic circulation and the cerebrospinal fluid by a mass spectrometry-gas chromatography assay developed specifically for this purpose. Pharmacokinetic and dose-response studies suggested that an oral dose of 20 mg/kg was optimal for behavioral and endocrine effects. We then administered this dose in a double-blind, placebo-controlled fashion to monkeys exposed to an intense social stressor: namely, placement of two unfamiliar males in adjacent cages separated only by a transparent Plexiglas screen. Antalarmin significantly inhibited a repertoire of behaviors associated with anxiety and fear such as body tremors, grimacing, teeth gnashing, urination, and defecation. In contrast, antalarmin increased exploratory and sexual behaviors that are normally suppressed during stress. Moreover, antalarmin significantly diminished the increases in cerebrospinal fluid CRH as well as the pituitary-adrenal, sympathetic, and adrenal medullary responses to stress. We conclude that CRH plays a broad role in the physiological responses to psychological stress in primates and that a CRH type 1 receptor antagonist may be of therapeutic value in human psychiatric, reproductive, and cardiovascular disorders associated with CRH system hyperactivity.
During threatening situations, a reflexive, coordinated series of behavioral and physiological alterations occurs to promote survival. Fear-related behaviors such as arousal and suppression of sexual activity serve a protective function and shift the focus exclusively on the perceived threat. The hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system are activated to promote cardiovascular and metabolic adaptation. At the same time, neurovegetative functions, whose execution might impair survival in a life-threatening situation, as well as endocrine programs for growth and reproduction are all suspended in the diversion of energy toward either escape or confrontation (1). Although several neuroanatomic substrates and hormonal systems contribute to this concerted response, studies in rodents show that CRH is capable of triggering almost the entire repertoire of behavioral, neuroendocrine, autonomic, and neurovegetative responses to stress (2).
On the basis of its effects in rodents, many hypothesized that CRH plays an important role in human disorders of the mood (3), growth (4, 5), and reproduction (6). It was therefore essential to verify the relevance of CRH to the primate stress response to understand the organization of the stress system in primates as well as the pathophysiology of disorders associated with a dysregulated stress system, such as anxiety and depression (7). Moreover, because of their marked similarities to humans in genetic make-up, neurophysiology, and neuroanatomy, non-human primates are far more suitable than rodents to model closely interrelated physiological and behavioral processes (8).
Here we synthesized the lipophilic nonpeptide corticotropin-releasing hormone (CRH) type 1 receptor (CRH-R1) antagonist, antalarmin, N-butyl-N-ethyl-[2,5,6-trimethyl-7-(2,4,6)trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl] amine, first described by Chen (9), and examined it in rhesus macaques. Antalarmin is related to the pyrrolopyrimidine CP 154,526 (10), by the addition of a methyl group at the 6-position of the pyrrolopyrimidine ring system (11). The lipophilic nature of antalarmin should result in its wide distribution to brain sites involved in the control of behavior, endocrine, and autonomic functions. To reliably study the effects of antalarmin in vivo, we developed a mass-spectrometry gas chromatographic assay for its measurement and studied its pharmacokinetics. We then conducted a dose-response study to examine the effects of the drug on the behavioral and physiological responses to stress. To evaluate behavioral responses, we used the intruder paradigm that involves pairing of two unfamiliar males rhesus monkeys in virtually one cage, an intense, well characterized social stressor that reliably allows quantitative assessment of anxiety-related behaviors in non-human primates (12–14).
We asked the following questions: (i) Does antalarmin gain access to the primate systemic circulation and central nervous system after oral administration? (ii) Does antalarmin influence classic behavioral responses to social stress? (iii) Does antalarmin attenuate the HPA axis response to stress, and, if so, does it cause adrenal insufficiency? (iv) Does antalarmin affect indices of the autonomic response to stress? and (v) How do cerebrospinal fluid concentrations of CRH respond to psychosocial stress, and does antalarmin modify such a response?
Methods
Measurement of Antalarmin in Biological Fluids.
Hexane extracts of biological samples [plasma, urine, and cerebrospinal fluid (CSF)] were analyzed by using a Hewlett–Packard Model 6890 gas chromatograph equipped with a Model 5973 mass selective detector. The analytical column used was a 15 M × 250 μm DB-1 capillary (J & W Scientific, Folsom, CA), with antalarmin eluting at 15.7 min under the operating conditions. Quantification of antalarmin was accomplished by using electron impact ionization with single ion monitoring of 349 m/z, representing the loss of an ethyl group from the amine. The area of the peak corresponding to antalarmin was compared with a calibration curve generated by using six standards from 0.002 μg/ml to 50 μg/ml and plotted log (concentration) vs. log (area) with a correlation of 0.99993.
Pharmacokinetics of Antalarmin in Rhesus Macaques.
