Abstract
Introduction
Functional abdominal symptoms are very common and account for nearly two million primary care consultations in Britain every year and produce significant morbidity. The aims of this study were to evaluate the impact of two self‐help interventions on consultation rates and symptom severity in patients with a primary care diagnosis of irritable bowel syndrome.
Methods
A total of 420 patients from 54 primary care centres were randomised either to receive self‐help information in the form of a guidebook or the guidebook plus a “self‐help” group meeting or to be in a control group receiving neither intervention. Data were collected using questionnaires and primary care records.
Results
At one year, patients in the guidebook group had a 60% reduction in primary care consultations (p<0.001) and a reduction in perceived symptom severity (p<0.001) compared with controls. Allocation to the self‐help group conferred no additional benefit. Actual symptom scores did not change significantly in any group. Costs per patient were reduced by £73 (confidence interval £43, £103) or 40% per year.
Conclusion
Introduction of a self‐help guidebook results in a reduction in primary care consultations, a perceived reduction in symptoms, and significant health service savings. This suggests that patients attending their primary care physician with functional abdominal symptoms should be offered self‐help information as part of their management.
Keywords: irritable bowel syndrome, functional bowel disease, self‐help, primary care, self‐management
The term functional bowel disorder describes an array of symptoms, including abdominal pain and disturbed bowel function without any obvious underlying pathological cause. Arbitrary criteria such as Manning, Rome I, and Rome II have been developed to define categories of these symptoms (for example, irritable bowel syndrome (IBS)), but patients with symptoms falling outside these criteria are nevertheless frequently categorised as having IBS in both primary and secondary care.1 Poor agreement in the diagnosis of IBS between primary care physicians and Rome II criteria has recently been reported in a large Scandinavian study.2 The community prevalence of IBS is high, varying widely according to the definitions used, but ranges between 10% and 15% in North America3 and between 6.2% and 12% in Europe.4 The reported rates of medical consultations also vary and range from 10% to 50%.5,6 English national consultation statistics indicate approximately 300 consultations/10 000 population/year for functional bowel symptoms in primary care, equating to approximately 1.8 million consultations in Britain per year.7 The economic impact of IBS in the eight major industrialised nations (incorporating healthcare and societal costs) has been estimated at $41 billion/year.8
Anxiety is a major accompaniment of symptoms of functional bowel disorders9,10 and stresses of daily life have been strongly linked with IBS.11,12 A survey of 148 patients with IBS concluded that patients would have coped better if they had been provided with more information about IBS, including details of aetiology and treatment.10 This led Van der Horst et al to suggest that general practitioners (GPs) should promote and reinforce education based self‐care activities in patients with IBS.13
Self‐help groups are considered to offer a currently underexploited role in improving symptom coping and supporting patients with chronic illness.14 Members of groups can provide each other with support, understanding, and solidarity to counteract the isolation of managing a chronic condition alone.15 There are no studies evaluating either type of self‐care intervention in functional bowel disorders but trials in other diseases have shown that a reduction in consultation rates of up to 60% is achievable without compromising clinical outcomes.16,17,18,19
The primary aim of this study was to test the impact of two self‐help interventions (a comprehensive self‐help guidebook and a self‐help group) on primary care consultation rates and global IBS symptom severity in patients with functional bowel disease. A secondary aim was to evaluate attendant changes in a range of other health outcomes, including use of secondary care resources and self‐care, together with impact on general health and quality of life.
Methods
Sample
A total of 458 consecutive patients, aged 18 years and over, attending their primary care physician with functional gastrointestinal symptoms diagnosed as IBS by either the GP or specialist (if they had previously been referred) but not necessarily fulfilling Rome II criteria, were invited to participate in the trial if they had consulted with similar symptoms on at least one previous occasion in the past year. They were provided with information about the trial and their details forwarded to the trial coordinator who arranged to meet them to obtain consent and collect preliminary data. Patients unable to read or understand English were excluded as they would be incapable of making use of the guidebook or completing the required questionnaires. All participating patients provided written informed consent and the study was approved by the relevant local research ethics committees.
