Abstract
Background
Bile acid induced apoptosis in hepatocytes can be antagonised by nuclear factor κB (NFκB) dependent survival pathways. Sulfasalazine modulates NFκB in different cell types. We aimed to determine the effects of sulfasalazine and its metabolites sulfapyridine and 5‐aminosalicylic acid (5‐ASA) on bile acid induced apoptosis in hepatocytes.
Methods
Apoptosis was determined by caspase assays and immunoblotting, NFκB activation by electrophoretic mobility shift assay and reporter gene assays, generation of reactive oxygen species (ROS) fluorometrically, bile secretion gravimetrically, and bile acid uptake radiochemically and by gas chromatography in HepG2‐Ntcp cells and isolated perfused rat livers.
Results
Glycochenodeoxycholic acid (GCDCA 75 µmol/l) induced apoptosis was reduced by sulfasalazine dose dependently (1–1000 µmol/l) in HepG2‐Ntcp cells whereas its metabolites 5‐ASA and sulfapyridine had no effect. Sulfasalazine significantly reduced GCDCA induced activation of caspases 9 and 3. In addition, sulfasalazine activated NFκB and decreased GCDCA induced generation of ROS. Bile acid uptake was competitively inhibited by sulfasalazine. In perfused rat livers, GCDCA (25 µmol/l) induced liver injury and extensive hepatocyte apoptosis were significantly reduced by simultaneous administration of 100 µmol/l sulfasalazine: lactate dehydrogenase and glutamate‐pyruvate transaminase activities were reduced by 82% and 87%, respectively, and apoptotic hepatocytes were observed only occasionally. GCDCA uptake was reduced by 45 (5)% when sulfasalazine was coadministered. However, when 50% of GCDCA (12.5 µmol/l) was administered alone, marked hepatocyte apoptosis and liver injury were again observed, questioning the impact of reduced GCDCA uptake for the antiapoptotic effect of sulfasalazine.
Conclusion
Sulfasalazine is a potent inhibitor of GCDCA induced hepatocyte apoptosis in vitro and in the intact liver.
Keywords: bile secretion, cholestasis, cell signalling, death receptor, liver disease
Cholestasis is a common feature of many human liver diseases. Elevated bile acid concentrations in hepatocytes, a hallmark of cholestasis, promote liver cell death resulting in liver injury and liver cirrhosis.1 Toxic bile acids induce hepatocellular apoptosis, thereby providing a cellular mechanism for bile acid mediated liver injury.2,3,4 The glycine and taurine conjugates of chenodeoxycholic acid (GCDCA, TCDCA) are the predominant dihydroxy bile acids in cholestatic patients and have been held responsible for cholestasis associated liver injury.5 Glycochenodeoxycholic acid (GCDCA) is thought to induce hepatocyte apoptosis by a Fas death receptor dependent process that is independent of Fas ligand6 but induces oligomerisation of Fas by increasing cell surface trafficking of Fas.7 GCDCA induced generation of reactive oxygen species followed by epidermal growth factor receptor dependent tyrosine phosphorylation of Fas also appears to be required for GCDCA induced Fas signalling.4 The transcription factor nuclear factor κB (NFκB) has been shown to reduce hepatocyte apoptosis induced by toxic bile acids,8 tumour necrosis factor α (TNF‐α), and during liver regeneration.9 Thus NFκB is an important factor of several antiapoptotic signalling cascades in the liver.
Sulfasalazine was synthesised in 1942 to combine an antibiotic, sulfapyridine (SPD), and an anti‐inflammatory agent, 5‐aminosalicylic acid (5‐ASA), for the treatment of rheumatoid arthritis.10 Later, sulfasalazine was also used successfully in the treatment of inflammatory bowel diseases. Although this drug has been used for decades, its mechanism of action remains a matter of debate. Numerous pharmacological and biochemical effects have been described, including modulatory effects on leucocyte function.11 Recently, it has been shown that sulfasalazine is a potent and specific inhibitor of NFκB in human colon epithelial cells.12 In these cells, sulfasalazine seems to be a direct inhibitor of IκB kinases α and β by antagonising adenosine triphosphate binding.13 However, it is not known if sulfasalazine or its metabolites can also modify NFκB signalling in hepatocytes.
The overall objectives of this study were therefore to examine the effects and potential mechanisms of sulfasalazine and its metabolites on GCDCA induced apoptosis in hepatocytes. To address these objectives we used a human hepatoma cell line stably transfected with the bile acid transporter sodium taurocholate cotransporting polypeptide (Ntcp) and isolated perfused rat livers.