Eight adult male rhesus monkeys belonging to the Laboratory of Comparative Ethology (National Institute of Child Health and Human Development) and the Laboratory of Clinical Studies (National Institute of Alcohol Abuse and Alcoholism) at the National Institutes of Health Animal Center in Poolesville, MD were used for this study. First, oral antalarmin at a dose of 20 mg/kg, in flavored tablet form, was administered. Each monkey received a single dose on an empty stomach at 8:00 a.m. Subjects were anesthetized by ketamine HCl (15 mg/kg intramuscularly) on three separate occasions over the next 330 min, and blood samples were obtained at 30, 45, 60, 90, 120, 180, and 330 min. CSF samples were collected by cisternal puncture at 60, 120, 180, and 330 min. Intravenous administration of the same dose in the same subjects followed 2 weeks later. For i.v. injections, the animals were anesthetized from the beginning, and antalarmin was dissolved in ethanol/cremophor/water (5:5:90, vol/vol/vol) and injected over a period of 2 min. After the injection, 5 ml of blood were obtained from each individual at 2, 5, 10, 15, 30, 45, 60, 90, 120, 180, and 330 min after dosing. Cerebrospinal fluid samples were collected in the same fashion described after the oral dose. Pharmacokinetic analysis was aided by saam ii 1.1.1 software (SAAM Institute, Seattle) using a three-compartmental module (15, 16). This experiment was approved by the Animal Care and Use Committee of the National Institute of Alcohol Abuse and Alcoholism and was conducted with strict adherence to the National Institutes of Health Guidelines for Using Animals in Intramural Research.
Determination of the Optimal Dose of Antalarmin for Behavioral and Physiological Effects.
Eight adult male rhesus monkeys from the same colony were used for this experiment to study the effects of 0, 5, 10, 20, and 40 mg/kg doses of antalarmin. Animals were randomly assigned to treatment groups of four, and there was a washout period of 7 days between treatments. Doses were given at 8:00 a.m. in a Primatreat banana-flavored tablet form (Bioserv, Frenchtown, NJ) after an overnight fast. Ninety minutes later, monkeys were exposed to acute psychosocial stress for 30 min, and their behavior was recorded as described later. Immediately thereafter, animals were anesthetized by intramuscular ketamine hydrochloride (15 mg/kg) to collect femoral venous blood and cisternal cerebrospinal fluid. The animals were closely monitored at all times and, for 24 h after the completion of the experiment, for potential side effects from the drug or ketamine administration. This experiment was approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development and was conducted according to the National Institutes of Health Guidelines for Using Animals in Intramural Research.
Acute Psychosocial Stress (the Intruder Paradigm).
Rhesus macaques show complex social interactions with one another. To assess anxiety, we used an “intruder paradigm” that has been shown to yield reproducible estimates of behaviors such as anxiety and aggression. Two male rhesus macaques unfamiliar with one another were placed in adjacent cages separated by a transparent Plexiglas screen. This paradigm consistently provoked male macaques to exhibit several features of intense stress. Animal responses were frequently violent, and, if physical contact were permitted, the session would have had to be terminated to rescue animals from serious injuries. We assessed anxiety by using a panel that totaled the following anxiety-related acts: fear grimacing, audible teeth grinding, body tremors, nondirected open mouth expression, urination, and defecation. Aggression was quantified by a panel of responses that included shaking the cage, hitting the Plexiglas panel, and ear wiggling (12, 14, 17). In addition, we assessed exploration and sexual activity (i.e., masturbation), behaviors that ordinarily occur only in safe, nonstressful environments (18). Each score consisted of the total number of occurrences over the 30-min pairing time of all behaviors. Because the response of rhesus macaques to the intruder paradigm significantly declines after the first 30 min, we designed the experiment so that the pairing lasted for 30 min. All introductions were carried out in a neutral environment: that is, a room unfamiliar to both subjects. A fully trained primatologist with extensive experience in using this model (K.P.W.) blindly scored animal behaviors. Behavioral data were simultaneously and continually collected for both male macaques over the 30-min pairing time.
A Double-Blind Placebo-Controlled Trial to Study Possible Effects of Oral Antalarmin (20 mg/kg) on Behavioral, Endocrine, and Autonomic Responses to Stress.
Six adult male macaques weighing 9–13 kg were used for this experiment. Every animal was introduced to all of the others, once on drug and once on placebo, thus generating a total of 60 sessions. Both the drug (200 mg/5 g tablet) and placebo (0 mg/5 g tablet) were incorporated in a Primatreat tablet form of flavored banana taste. Tablets were breakable so that each animal received his dose based on body weight (20 mg/kg). The sequence of treatments was randomly assigned, and there was a washout period of 7–10 days between experiments. One hundred and fifty minutes after receiving the oral dose, animals were exposed to the same intruder paradigm of acute stress described earlier for thirty minutes, and their behaviors scored. Immediately thereafter, subjects were anaesthetized (ketamine HCl, 15 mg/kg i.m.), and their femoral venous blood obtained for measurements of ACTH (corticotropin), cortisol, norepinephrine, epinephrine, and antalarmin. In addition, cisternal cerebrospinal fluid samples were collected and assayed for CRH and arginine vasopressin (AVP).