Randomisation
Patients were randomly allocated to one of three groups using a central telephone randomisation system based on minimisation.20,21 Groups were stratified according to duration of illness, frequency of primary care visits, age, and sex.
Patients in group 1 received a comprehensive self‐help guidebook produced following a series of focus group meetings with other IBS patients who described the information they required to help them cope with their symptoms better. The guidebook contained information about lifestyle, diet, and pharmacological and alternative therapies, and was based on up to date evidence and patients' own anecdotal experiences; details of the guidebook and its development have been published elsewhere.22 Patients in group 2 were given the guidebook and invited to participate in a one‐off self‐help group meeting (8–12 patients) facilitated by the trial coordinator. The session was scheduled for two hours during which patients shared their experiences of living with their functional bowel symptoms and described approaches which helped them to manage their illness.
Patients in group 3 (control group) continued to receive their usual care at the discretion of the primary care physician. Patients in all groups were informed that they were free to continue to visit their primary care physician without restriction.
Data collection
Patients who consented to participate in the trial provided data at trial entry and at one year. Further data were obtained from the primary care records at the end of the study.
Primary outcomes
The main outcomes of this study were the number of primary care consultations recorded from the primary care records, and patients' clinical global impression scores. The global impression scale requires patients to rate two items: the severity of their IBS symptoms on a seven point scale from unbearable to no symptoms; and improvement in symptoms, also on a seven point scale (from very much worse to very much improved). This global impression scale has been reported to be a sensitive measure of overall change in patients with IBS.23
Secondary outcomes
Other outcomes of interest were hospital consultation rates, symptom severity, quality of life scores, health status, and costs to the health service.
Hospital consultation rates
Hospital visits (by those receiving hospital follow up) were collected from patient self‐report.
Symptom severity
Patients rated their symptoms using four visual analogue scales representing severity of abdominal pain, abdominal distension, constipation, and diarrhoea.24,25 Additional questions allowed us to identify whether the Rome II criteria for IBS26 had been fulfilled.
Quality of life
Patients completed the IBS‐QOL, a disease specific instrument for measuring quality of life.27
Health status
The GHQ‐2828 and the SF‐3629 were used to measure health status. The GHQ‐28 is a 28 item measure of general psychological well being. The SF‐36 has eight subscales rating physical function, physical role limitation, mental health, emotional role limitation, social function, energy and vitality, bodily pain, and health perceptions.
Economic data
Costs to the National Health Service were calculated based on primary and secondary care consultation rates and costs of prescription drugs. A visit to a GP was costed at £20, the national average cost of a surgery consultation.30 A hospital visit was costed at £73, the national average cost of a gastroenterology outpatient follow up attendance with no investigation or procedure.31 Data on any inpatient care for IBS related symptoms were not collected. Data were collected from patients about prescribed medications but not dosage regimens. National Health Service drug costs were therefore estimated on the basis of costs of average dosages reported in the British National Formulary.
Sample size
As the study was designed to be a pragmatic trial of a self‐care intervention in a primary care setting, sample size was determined by the rate at which eligible patients presented themselves within the period available for recruitment. All eligible patients who consulted for functional bowel symptoms over a period of 22 months were therefore offered the opportunity to participate in the trial. A total of 420 patients accepted and were randomised to one of the three groups.
Statistical methods
The study evaluated three primary outcomes and 21 secondary outcomes. Following the recommendations of Bender and Lange,32 the primary outcomes were subjected to a confirmatory analysis in which the alpha levels for significance were adjusted for multiple end points related to a single experimental question (for this purpose we used Holm's modified Bonferroni method33) while secondary outcomes were subjected to an exploratory analysis in which alpha levels were not adjusted and more emphasis was given to descriptive interpretation.32 A resource cost analysis is also presented in the form of an exploratory analysis but with treatment effects expressed in monetary values.