Material and methods
Reagents
ZVAD‐FMK was from Promega (Madison, Wisconsin). 5‐(and‐6)‐Carboxy‐2′ 7′‐dichloro‐dihydrofluorescein diacetate (carboxy‐H2DCFDA) was from Molecular Probes (Eugene, Oregon, USA).[3H]‐Taurocholate was from Perkin Elmer (Boston, Massachusetts, USA). Antibodies against cleaved caspase 9 and cleaved caspase 3 were purchased from Cell Signalling (Beverly, Massachusetts, USA). MG132 was from Calbiochem (La Jolla, California, USA). GCDCA, sulfasalazine, SPD, 5‐ASA, dimethyl sulfoxide (DMSO), staurosporine, and all other reagents were obtained from Sigma Chemical (St Louis, Missouri, USA).
Cell culture
HepG2‐Ntcp cells14 were grown at 37°C under 5% CO2 in MEM (pH 7.4) containing 10% fetal bovine serum, 1% non‐essential amino acids, 2 mmol/l l‐glutamine, 1 mmol/l sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B.
[3H]Taurocholic acid uptake
Confluent HepG2‐Ntcp cells were washed with a buffer containing 100 mmol/l NaCl, 2 mmol/l KCl, 1 mmol/l CaCl2, 1 mmol/l MgCl2, 5.5 mmol/l d‐glucose, and 10 mmol/l N‐[2‐hydroxyethyl]piperazine‐N′‐[2‐ethane sulfonic acid] (pH 7.5, 37°C). After incubation in NaCl medium containing 1 µCi/ml [3H]taurocholic acid (TCA) and 10 µmol/l unlabelled TCA at 37°C for 20 minutes, cells were washed with an ice cold NaCl medium containing 1 mmol/l unlabelled TCA and lysed with 0.5 ml Triton X‐100 (1%, v/v). Aliquots of 400 µl were dissolved in 10 ml of scintillation cocktail (Ultima Gold Canberra Packard, Frankfurt/Main, Germany). Radioactivity was quantified using a liquid scintillation analyser (Packard Instrument Co., Frankfurt, Germany).
Caspase assays
Caspase 3/7, and caspase 9 activation were determined in subconfluent HepG2‐Ntcp cells treated with GCDCA in the absence or presence of sulfasalazine or the pancaspase inhibitor ZVAD‐FMK at the indicated concentrations and time intervals. Commercially available caspase assay kits from Promega were performed according to the recommendations of the manufacturer.
Plasmids and transfection
Luciferase reporter plasmids p105 (cona‐luc) and p106 (κB‐cona‐luc) for NFκB reporter gene assays have been previously described.8 The TK‐Renilla‐CMV plasmid was purchased from Promega and used to normalise for transfection efficiency in luciferase assays. HepG2‐Ntcp cells at a confluence of approximately 50% were transiently transfected using FuGENE (Roche, Mannheim, Germany) and used 48 hours after transfection.
Electrophoretic mobility shift assay (EMSA)
HepG2‐Ntcp cells were stimulated with diluent or sulfasalazine at different concentrations. Nuclear proteins (6 μg) and non‐specific competitor poly(dIdC) (3 μg) were incubated in binding buffer (100 mM HEPES, 300 mM KCl, 20% Ficoll, 0.05% NP‐40, 0.5 mg/ml bovine serum albumin) with 3.5 pmol of double stranded DNA oligonucleotide containing a NFκB consensus binding sequence (5′‐AGT TGA GGG GAC TTT CCC AGG C‐3′) that was labelled with [γ‐32P]‐ATP using T4 polynucleotide kinase (Promega). Protein‐DNA complexes were separated from the unbound DNA probe by electrophoresis through 5% native polyacrylamide gels.
Luciferase reporter gene assay
HepG2‐Ntcp cells were cotransfected with 0.2 µg of TK‐Renilla‐CMV and 1.5 µg of either p105 or p106. Forty eight hours later, cells were cultured in serum free MEM for 18–24 hours and then stimulated with bile acids and/or sulfasalazine for one hour. Both firefly and Renilla luciferase activities were quantitated using dual reporter gene assays from Promega, according to the manufacturer's instructions using a TD 20/20‐Luminometer (Software Turner Design Version 2.0.1, Turner Designs Inc., California, USA). Background luciferase expression, as determined in cells transfected with p105, was subtracted from p106 values.