Hormonal Assays.
Cisternal CSF and blood samples were obtained immediately after the 30-min pairing under ketamine anesthesia (15 mg/kg i.m.). The CSF was immediately aliquoted in cryotubes and frozen in liquid nitrogen. Blood samples were immediately placed on wet ice and centrifuged at 4°C for 20 min. The plasma was then aliquoted and immediately frozen in liquid nitrogen. CSF and plasma samples were stored at −70°C until assayed. CSF was assayed for CRH and AVP by RIA coupled with C18 Sep-pak extraction using a methodology described previously (19, 20). Extracted samples were concentrated 3-fold at the time of their reconstitution in the assay buffer. The sensitivity of the assays was 15 pg/ml and 0.5 pg/ml for CRH and AVP, respectively. AVP was measured because of its involvement in driving the HPA axis during stress (21, 22) and as a reference for the specificity of potential antalarmin actions in the central nervous system. Plasma ACTH and cortisol were assayed as described (20). All of the aforementioned assays were run in duplicate, and the interassay coefficients of variation (CVs) were 10.4, 6.6, 8.5, and 9.5% for CRH, AVP, ACTH, and cortisol, respectively. Plasma epinephrine and norepinephrine were assayed by a liquid-chromatographic method described previously (23). Intraassay coefficients of variation (CVs) were 6.45, 10.8, 9.1, 8.9, 9.6, and 11.7 for CRH, AVP, ACTH, cortisol, epinephrine, and norepinephrine, respectively.
Statistical Analysis.
For each subject under drug treatment, values of the five sessions for a given parameter were averaged and compared with the same subject's average value under placebo treatment. Therefore, the means of 30 measures with each treatment were obtained as pertains to each individual animal to yield an n of 6. Results were considered to be statistically significant at a P level of 0.05 or less as analyzed by paired, two-tailed Student's t tests. Pearson r correlation coefficient was used to express the degree of linear association between two variables. The 95% confidence interval and squared r were considered in calculating the two-tailed P value for the significance of the correlation. All statistical analyses were aided by data desk 6.0 (Data Description, Ithaca, NY) and confirmed by GraphPad (San Diego) prism 2.0 softwares for Macintosh computer.
Results
Antalarmin Pharmacokinetics in Adult Male Macaques.
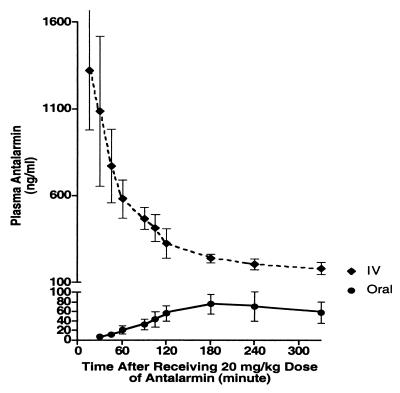
Animals attained detectable concentrations of antalarmin after oral administration, and the average plasma and CSF concentrations observed 180 min after the oral dose of 20 mg/kg were 76 and 9.8 ng/ml, respectively. The total clearance of antalarmin was 4.46 liters/h⋅kg, its elimination half-life was 7.82 h, and its oral bioavailability was 19.3% (Fig. 1).
Figure 1.
Pharmacokinetics of antalarmin in adult male rhesus macaques after the administration of 20 mg/kg orally and intravenously on two separate occasions. n = 8, mean ± SD. The total clearance of antalarmin was 4.46 liters/h⋅kg, the elimination half-life was 7.82 h, and its oral bioavailability was 19.3%, as determined by a three-compartmental module pharmacokinetic analysis aided by saam ii 1.1.1 software.
Dose-Response Studies.
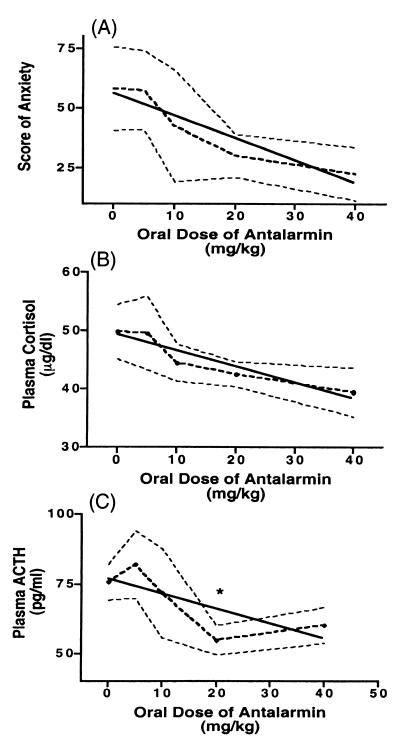
The only dose of antalarmin to significantly decrease plasma ACTH response to the intruder paradigm of stress was 20 mg/kg (from 75.75 ± 6.498 to 54.88 ± 5.29 pg/ml, P = 0.047) (Fig. 2). Trends for dose-response correlations were obtained at oral doses of 0, 5, 10, 20, and 40 mg/kg with plasma cortisol (r = −0.379, P = 0.068), plasma ACTH (r = −0.375, P = 0.1), and the score of anxiety (r = −0.362, P = 0.08), despite the small number of the subjects (n = 4). No statistical difference between the doses of 20 mg/kg and 40 mg/kg was found with plasma cortisol (42.55 ± 2.14 to 39.45 ± 4.20 μg/dl, P = 0.53), plasma ACTH (54.88 ± 5.29 to 60.25 ± 6.42 pg/ml, P = 0.54), or the score of anxiety (30.00 ± 9.13 to 22.50 ± 11.09, P = 0.62).