The main analyses were conducted using the multiple regression procedures in STATA version 8.34 GP practice was designated as the cluster variable and robust estimates of variance adopted. The study had two intervention groups (guidebook alone and guidebook plus self‐help group) and a control group. To partition the effect of the guidebook from that of the self‐help group, two binary dummy variables were used in each analysis (guidebook yes/no; self‐help group yes/no) in place of the three level group variable. These two components were treated as addressing different experimental questions when adjusting alpha for the tests of the primary outcomes (see above).
Missing data
Twelve month questionnaire data were missing for 56 patients (13%). To account for this, logistic regression was used to estimate the probability of questionnaire return on the basis of patient characteristics. The inverse of these probabilities was then assigned to individual cases as weights in the analysis.
Covariates
Potential covariates in each analysis were the baseline values of the other primary and secondary outcomes, plus patient characteristics (sex, age, marital status, education, years with condition, family history of IBS, use of information sources, Eysenck neuroticism, extroversion, and psychoticism scores). Covariates were introduced into each analysis in a forward stepwise manner (alpha for entry = 0.05).
All analysis was performed on an intention to treat basis. Two sets of analyses were run. For each outcome the first (principal) analysis tested for effects of the guidebook and the self‐help group while controlling for the baseline values of the outcome as a covariate. The second analysis repeated the first while also incorporating probability weights to adjust for missing data and controlling for significant covariates. In addition, where data were skewed and the principal analysis gave p<0.10, bootstrapping (using 10 000 repetitions and percentile confidence intervals) was used to confirm the statistical significance of the result. Neither the second analysis nor bootstrapping made a substantial difference to any of the results (either to the p value or effect size); therefore, only the results of the principal analysis are presented below. All confidence intervals (CI) are given at the 95% level.
Results
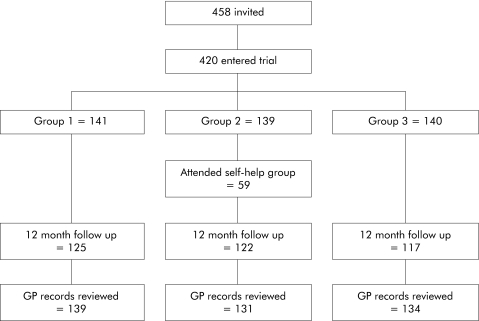
A total of 458 patients, drawn from 54 GP practices, were invited to participate in the trial. Thirty four patients declined and a further four agreed to participate but missed the recruitment deadline; hence 420 were randomised (fig 1). Mean age of the participants was 40 years (SD 14.4); 89% (n = 370) were female, 49% (n = 206) were married or cohabiting, and 13% (n = 55) were widowed, separated, or divorced. Forty seven per cent of patients (n = 198) were in full time employment and 34% (n = 144) were educated to degree level. On average, patients had suffered bowel symptoms for an average of 6 years (SD 7.2) and 38% satisfied Rome II criteria. Only 59 of 139 patients in the self‐help group actually attended the meeting. Most patients did not provide an explanation for non‐attendance although additional qualitative data showed that some patients were unwilling to discuss bowel related symptoms with strangers.
Figure 1 Flow diagram illustrating the study design. GP, general practitioner.
Primary outcomes (table 1)
Table 1 Primary outcomes.
| Outcome | n | Baseline | 1 year | |
|---|---|---|---|---|
| GP visits (primary outcome) | ||||
| Control group | 136 | 2.75 (1.36) | 2.26 (2.04) | Guidebook effect: −1.56 (−1.98, −1.15); p<0.001* (p<0.017)† Self‐help group effect: 0.00 (−0.28, 0.28); p = 0.990 (p>0.05)† |
| Guidebook group | 141 | 3.69 (2.41) | 1.03 (1.60) | |
| Guidebook and self‐help group | 138 | 3.51 (2.27) | 0.96 (1.42) | |
| Global impressions scale—severity | ||||
| Control group | 117 | 3.29 (0.88) | 3.93 (1.31) | Guidebook effect: 0.25 (−0.05, 0.56); p = 0.102 (p>0.05)† Self‐help group effect: −0.14 (−0.49, 0.21); p = 0.433 (p>0.017)† |
| Guidebook group | 125 | 3.14 (0.84) | 4.15 (1.41) | |
| Guidebook and self‐help group | 122 | 3.23 (0.98) | 4.03 (1.33) | |
| Global impressions scale—change | ||||
| Control group | 117 | NA | 4.63 (1.41) | Guidebook effect: 0.51 (0.23, 0.79); p = 0.001* (p<0.025)† Self‐help group effect: −0.09 (−0.38, 0.21); p = 0.559 (p>0.025)† |
| Guidebook group | 125 | NA | 5.14 (1.20) | |
| Guidebook and self‐help group | 122 | NA | 5.06 (1.27) |
Values are mean (SD).