Measurement of reactive oxygen species (ROS)
Confluent HepG2‐Ntcp cells were incubated with carboxy‐H2DCFDA (4 µmol/l) for four hours at 37°C, washed three times, incubated with GCDCA in the absence or presence of sulfasalazine at the indicated concentrations for two hours at 37°C, and quantitated in a CytoFluor 4000 reader (Perseptive Biosystems, Weiterstadt, Germany) using an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Immunoblot analysis
Subconfluent cells were treated with staurosporine (5 µmol/l) or GCDCA (75 µmol/l) in the absence or presence of sulfasalazine, washed with phosphate buffered saline, homogenised in ice cold lysis buffer (20 mM Tris‐HCl pH 8, 150 mM NaCl, 2 mM EDTA, 1% Triton, 100 µM vanadate, 10 mM NaF), incubated for five minutes on ice, sonicated, and centrifuged for five minutes at 14000 g and 4°C. The supernatant was resolved by 12.5% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis, transferred to Immobilon‐P membranes (Millipore, Eschborn, Germany), and probed against the appropriate primary antibody at a dilution of 1:1000 in 5% milk/TBS‐T overnight. Peroxidase conjugated goat antirabbit IgG antibody (Santa Cruz Biotechnologies, Santa Cruz, California, USA) was incubated at a dilution of 1:4000. Membranes were stripped and reprobed with anti‐β‐actin antibody (1:3500, Sigma) to ensure equal loading.
Animals
Male Sprague‐Dawley rats (229 (16) g) were obtained from Charles River (Sulzfeld, Germany). They were subjected to a 12 hour day‐night rhythm with unlimited access to food and water.
Isolated rat liver perfusion
The technical procedure used has been described previously.15 In brief, livers were perfused in a non‐recirculating fashion with Krebs‐Ringer bicarbonate solution at 37°C at a constant flow rate of 4.0–4.5 ml/min/g liver for 90 minutes. After 20 minutes, sulfasalazine (or the carrier DMSO only, 0.001% v/v) was continuously infused for 70 minutes to reach a final concentration of 100 µmol/l in the portal vein. After 30 minutes, the bile acid GCDCA (or the carrier DMSO only, 0.1% v/v) was infused for 60 minutes at a continuous rate to reach a final concentration of 25 µmol/l or 12.5 µmol/l in the portal vein. Hepatovenous efflux of lactate dehydrogenase (LDH) and glutamate‐pyruvate transaminase (GPT) as indicators of liver cell damage were measured by use of standard enzymatic tests.16 Bile flow was measured gravimetrically in prepared tubes.
Immunofluorescence microscopy
Activated caspase 3 and cytokeratin intermediate filament alterations, typical of apoptotic cell death, were studied as described previously.17 For quantification, caspase 3 positive hepatocytes with concomitant cytokeratin intermediate filament breakdown were counted in 20 different high power fields per sample and expressed as x‐fold increase over control.
Determination of bile acids
GCDCA concentrations in the hepatovenous effluate were determined as described previously.18 Briefly, bile acids were extracted with Bond‐Elut C18 cartridges (Analytichem International, San Diego, California, USA). Deconjugated bile acids were isolated by extraction on Lipidex 1000 (Packard Instruments, Groningen, the Netherlands) and were then methylated and trimethylsilylated. Capillary gas chromatography was performed using a Carlo Erba Fractovap 4160 analyser (Carlo Erba, Hofheim, Germany). Bile acid derivates were separated on a silica capillary CP Sil 19 CB column (Chrompack, Middelburg, the Netherlands). Eluted bile acid derivates were detected by a flame ionisation detector.
Statistics
Results from at least three independent experiments are expressed as mean (SD). Differences between groups were compared using an analysis of variance for repeated measures and a post hoc Bonferroni test to compare for multiple comparisons.
Results
Does sulfasalazine or its metabolites modulate bile acid induced apoptosis in vitro?