Figure 2.
Effects of increasing doses of oral antalarmin treatment (0, 5, 10, 20, and 40 mg/kg) on adult male rhesus macaques' scores of anxiety (A) (r = −0.362, P = 0.08), plasma cortisol (B) (r = −0.379, P = 0.068), and plasma ACTH (C) (r = −0.375, P = 0.1) during introductions of two unfamiliar subjects in adjacent chambers separated only by a transparent screen for 30 min. n = 4, mean ± SE. *, P < 0.05.
Behavioral and Biological Responses to Stress.
Exposure of adult male rhesus macaques to acute psychosocial stress precipitated significant anxiety and agitation (e.g., hitting the Plexiglas panel, cage shaking, ear wiggling, audible teeth grinding, nondirected open mouth expression, grimacing, body tremors, urination, avoidance, and defecation). Stress also produced significant increases in plasma ACTH (48.67 ± 4.33 to 67.5 ± 4.52 pg/ml, P = 0.0006), cortisol (27.28 ± 2.32 to 44.95 ± 1.55 μg/dl, P = 0.0003), epinephrine (71.83 ± 12.16 to 171.6 ± 17.74 pg/ml, P = 0.002), and norepinephrine (131.5 ± 22.43 to 338.8 ± 37.5 pg/ml, P = 0.0007). Social stress also increased CSF CRH concentrations (from 57.68 ± 2.83 to 101.5 ± 15.0 pg/ml, P = 0.018). CSF AVP did not significantly change (1.9 ± 0.33 to 2.1 ± 0.45 pg/ml). All values are expressed as the mean ± SE.
Behavioral and Biological Effects of Antalarmin.
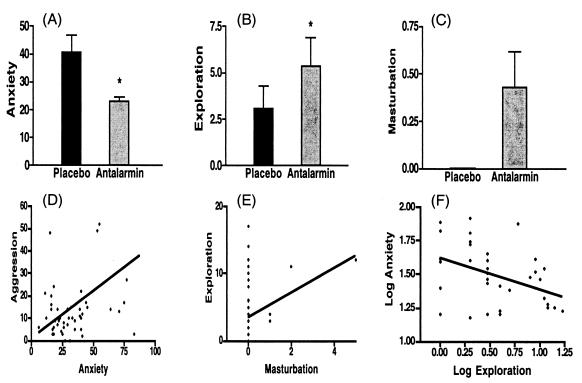
Fig. 3 shows that antalarmin significantly diminished the behavioral index of anxiety in response to acute social stress from 40.53 ± 6.31 to 23.17 ± 1.31 times/30 min (P = 0.02). In addition, it enhanced exploration (from 3.07 ± 1.22 to 5.38 ± 1.50 times/30 min, P = 0.02) and precipitated masturbation in two-thirds of the animals (from 0.00 ± 0.0 to 0.43 ± 0.19 times/30 min). The 95% confidence interval of the latter ranged from −0.05 to 0.91. Individual values of exploration significantly correlated with those of masturbation (r = 0.409, P = 0.004). Although the reduction in the score of aggression by antalarmin treatment (from 36.12 ± 4.38 to 23.83 ± 2.40) was not statistically significant (P = 0.062), individual aggression scores significantly correlated with those of anxiety (r = 0.457, P = 0.001). After log transformation, the negative correlation between individual anxiety scores and individual exploration scores was statistically significant (r = −0.41, P = 0.013).
Figure 3.
Effects of antalarmin treatment (20 mg/kg orally) on adult male macaques' behavior during introduction of two unfamiliar subjects in adjacent cages separated by a transparent Plexiglas plate for 30 min starting 150 min after receiving the drug or placebo in a blind fashion. Every animal was paired with all of the others, thus generating 30 sessions each. Anxiety score (A) totaled the number of occurrences over the 30-min stress period of fear grimacing, audible teeth grinding, body tremors, open mouth expressions, urination, and defecation. Exploration score (B) represented the total number of times an animal approached and played with a toy in his cage or expressed unapprehensive curiosity in the surrounding environment. Masturbation score (C) considered the total number of times a male monkey entertained by sexual manipulation of his penis. n = 6, mean ± SE. *, P < 0.05. Individual values of exploration significantly correlated with those of masturbation (E) (r = 0.409, P = 0.004), and individual aggression scores significantly correlated with those of anxiety (D) (r = 0.457, P = 0.001). After log transformation, the negative correlation between individual anxiety scores and individual exploration scores was statistically significant (F) (r = −0.41, P = 0.013).