†p values for significance (at alpha = 0.05) are based on Holm's modified Bonferroni method.32
*Statistically significant result
Clinician visits
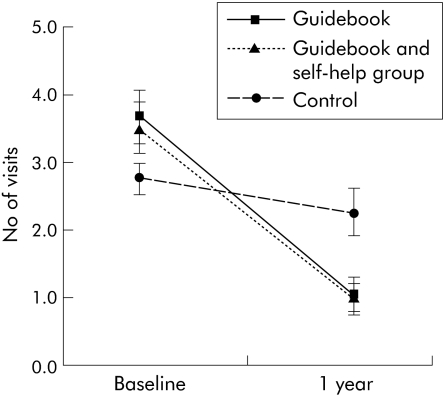
Primary care visits declined in all three groups, although by a significantly wider margin (p<0.001) among patients who received the guidebook (fig 2). A reduction in all groups would be expected (regression to the mean) as patients had to have had at least one visit at baseline in order to be recruited into the study but may not have had any visits during the course of the trial.
Figure 2 General practitioner visits in the three groups. Values are mean and 95% confidence interval.
The estimated effect of the guidebook was to reduce GP visits in the trial year by an average of 1.56 visits (95% CI 1.15, 1.98). This represents a 60% reduction in visits compared with what would have been expected if these patients had not been provided with the guidebook (that is, mean of actual visits plus 1.56). Allocation to the self‐help group had no additional impact on numbers of visits.
Global impressions scale (severity)
Neither the guidebook nor membership of the self‐help group had an effect on scores on the severity subscale of the global impressions scale at one year.
Global impressions scale (perception of change)
Patients who received the guidebook reported a higher degree of perceived improvement in IBS symptoms relative to the start of the trial (p<0.001). The mean effect was 0.51 (95% CI 0.23; 0.79) of a point on the (seven point) scale compared with the control group. Membership of the self‐help group had no additional impact on perceptions of change in IBS symptom severity.
Secondary outcomes
Hospital outpatient visits
Hospital visits during the trial year were significantly lower (p = 0.038) for patients who received the guidebook compared with those who did not receive it. Mean difference was 0.22 (95% CI 0.01, 0.42) visits after controlling for baseline levels. This is a 40% reduction compared with what would be expected for these patients had the guidebook not been provided. Being in the self‐help group had no additional effect.
Symptoms
There were no significant differences between the three groups with respect to pain (−1.40 (95% CI −8.45, 5.65)), bloating (−4.42 (95% CI −11.30, 2.46)), diarrhoea (−0.85 (95% CI −6.88, 5.18)), or constipation (−5.32 (95% CI −11.80, 1.16)). All differences favoured the treatment groups however.
Self‐care activities
There was evidence that the use of dietary treatments (0.19 (95% CI 0.01, 0.37); p = 0.035) and relaxation therapy (0.23 (95% CI 0.06, 0.41); p = 0.011) were higher at the end of the trial for the guidebook groups but no differences with respect to the use of exercise (0.04 (95% CI −0.19, 0.27)), alternative products (0.09 (95% CI −0.11, 0.29)), or complementary therapies (0.01 (95% CI −0.13, 0.15)) were found.
Quality of life
There were no differences between groups in quality of life scores at the end of the trial, as measured by the IBS‐QOL (1.96 (95% CI −1.36, 5.27)).