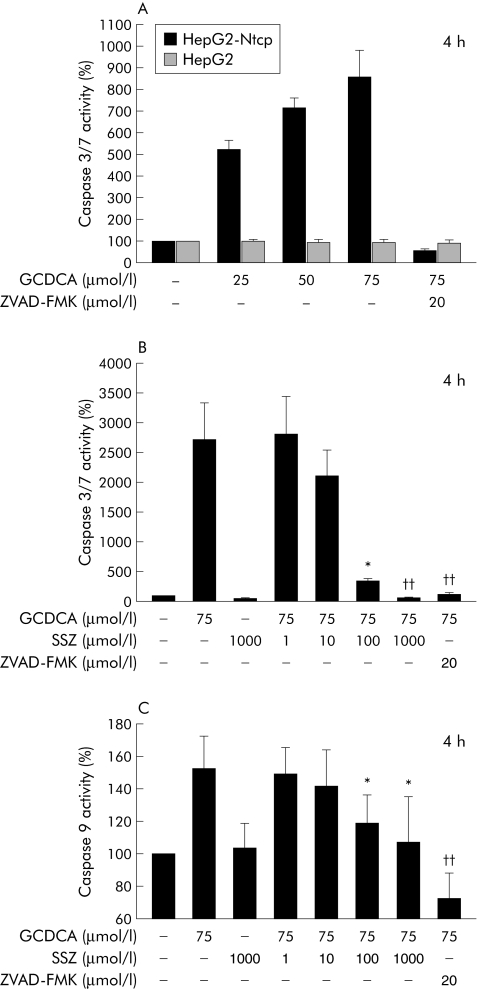
The bile acid transporting human hepatoma cell line HepG2‐Ntcp14 was used for the in vitro studies. GCDCA effectively induced apoptosis in this cell line while parent non‐bile acid transporting HepG2 cells were not sensitive to GCDCA induced apoptosis (fig 1A). Based on these results, 75 µmol/l of GCDCA was used for the remainder of the in vitro studies.
Figure 1 Sulfasalazine reduced bile acid induced apoptosis in a hepatoma cell line. (A) HepG2‐Ntcp and parent HepG2 cells were treated with diluent or glycochenodeoxycholic acid (GCDCA) at the indicated concentrations for four hours. The pancaspase inhibitor ZVAD‐FMK (20 µmol/l) was used to demonstrate the specificity of the assay. Apoptosis was quantified by measuring caspase 3/7 activity and expressed as a percentage over controls (set as 100%). (B) HepG2‐Ntcp cells were treated with GCDCA (75 µmol/l) in the presence or absence of sulfasalazine (SSZ) at the indicated concentrations. Apoptosis was quantified by measuring caspase 3/7 activity (*p<0.05; ††p<0.01 v GCDCA). (C) HepG2‐Ntcp cells were treated with GCDCA (75 µmol/l) in the presence or absence of SSZ at the indicated concentrations. Apoptosis was quantified by measuring caspase 9 activity (*p<0.05; ††p<0.01 v GCDCA). Results are mean (SD) of six independent experiments.
A dose dependent reduction in GCDCA induced apoptosis was observed when sulfasalazine was used in combination with 75 µmol/l of GCDCA. After four hours, sulfasalazine 1000 µmol/l reduced GCDCA induced caspase 3/7 activity to control levels; 100 µmol/l resulted in a 80 (12)% reduction and 10 µmol/l in a 25 (7)% reduction in caspase 3/7 activity (fig 1B). Sulfasalazine 1 µmol/l had no effect on GCDCA induced apoptosis. Because HepG2‐Ntcp is a hepatoma cell line, we also repeated this experiment using 18 hour cultured primary mouse hepatocytes and confirmed the dose dependent antiapoptotic effect of sulfasalazine on GCDCA induced apoptosis which was nearly identical to that shown in fig 1B (n = 3; data not shown).
Hepatocytes are considered to be type II cells in which the mitochondrial pathway is essential to induce apoptosis.19 Therefore, the effects of sulfasalazine on GCDCA induced caspase 9 activation were examined. In these experiments, sulfasalazine reduced GCDCA induced caspase 9 activation in a dose dependent manner similar to the reduction in the effector caspases 3/7 shown above (fig 1C).
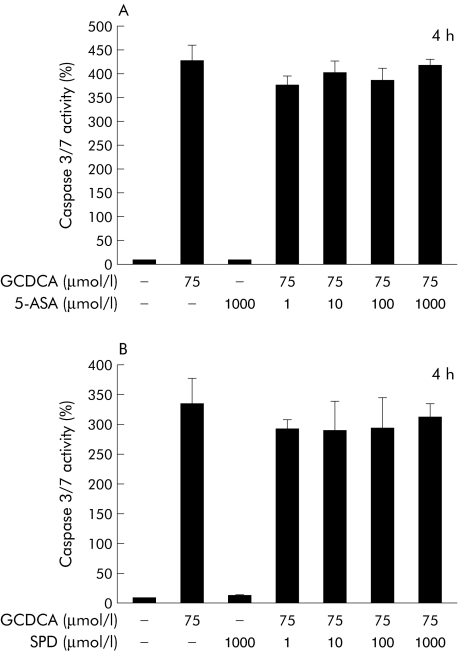
We next evaluated the effects of the two metabolites of sulfasalazine, 5‐ASA and sulfapyridine, in the same experimental system. In contrast with sulfasalazine, neither 5‐ASA nor sulfapyridine had any effect on GCDCA induced apoptosis, as measured by caspase 3/7 activity (fig 2A, 2B). The combination of 5‐ASA and sulfapyridine in the same ratio as present in sulfasalazine also did not alter GCDCA induced apoptosis (data not shown). These data indicate that sulfasalazine, but not its metabolites, is a potent inhibitor of GCDCA induced apoptosis in hepatocytes.