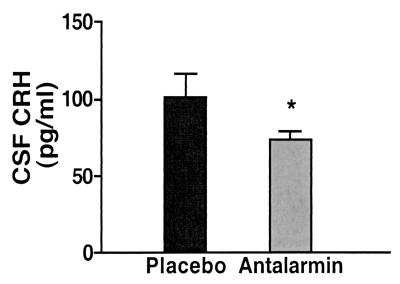
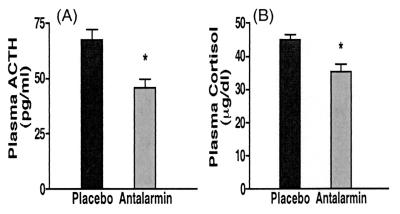
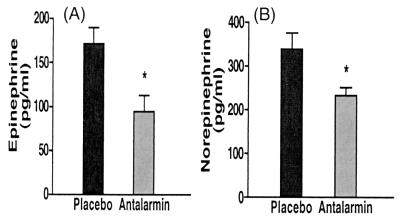
Centrally, antalarmin significantly reduced the stress-induced rise of cerebrospinal fluid CRH levels (from 101.5 ± 15.0 to 73.88 ± 5.28 pg/ml, P = 0.04) (Fig. 4). We have shown that the animals' individual scores of anxiety significantly correlate with their CSF concentration of CRH.lAntalarmin's suppression of anxiety scores and CSF levels of CRH was paralleled by similar significant effects on the HPA axis responses to stress, namely plasma ACTH (from 67.5 ± 4.52 to 45.5 ± 3.99 pg/ml, P = 0.003) and cortisol concentrations (from 44.95 ± 1.55 to 35.23 ± 2.34 μg/dl, P = 0.02) (Fig. 5). Antalarmin also significantly reduced plasma norepinephrine (from 338.8 ± 37.5 to 234.2 ± 18.07 pg/ml, P = 0.03) and epinephrine (from 171.6 ± 17.74 to 94.18 ± 18.14 pg/ml, P = 0.017) responses to social stress (Fig. 6).
Figure 4.
Effects of antalarmin treatment (20 mg/kg orally) on adult male macaques' CSF concentrations of CRH during introduction of two unfamiliar subjects in adjacent cages separated by a transparent Plexiglas plate for 30 min starting 150 min after receiving the drug or placebo in a blind fashion. *, P = 0.04.
Figure 5.
Effects of antalarmin treatment (20 mg/kg orally) on adult male macaques' plasma concentrations of ACTH (A) and cortisol (B) during introduction of two unfamiliar subjects in adjacent cages separated by a transparent Plexiglas plate for 30 min starting 150 min after receiving the drug or placebo in a blind fashion. *, P < 0.02.
Figure 6.
Effects of antalarmin treatment (20 mg/kg orally) on adult male macaques' plasma concentrations of epinephrine (A) and norepinephrine (B) during introduction of two unfamiliar subjects in adjacent cages separated by a transparent Plexiglas plate for 30 min starting 150 min after receiving the drug or placebo in a blind fashion. *, P < 0.03.
Discussion
Antalarmin significantly influenced the behavioral and physiological responses of male rhesus macaques to an intense social stressor: namely, intrusion of a nonfamiliar male. Antalarmin significantly decreased the stress-induced increases in anxiety-like behaviors, plasma ACTH, cortisol, norepinephrine, and epinephrine. CSF CRH, but not CSF AVP, concentrations increased as a result of exposure to psychosocial stress, an effect that was suppressed by antalarmin treatment as well. We have shown a correlation between anxiety scores and the animals' CSF concentration of CRH,l in addition to a significant shift in the regression line to the right by antalarmin treatment: thus, for the same CRH concentration, the animals' scores of anxiety were significantly lower after CRH-R1 antagonism (K.E.H., unpublished work). Conversely, antalarmin significantly increased exploration and precipitated sexual activity in four of the six monkeys during confrontation. These responses ordinarily occur only in an environment that seems safe and rarely, if ever, occur during moderate stress (18, 24).
Previous studies have shown that the intracerebroventricular administration of CRH to non-human primates causes both anorexia and arousal (25, 26). However, unless there were procedural controls, these changes could simply reflect nonspecific aversive effects of the procedure. Wel and others (27) have observed elevations of CSF CRH levels in stressed non-human primates. However, the increases in CSF CRH during stress could be a noncrucial intermediary in a chain of events occurring during the course of the stress response. In contrast, the administration of a nonpeptide CRH receptor antagonist that is orally absorbed and crosses the blood–brain barrier is more likely to reflect the physiological actions of CRH. The latter obviates the need for inserting an intracerebroventricular cannula, reaches the entire brain, rather than neural structures adjacent to the ventricular system, and blocks the endogenous effects of an important mediator of the stress response.