Health status
There was no evidence that the intervention impacted on GHQ scores (−0.28 (95% CI −1.37, 0.80)). Of the eight dimensions of the SF‐36, post‐trial scores on the health perceptions scale were significantly higher (p = 0.029) for patients who received the guidebook (5.11 (95% CI 0.55, 9.68)). No significant associations were found between the guidebook and scores on the remaining SF‐36 dimensions, although all differences favoured the guidebook (physical function 2.85 (95% CI −1.03, 6.72); physical role limitation 2.07 (95% CI −4.97, 9.11); emotional role limitation 2.25 (95% CI −7.08, 11.57); social function 0.55 (95% CI −2.86, 3.97); mental health 2.05 (95% CI −1.23, 5.33); energy and vitality 1.87 (95% CI −3.34, 7.08); bodily pain 2.75 (95% CI −2.21, 7.70); and change in health 3.89 (95% CI −1.65, 9.42)). Scores on the physical role limitation scale were significantly improved (p = 0.026) for those assigned to the self‐help group (6.80 (95% CI 0.85, 12.75)).
National Health Service resource use analysis (table 2)
Table 2 National Health Service resource use.
| Outcome | n | Baseline | 1 year | |
|---|---|---|---|---|
| Cost of GP visits | ||||
| Control group | 136 | £55.15 (27.27) | £45.15 (40.81) | Guidebook effect: −£31.30 (−39.60, −22.99); p<0.001* Self‐help group effect: −£0.04(−5.70, 5.62); p = 0.990 |
| Guidebook group | 141 | £73.76 (48.29) | £20.58 (32.0) | |
| Guidebook and self‐help group | 138 | £70.29 (45.35) | £19.28 (28.43) | |
| Cost of hospital visits | ||||
| Control group | 136 | £37.57 (76.94) | £41.87 (78.29) | Guidebook effect: −£15.85 (−£30.82, −£0.88); p = 0.038* Self‐help group effect: −£6.03 (−£21.14, £9.08); p = 0.427 |
| Guidebook group | 141 | £42.97 (94.81) | £27.44 (71.17) | |
| Guidebook and self‐help group | 138 | £46.02 (85.09) | £22.22 (54.80) | |
| Cost of prescribed drugs | ||||
| Control group | 133 | £86.77 (84.65) | £74.41 (95.35) | Guidebook effect: −£24.23 (−46.12, −2.33); p = 0.031* Self‐help group effect: 12.21 (−6.29, 30.71); p = 0.191 |
| Guidebook group | 139 | £118.27 (103.62) | £65.55 (87.61) | |
| Guidebook and self‐help group | 131 | £102.51 (84.41) | £70.08 (88.73) | |
| Total resource costs | ||||
| Control group | 133 | £177.02 (128.64) | £159.14 (145.39) | Guidebook effect: −£72.74 (−102.63, −42.84); p = 0.000* Self‐help group effect: 7.53 (−20.41, 35.48); p = 0.591 |
| Guidebook group | 139 | £235.24 (186.46) | £111.67 (138.42) | |
| Guidebook and self‐help group | 131 | £216.11 (138.29) | £110.89 (132.99) |
Values are mean (SD).
*Statistically significant result.
The cost to the National Health Service of GP visits during the trial year was significantly lower (p<0.001) for patients who received the guidebook compared with controls, by an average of £31 (95% CI £23, £40) after adjustment for baseline levels. Hospital visit costs were also significantly lower (p = 0.038), by an average of £16 (95% CI £1, £31), as were the costs of prescribed drugs (mean difference of £24 (95% CI £2, £46); p = 0.031). The guidebook was estimated to reduce total resource costs (GP and hospital visits plus prescribed drugs) by £73 per patient (95% CI £43, £103; p<0.001) compared with the costs for patients without the guidebook. This represents a cost saving of approximately 40%. Allocation to the self‐help group had no effect on any aspect of National Health Service resource usage.
Effect of Rome II
There was no difference in outcomes in any of the measured variables when patients who did or did not fulfil the Rome II criteria were compared.