Figure 2 5‐Aminosalicylic acid and sulfapyridine did not affect bile acid induced apoptosis. HepG2‐Ntcp cells were treated with diluent or glycochenodeoxycholic acid (GCDCA 75 µmol/l) in the presence or absence of 5‐aminosalicylic acid (5‐ASA) (A) or sulfapyridine (SPD) (B) at the indicated concentrations for four hours. Apoptosis was quantified by measuring caspase 3/7 activity and expressed as a percentage over controls (set at 100%). Results are mean (SD) of three independent experiments.
Is NFκB activity in HepG2‐Ntcp cells induced by sulfasalazine?
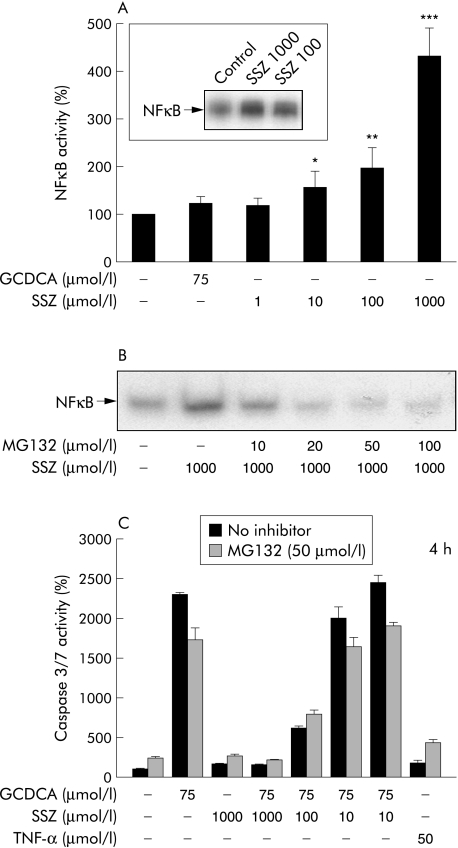
The effect of sulfasalazine on NFκB activation in HepG2‐Ntcp cells was examined by EMSA and luciferase reporter gene assays. Sulfasalazine induced NFκB activity in a dose dependent manner in HepG2‐Ntcp cells after one hour of incubation (fig 3A). NFκB activation by 100 and 1000 µmol/l sulfasalazine was also shown by EMSA (fig 3A, inset). In comparison, GCDCA 75 µmol/l did not significantly affect NFκB activity. Thus sulfasalazine appears to be an inducer of NFκB in hepatoma cells. To assess the potential role of sulfasalazine induced NFκB activation on its beneficial effect on GCDCA induced apoptosis, the NFκB inhibitor MG132 (carbobenzoxyl‐leucinyl‐leucynil‐leucynal)20 was used for additional experiments. At a concentration of 50 µmol/l, MG132 reduced sulfasalazine induced NFκB activation to control levels, as demonstrated by EMSA (fig 3B) and reporter gene assays (not shown). However, the effect of sulfasalazine on GCDCA induced caspase 3/7 activity was not altered by MG132 (fig 3C), indicating that this mechanism is not relevant for the beneficial effects of sulfasalazine on GCDCA induced apoptosis.