To our knowledge, this is the first report of the effects of a nonpeptide CRH-R1 antagonist on behavioral and physiological responses to stressful stimuli in primates. Previous studies have shown that other pyrrolopyrimidine CRH-R1 antagonists gain entry to the central nervous system after peripheral administration to rats and exert anxiolytic effects comparable to those of buspirone and diazepam (10, 28). CRH-R1 antagonists also suppress CRH-induced acoustic startle, and the CRH-induced increases in locus ceruleus neuronal firing (29). We have previously shown that the administration of antalarmin to the rat produces biological effects similar to standard anti-anxiety and antidepressant drugsm and blocks not only the expression of classical fear conditioning but its development as well (30).
The role of CRH and its receptors in the stress response has been elucidated to a far greater extent in rodents than in primates. CRH-R1 receptors are localized in the primate neocortex, amygdala, hippocampus, hypothalamus, and in the locus ceruleus (31). It is likely that CRH receptors in these loci transduce the effects of CRH on the stress-induced changes in behavior, HPA axis, and sympathetic and adrenal medullary activity. Our data here show that antalarmin, at the oral dose of 20 mg/kg, attenuates the HPA axis and plasma catecholamine responses to a social stress without completely abolishing these responses. We have previously shown that long-term administration of antalarmin to the rat decreases basal HPA axis function and suppresses stress-induced HPA axis activation only partially (32, 33). Therefore, we suggest that antalarmin or other CRH-R1 antagonists, at appropriate doses to decrease behavioral effects to stress, would not impair the physiologic functioning of the HPA axis.
In addition to its effects on the HPA axis, studies in rodents reveal that CRH plays significant roles in the regulation of the gonadal, growth hormone, and thyroid axes, as well as in various components of the immune response and inflammation (34). In the reproductive system, CRH directly inhibits the release of luteinizing hormone-releasing hormone (LH-RH) and, via glucocorticoids, inhibits gonadotropin and sex steroid secretion as well as sex steroid effects at target tissues (6). CRH also inhibits the growth hormone axis via stimulation of somatostatin as well as through glucocorticoid-mediated inhibition of growth hormone secretion and interference with the tissue effects of somatomedin C (4). To add another level of complexity, CRH effects on the immune system could be anti- or pro-inflammatory (35, 36).
Our data indicate that CRH mediates the effects of psychological stress not only in increasing anxiety-related behaviors and inhibiting exploration and sexual behavior, but also in driving virtually the entire cascade of physiological responses characteristic of stress. This constitutes a fundamental concept in understanding the stress response in primates and, therefore, the pathophysiology of illnesses related to stress without other evidence of organic etiology. As an example, we suggest that a CRH-R1 antagonist may be helpful in the treatment of sexual disorders associated with stress such as psychogenic impotence and amenorrhea. Naloxone enhances gonadotropin secretion in amenorrheic women (37), which may be attributable to inhibition of CRH-driven central opioid pathways. β-endorphin that inhibits luteinizing hormone-releasing hormone (LH-RH) secretion derives from the arcuate nucleus proopiomelanocortin (POMC) neurons that respond to CRH emanating from the hypothalamic paraventricular nucleus (38).
Given the inhibitory roles of CRH and glucocorticoids on the thyroid and GH axes, a CRH antagonist could be potentially useful in the treatment of psychosocial growth retardation, euthyroid sick syndrome, and peripheral inflammatory disorders involving mast cell degranulation, such as stress-induced asthma and urticaria (22, 39). CRH may be involved in the pathogenesis of functional gastrointestinal disorders as irritable bowel syndrome (40–42), which may be another therapeutic benefit for CRH-R1 antagonists. In the upper gut, we have observed significant attenuation of stress-induced gastric ulcer by antalarmin.m In the light of the inhibitory role of antalarmin on stress-mediated plasma norepinephrine secretion in the primate, and the potential hypertensive effect of CRH (43), a CRH antagonist could also be useful in the treatment of hypertension.
The finding that antalarmin significantly attenuated the norepinephrine response during confrontation is of importance in the light of the role norepinephrine plays in encoding emotional memory. Norepinephrine not only produces immediate arousal and activates the amygdala, but also facilitates the relay of explicit, aversively charged memories from temporary storage in the amygdala to long-term storage in the hippocampus and striatum (44, 45). As noted, glucocorticoids also enhance arousal in addition to stimulating the amygdala and hypothalamic CRH pathways (46). Collectively, it is strongly suggested that CRH, norepinephrine, and glucocorticoids work together in mutually reenforcing positive feedback loops (47, 48), and, therefore, a CRH-R1 antagonist is likely to break the cycle not only by inhibiting transduction at CRH-R1 receptors but also by decreasing the activities of noradrenergic and glucocorticoid systems.