Discussion
This study has demonstrated that provision of a self‐help guidebook, designed with the aid of patients to help them deal with functional gastrointestinal symptoms, reduces primary care consultations by 60% compared with controls. Global symptom scores were not reduced by the intervention but patients perceived that their symptoms were improved and there was a trend towards increased self‐care activity. Although there was no impact on GHQ or SF‐36 scores and overall quality of life scores did not change significantly, the results are commensurate with other studies of self‐management which suggest that attitudes, such as self‐efficacy which are associated with increased self‐control, may help patients to manage their condition on a daily basis more than an actual reduction in levels or severity of symptoms.35 The guidebook may also compensate for poor or inadequate information and provide reassurance to patients concerned about more serious pathology by providing up to date information and anecdotal reports of other patients' experiences.
Although the great majority of secondary outcomes were unchanged, the trend on all was in favour of patients who received the guidebook. Importantly, the guidebook did not appear to impact negatively on patient health outcomes. This is evidenced by the confidence intervals around the effects which in nearly all cases suggest that if there were negative effects these were of small order.
Combining the guidebook with a self‐help group meeting did not improve results for any of the primary outcome measures but only 59 of 139 patients actually attended the sessions. Therefore, as a pragmatic intervention for patients with functional bowel disorders, self‐help groups appear to be unpopular.
We also found that the Rome II criteria were unimportant in predicting which patients benefited from the interventions and appear to have little relevance in a primary care setting.
The long term effects of the guidebook are unknown. It is possible that consultation rates could increase again with time if the guidebook effect fatigues. This will be assessed in a later study. There was no evidence that patients began consulting for other functional symptoms during the study as primary care consultations for non‐gastrointestinal symptoms were similar for all groups.
The trial benefited from a very high rate of follow up, with 87% of patients completing the final questionnaire and 96% primary care records reviewed. However, the limitations of the study should be noted. Participation was restricted to patients able to read and understand English so that we cannot assume that the findings of this study would be similar for patients of other cultures in whom English is not their first language. The sample was also restricted to patients presenting in primary care who had consulted on at least one other occasion in the previous year, and therefore patients with more quiescent symptoms are likely to be underrepresented. Finally, the study did not include hospital admission data although it is very unlikely that the intervention would influence rates of admission of patients in any of the groups.
In conclusion, the use of a self‐help educational guidebook in primary care patients with functional bowel symptoms results in a clear reduction in health service utilisation and costs without any deterioration in symptoms or other health outcomes. We therefore suggest that the intervention could be used as a firstline treatment for patients presenting with functional bowel symptoms in primary care. If the intervention was extended to the UK functional bowel disease population, annual savings of over £30 million could be expected.
Abbreviations
IBS - irritable bowel syndrome
GP - general practitioner
Footnotes
We are grateful to the Manchester, Stockport, and West Pennine Research and Development Liaison Group for funding this study.
Conflict of interest: None declared.
References
- 1.Thompson W G, Heaton K W, Smyth G T.et al Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 20004678–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandvik P O, Aabakken L, Farup P G. Diagnosing irritable bowel syndrome: poor agreement between general practitioners and the Rome II criteria. Scand J Gastroenterol 200439448–453. [DOI] [PubMed] [Google Scholar]
- 3. Saito YA, Schoenfeld P, Locke GR III, eds. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 2002971910–1915. [DOI] [PubMed] [Google Scholar]
- 4.Hungin A P, Whorwell P J, Tack J.et al The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 200317643–650. [DOI] [PubMed] [Google Scholar]
- 5.Talley N J, Gabriel S E, Harmsen W S.et al Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 19951091736–1741. [DOI] [PubMed] [Google Scholar]
- 6.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ 199230487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick A, Fleming D, Charlton J.Morbidity statistics from general practice. Fourth national study 1991–1992. Office of Population Censuses and Surveys. London: HMSO, 1995, series MB5 No 3.