Figure 3 Sulfasalazine induced nuclear factor κB (NFκB) activity. (A) Hep‐G2‐Ntcp cells were cotransfected with TK‐Renilla‐CMV and Luc‐NFκB (p106). Cells were stimulated with diluent (DMEM), sulfasalazine (SSZ) at the indicated concentrations, or glycochenodeoxycholic acid (GCDCA 75 µmol/l) for one hour. Cell lysates were prepared, and firefly and Renilla luciferase assays were performed. To control for transfection efficiency, the ratio of firefly to Renilla luciferase was calculated. The resulting values are presented as arbitrary units. The inset shows an electrophoretic mobility shift assay (EMSA) for NFκB after stimulation of HepG2‐Ntcp cells with 100 or 1000 µmol/l SSZ or medium only (control) for one hour. Results are mean (SD) of three independent experiments (*p<0.05; **p<0.01; ***p<0.001 v control). (B) HepG2‐Ntcp cells were preincubated for one hour with MG132 (carbobenzoxyl‐leucinyl‐leucynil‐leucynal) at the indicated concentrations and SSZ (1000 µmol/l) or diluent (as control) was added for one hour. Nuclear extracts were prepared and EMSAs were performed. (C) HepG2‐Ntcp cells were incubated with GCDCA, SSZ, or diluent (as control) in the absence or presence of the NFκB inhibitor MG132 (50 µmol/l) for four hours. Apoptosis was quantified by measuring caspase 3/7 activity and expressed as a percentage over control (set as 100%). Results are mean (SD) of three independent experiments.
Does sulfasalazine reduce GCDCA induced ROS generation?
To determine a possible effect of sulfasalazine on GCDCA induced ROS generation, HepG2‐Ntcp cells were loaded with the fluorescent dye carboxy‐H2‐DCFDA and then incubated with GCDCA (75 µmol/l) in the absence or presence of sulfasalazine. GCDCA significantly increased generation of ROS by nearly 30% compared with controls after two hours. A combination of GCDCA with sulfasalazine (1–1000 µmol/l) led to a dose dependent reduction in ROS generation which reached control levels using sulfasalazine 1000 µmol/l (table 1). As the proapoptotic Bcl‐2 proteins Bax and Bid have also been shown to be important for the mitochondrial damage during bile acid induced apoptosis,21 additional immunoblot experiments were performed. However, expression and activation of both Bax and Bid were not modulated by sulfasalazine (data not shown).
Table 1 Sulfasalazine reduced glycochenodeoxycholic acid (GCDCA) induced oxidative stress in HepG2‐Ntcp cells.
| Condition | Fold ROS formation |
|---|---|
| Control | 1 |
| GCDCA 75 µmol/l | 1.28 (0.05)* |
| Sulfasalazine 1000 µmol/l | 0.97 (0.02) |
| GCDCA 75 µmol/l+sulfasalazine 1 µmol/l | 1.28 (0.04) |
| GCDCA 75 µmol/l+sulfasalazine 10 µmol/l | 1.24 (0.01) |
| GCDCA 75 µmol/l+sulfasalazine 100 µmol/l | 1.09 (0.03)† |
| GCDCA 75 µmol/l+sulfasalazine 1000 µmol/l | 1.00 (0.03)†† |
HepG2‐Ntcp cells were loaded for four hours with the fluorescent dye carboxy‐H2‐DCFDA (4 µmol/l) to detect generation of reactive oxygen species (ROS). Then, cells were incubated with GCDCA in the absence or presence of sulfasalazine for two hours.
Data represent the n‐fold increase in carboxy‐H2‐DCFDA fluorescence compared with controls (set at 1).
Results are the mean (SD) of 12 independent experiments.
*p<0.01 v control; †p<0.05, ††p<0.01 v GCDCA.
Is bile acid uptake inhibited by sulfasalazine?
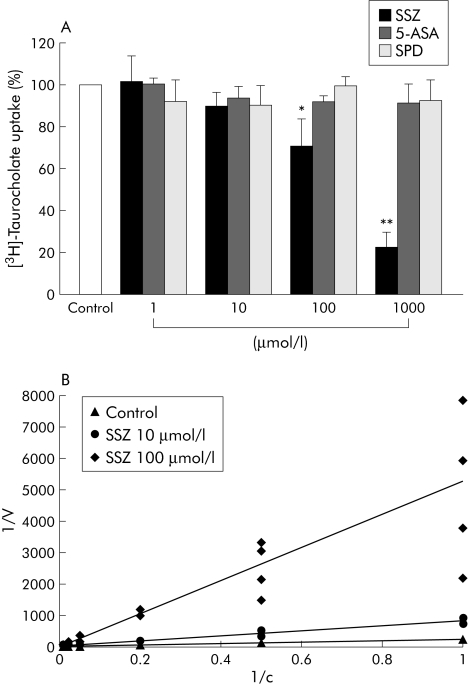
We next evaluated if sulfasalazine influenced bile acid uptake as another possible mechanism for its antiapoptotic properties. Bile acid uptake under control conditions was set as 100%. Sulfasalazine at a concentration of 1 µmol/l had no effect and at 10 µmol/l tended to reduce bile acid uptake in HepG2‐Ntcp cells, but the difference did not reach significance (fig 4A). However, using 100 µmol/l sulfasalazine, bile acid uptake was reduced to 71 (12)% compared with controls and to 23 (7)% at 1000 µmol/l, both significantly differences. In contrast, 5‐ASA and sulfapyridine did not affect bile acid uptake (fig 4A).