Over a decade ago, we postulated that the clinical and biochemical manifestations of melancholic depression (pathological hyperarousal, intense anxiety, HPA and sympathetic-adrenal medullary hyperactivity, inhibition of vegetative functions, and inability to switch the mood from the emotionally charged state) reflect activation of CRH and locus ceruleus-norepinephrine systems (47). Several lines of evidence followed to support this hypothesis (48); however, this study documents the involvement of CRH-R1 receptors in several features of this syndrome in primates. Given the data of this study, antalarmin exerted at least three effects of potential therapeutic value in melancholic depression: namely, the attenuation of CRH-mediated hypercortisolism, behavioral arousal, and sympathetic and adrenal medullary responses during stimuli perceived as threatening. In contrast to presently available antidepressants that generally take 2 weeks or more to exhibit any therapeutic efficacy, a CRH-R1 antagonist could potentially act more quickly. Moreover, in contrast to the side effect of sexual dysfunction seen with most available antidepressants (49), a CRH-R1 antagonist should not interfere with, and might even enhance, sexual performance.
In summary, our data strongly support the concept that CRH plays a far broader role in the primate stress response than had been previously demonstrated. This role suggests the possible use of CRH-R1 antagonists to prevent or ameliorate many disorders associated with hyperfunctioning of the CRH and/or noradrenergic systems, including mood, anxiety, gastrointestinal, cardiovascular, and reproductive disorders.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- CRH
corticotropin-releasing hormone
- CRH-R1
CRH type 1 receptor
- CSF
cerebrospinal fluid
- AVP
arginine vasopressin
Footnotes
Habib, K.E., Schulkin, J., Pushkas, J., Listwak, M., Champonx, M., Shannon, C., Chrousos, G. P., Suomi, S., Gold, P. W. & Hisley, J. D. (1999) Soc. Neurosci. Abstr. 25, 2066.
Habib, K. E., Rice, K. C., Chronsos, G. P. & Gold, P. W. (2000) FASEB J. 14, 388 (abstr.).
References
- 1.Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 2.Behan D P, Grigoriadis D E, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, De Souza E B. Mol Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- 3.Gold P W, Kling M A, Khan I, Calabrese J R, Kalogeras K, Post R M, Avgerinos P C, Loriaux D L, Chrousos G P. Adv Biochem Psychopharmacol. 1987;43:183–200. [PubMed] [Google Scholar]
- 4.Ghizzoni L, Vottero A, Street M E, Bernasconi S. J Clin Endocrinol Metab. 1996;81:1397–1400. doi: 10.1210/jcem.81.4.8636340. [DOI] [PubMed] [Google Scholar]
- 5.Barbarino A, Corsello S M, Della Casa S, Tofani A, Sciuto R, Rota C A, Bollanti L, Barini A. J Clin Endocrinol Metab. 1990;71:1368–1374. doi: 10.1210/jcem-71-5-1368. [DOI] [PubMed] [Google Scholar]
- 6.Chrousos G P. Am J Obstet Gynecol. 1999;180:S249–S250. doi: 10.1016/s0002-9378(99)70710-6. [DOI] [PubMed] [Google Scholar]
- 7.Charney D S, Bremner J D. In: The Neurobiology of Mental Illness. Charney D S, Nestler E J, Bunney B S, editors. New York: Oxford Univ. Press; 1999. pp. 494–517. [Google Scholar]
- 8.Higley J D, Suomi S J, Linnoila M. Biol Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y L. International Patent WO 9413676-A1. 1994. [Google Scholar]
- 10.Chen Y L, Mansbach R S, Winter S M, Brooks E, Collins J, Corman M L, Dunaiskis A R, Faraci W S, Gallaschun R J, Schmidt A, Schulz D W. J Med Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- 11.Webster E L, Lewis D B, Torpy D J, Zachman E K, Rice K C, Chrousos G P. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein I S, Gordon T P, Rose R M. In: Primate Aggression, Territoriality, and Xenophobia. Holloway R L, editor. London: Academic; 1974. pp. 211–240. [Google Scholar]
- 13.Kaplan J R. In: Behavior, Conservation and Ecology. Mitchell G, Erwin J, editors. 2, Part A. New York: Liss; 1986. pp. 455–492. [Google Scholar]
- 14.McKinney W T. In: Models of Mental Disorders: A New Comparative Psychiatry. McKinney W T, editor. New York: Plenum; 1988. pp. 97–124. [Google Scholar]
- 15.Fleishaker J C, Smith R B. J Clin Pharmacol. 1987;27:922–926. doi: 10.1002/j.1552-4604.1987.tb05591.x. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson A J, Jr, Kushner W. Annu Rev Pharmacol Toxicol. 1979;19:105–127. doi: 10.1146/annurev.pa.19.040179.000541. [DOI] [PubMed] [Google Scholar]