- 8.Fullerton S. Functional digestive disorders (FDD) in the year 2000—economic impact. Eur J Surg Suppl 199858262–64. [DOI] [PubMed] [Google Scholar]
- 9.Caudell K A. Psychophysiological factors associated with irritable bowel syndrome. Gastroenterol Nurs 19941761–67. [DOI] [PubMed] [Google Scholar]
- 10.Dancey C P, Backhouse S. Towards a better understanding of patients with irritable bowel syndrome. J Adv Nurs 1993181443–1450. [DOI] [PubMed] [Google Scholar]
- 11.Dancey C P, Taghavi M, Fox R J. The relationship between daily stress and symptoms of irritable bowel: a time‐series approach. J Psychosom Res 199844537–545. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead W E, Crowell M D, Robinson J C.et al Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 199233825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Horst H E, Schellevis F G, van Eijk J T.et al Managing patients with irritable bowel syndrome in general practice. How to promote and reinforce self‐care activities. Patient Educ Couns 199835149–156. [DOI] [PubMed] [Google Scholar]
- 14.Wagner E H, Davis C, Schaefer J.et al A survey of leading chronic disease management programs: are they consistent with the literature? Manag Care Q 1999756–66. [PubMed] [Google Scholar]
- 15.Trojan A. Benefits of self‐help groups: a survey of 232 members from 65 disease related groups. Soc Sci Med 198929225–232. [DOI] [PubMed] [Google Scholar]
- 16.Robinson A, Thompson D G, Wilkin D.et al Northwest Gastrointestinal Research Group. Guided self‐management and patient‐directed follow‐up of ulcerative colitis: a randomised trial, Lancet 2001358976–981. [DOI] [PubMed] [Google Scholar]
- 17.Hansen B W. A randomized controlled trial on the effect of an information booklet for young families in Denmark. Patient Educ Couns 199016147–150. [DOI] [PubMed] [Google Scholar]
- 18.Kemper D W, Lorig K, Mettler M. The effectiveness of medical self‐care interventions: a focus on self‐initiated responses to symptoms. Patient Educ Couns 19932129–39. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy A P, Nelson E, Reeves D.et al A randomised controlled trial to assess effectiveness and cost of a patient orientated self‐management approach to chronic inflammatory bowel disease. Gut 2004531639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taves D R. Minimisation: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 197415443–453. [DOI] [PubMed] [Google Scholar]
- 21.Treasure T, MacRae K D. Minimisation: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups; minimisation does. BMJ 1998317362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy A, Robinson A, Rogers A. Incorporating patients' views and experiences of life with IBS in the development of an evidence based self‐help guidebook. Patient Educ Couns 200350303–310. [DOI] [PubMed] [Google Scholar]
- 23.Klein K D. Assessment of treatment outcome in the functional gastrointestinal disorders. In: Corazziani E, ed. Approach to the patient with chronic gastrointestinal disorders. Milan, Italy: Messaggi, 1999545–556.
- 24.Benoussan A, Talley N J, Hing M.et al Treatment of irritable bowel syndrome with Chinese herbal medicine. A randomised controlled trial. JAMA 19982801585–1589. [DOI] [PubMed] [Google Scholar]
- 25.Francis C Y, Morris J, Whorwell P J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 199711395–402. [DOI] [PubMed] [Google Scholar]
- 26.Thompson W G, Longstreth G F, Drossman D A.et al Functional bowel disorders and functional abdominal pain. Gut 199945(suppl 2)II43–II47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patrick D L, Drossman D A, Frederick I O.et al Quality of life in persons with irritable bowel syndrome. Dig Dis Sci 199843400–411. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg D, Williams P.A users' guide to the general health questionnaire. Windsor: NFER‐Nelson, 1988
- 29.Ware J.SF‐36 physical and mental health summary scales: A user's manual. Boston: The Health Institute, 1994
- 30.Unit costs of health and social care. Personal Social Services Research Unit. Kent, UK: University of Kent 2003
- 31.NHS Reference costs 2002. London: Department of Health, 2002
- 32.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol 200154343–349. [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979665–70. [Google Scholar]
- 34.StataCorp Stata Statistical Software: Release 8.0. College Station, Texas: Stata Corporation, 2003
- 35.Lorig K, Sobel D S, Stewart A L.et al Evidence suggesting that a chronic disease self‐management programme can improve health status whilst reducing hospitalisation. Med Care 1999375–14. [DOI] [PubMed] [Google Scholar]