Figure 4 Sulfasalazine competitively inhibited bile acid uptake. (A) HepG2‐Ntcp cells were incubated with[3H]‐taurocholic acid (1 mCi/ml) in the absence or presence of sulfasalazine (SSZ), 5‐aminosalicylic acid (5‐ASA), or sulfapyridine (SPD) at the indicated concentrations for 10 minutes. Cells were then washed, lysed, and radioactivity measured in an automated counter. Results are mean (SD) of six independent experiments and are expressed as a percentage of controls (*p<0.05; **p<0.01 v control). (B) HepG2‐Ntcp cells were incubated with[3H]‐taurocholic acid (1 mCi/ml) at different concentrations (0–100 µmol/l) in the absence or presence of SSZ (10 and 100 µmol/l). The corresponding Km and Vmax values were calculated after measuring intracellular radioactivity and plotted as a Lineweaver‐Burk graph.
To determine the mechanism of sulfasalazine induced reduction of bile acid uptake, the kinetics of bile acid uptake in the absence or presence of sulfasalazine were evaluated. The Km under control conditions was 42 µmol/l and increased to 99 µmol/l (at 10 µmol/l sulfasalazine) and to 263 µmol/l (at 100 µmol/l sulfasalazine). Vmax values were nearly unchanged (0.24 (0.03) nmol/min/mg protein) under all experimental conditions. Inhibition was thus competitive, as also shown in the Lineweaver‐Burk plot (fig 4B).
Does sulfasalazine also inhibit apoptosis that is not induced by bile acids?
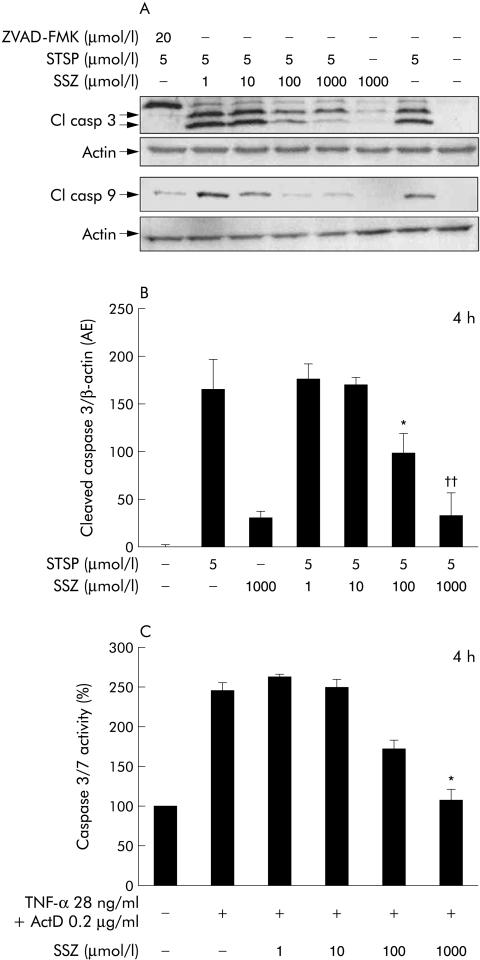
Staurosporine, a non‐specific kinase inhibitor, induces apoptosis through disruption of mitochondrial function.22 At a concentration of 5 µmol/l, staurosporine readily induced cleavage of caspase 9 and caspase 3 in HepG2‐Ntcp cells, which was inhibited by the pancaspase inhibitor ZVAD‐FMK (fig 5A). Treatment with staurosporine and sulfasalazine (1–1000 µmol/l) resulted in a dose dependent reduction in both caspase 9 and caspase 3 cleavage. Densitometric analysis of the cleaved caspase 3 immunoblots (normalised to β‐actin) revealed that staurosporine induced caspase 3 cleavage was significantly reduced when 100 or 1000 µmol/l sulfasalazine were administered (fig 5B). Similar results were obtained for cleaved caspase 9 (data not shown). Sulfasalazine also dose dependently reduced caspase 3/7 activity when TNF‐α and actinomycin D were used as an apoptotic stimulus that requires Fas signalling (fig 5C). These results indicate that inhibition of intracellular apoptosis signalling might be more important for the antiapoptotic effects of sulfasalazine than the observed reduction in bile acid uptake.