- 17.Vellucci S V. Pharmacol Ther. 1990;47:167–180. doi: 10.1016/0163-7258(90)90085-g. [DOI] [PubMed] [Google Scholar]
- 18.Higley J D, Hopkins W D, Thompson W W, Byrne E A, Hirsch R M, Suomi S J. Dev Psychol. 1992;28:1163–1171. [Google Scholar]
- 19.De Bellis M D, Gold P W, Geracioti T D, Jr, Listwak S J, Kling M A. Am J Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- 20.Chrousos G P, Schulte H M, Oldfield E H, Gold P W, Cutler G B, Jr, Loriaux D L. N Engl J Med. 1984;310:622–626. doi: 10.1056/NEJM198403083101004. [DOI] [PubMed] [Google Scholar]
- 21.de Goeij D C, Kvetnansky R, Whitnall M H, Jezova D, Berkenbosch F, Tilders F J. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- 22.Smith M A, Kling M A, Whitfield H J, Brandt H A, Demitrack M A, Geracioti T D, Chrousos G P, Gold P W. Horm Res. 1989;31:66–71. doi: 10.1159/000181089. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhofer G, Goldstein D S, Stull R, Keiser H R, Sunderland T, Murphy D L, Kopin I J. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 24.Baldwin J D. In: Behavior, Conservation and Ecology. Mitchell G, Erwin J, editors. 2, Part A. New York: Liss; 1986. pp. 295–326. [Google Scholar]
- 25.Glowa J R, Gold P W. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- 26.Kalin N H. Fed Proc. 1985;44:249–253. [PubMed] [Google Scholar]
- 27.Coplan J D, Andrews M W, Rosenblum L A, Owens M J, Friedman S, Gorman J M, Nemeroff C B. Proc Natl Acad Sci USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenck F, Widmer U, Moreau J L. Eur J Pharmacol. 1996;309:195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- 29.Holsboer F. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 30.Deak T, Nguyen K T, Ehrlich A L, Watkins L R, Spencer R L, Maier S F, Licinio J, Wong M L, Chrousos G P, Webster E, Gold P W. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez M M, Young L J, Plotsky P M, Insel T R. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 32.Wong M L, Webster E L, Spokes H, Phu P, Ehrhart-Bornstein M, Bornstein S, Park C S, Rice K C, Chrousos G P, Licinio J, Gold P W. Life Sci. 1999;65:L53–L58. doi: 10.1016/s0024-3205(99)00268-4. [DOI] [PubMed] [Google Scholar]
- 33.Bornstein S R, Webster E L, Torpy D J, Richman S J, Mitsiades N, Igel M, Lewis D B, Rice K C, Joost H G, Tsokos M, Chrousos G P. Endocrinology. 1998;139:1546–1555. doi: 10.1210/endo.139.4.5938. [DOI] [PubMed] [Google Scholar]
- 34.Chrousos G P. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 35.Karanth S, Lyson K, McCann S M. Proc Natl Acad Sci USA. 1993;90:3383–3387. doi: 10.1073/pnas.90.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster E L, Torpy D J, Elenkov I J, Chrousos G P. Ann NY Acad Sci. 1998;840:21–32. doi: 10.1111/j.1749-6632.1998.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 37.Krause B, Moller S, Goretzlehner G, Ulrich U, Matuszewski F, Wodrig W, Weber A. Exp Clin Endocrinol. 1992;99:113–115. doi: 10.1055/s-0029-1211149. [DOI] [PubMed] [Google Scholar]
- 38.Laatikainen T J. Ann Med. 1991;23:489–496. doi: 10.3109/07853899109150508. [DOI] [PubMed] [Google Scholar]
- 39.Theoharides T C, Singh L K, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 40.Tache Y, Martinez V, Million M, Rivier J. Can J Gastroenterol. 1999;13, Suppl. A:18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- 41.Swiatkowski M, Rybakowski J K. J Affective Disord. 1993;28:199–202. doi: 10.1016/0165-0327(93)90105-s. [DOI] [PubMed] [Google Scholar]
- 42.Fukudo S, Nomura T, Hongo M. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutkowska J, Jankowski M, Mukaddam-Daher S, McCann S M. Proc Natl Acad Sci USA. 2000;97:483–488. doi: 10.1073/pnas.97.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferry B, Roozendaal B, McGaugh J L. Biol Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 45.Liang K C, Chen L L, Huang T E. Chin J Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- 46.Quirarte G L, Roozendaal B, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold P W, Goodwin F K, Chrousos G P. N Engl J Med. 1988;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 48.Wong M L, Kling M A, Munson P J, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon I E, Geracioti T D, Jr, DeBellis M D, et al. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellison J M. Harvard Rev Psychiatry. 1998;6:177–189. doi: 10.3109/10673229809000328. [DOI] [PubMed] [Google Scholar]