Figure 5 Sulfasalazine reduced staurosporine and tumour necrosis factor α/actinomycin D induced apoptosis. (A) Subconfluent HepG2‐Ntcp cells were treated with diluent or staurosporine (STSP) in the absence or presence of sulfasalazine (SSZ) or the pancaspase inhibitor ZVAD‐FMK at the indicated concentrations for four hours. Equivalent amounts of proteins were immunoblotted with antibodies against cleaved (Cl) caspase 3 or cleaved caspase 9. Membranes were then stripped and reprobed with an anti‐β‐actin antibody to ensure equal loading in an identical procedure. Representative blots from three independent experiments are shown. (B) In addition, densitometry of cleaved caspase 3 and β‐actin was performed and expressed as the ratio cleaved caspase 3/β‐actin. Results are mean (SD) of three independent experiments (*p<0.05, ††p<0.01 v STSP). (C) HepG2‐Ntcp cells were treated with tumour necrosis factor α (TNF‐α 28 ng/ml) in combination with actinomycin D (ActD 0.2 µg/ml) in the presence or absence of SSZ at the indicated concentrations. Apoptosis was quantified by measuring caspase 3/7 activity. Results are mean (SD) of three independent experiments (*p<0.05 v TNF‐α/ActD).
Does sulfasalazine ameliorate GCDCA induced liver damage in the intact organ?
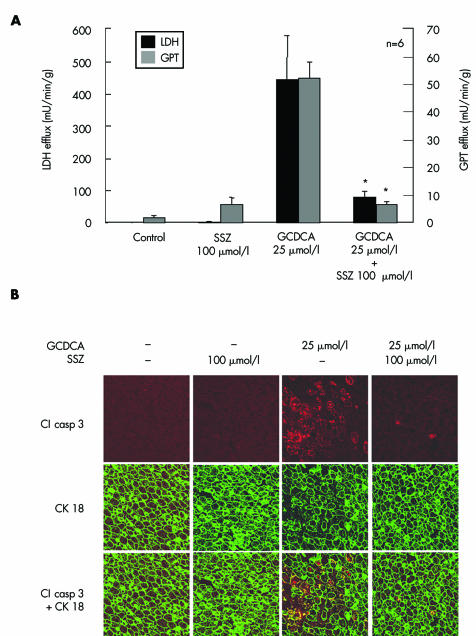
Livers of Sprague‐Dawley rats were perfused with sulfasalazine (100 µmol/l) or the carrier DMSO only (0.1% v/v) for 70 minutes beginning at 20 minutes. GCDCA (25 µmol/l) was infused for 60 minutes beginning at 30 minutes. We have recently demonstrated that GCDCA (25 µmol/l) induces widespread hepatocyte apoptosis and liver damage in perfused rat livers.23 In contrast, GCDCA induced apoptosis and liver injury were significantly reduced by simultaneous perfusion with 100 µmol/l sulfasalazine: LDH and GPT activities were reduced by 82% and 87%, respectively, compared with GCDCA perfused livers (fig 6A). Correspondingly, apoptotic hepatocytes were only observed occasionally compared with the extensive hepatocyte apoptosis (>100‐fold compared with controls) observed after treatment with GCDCA alone (fig 6B). Sulfasalazine by itself did not cause liver damage or hepatocyte apoptosis. Thus sulfasalazine distinctly reduces GCDCA induced apoptosis and liver damage in the intact liver also.
Figure 6 Sulfasalazine reduced bile acid induced liver injury and apoptosis in perfused rat livers. Rat livers were perfused with 25 µmol/l glycochenodeoxycholic acid (GCDCA) or the carrier dimethyl sulfoxide only, in the absence or presence of 100 µmol/l sulfasalazine (SSZ) for 60 minutes. (A) After 55 minutes of bile acid administration, lactate dehydrogenase (LDH) and glutamate‐pyruvate transaminase (GPT) activities in the hepatovenous effluate were determined photometrically. Results are mean (SD) of six independent experiments. (B) Hepatocyte apoptosis was determined by immunohistochemistry of activated cleaved (Cl) caspase 3 and double immunofluorescence labelling for activated caspase 3 and cytokeratin (CK) 18. After 60 minutes, double immunofluorescence labelling for CK 18 (green) and activated caspase 3 (red and yellow due to colocalisation with CK 18) in GCDCA treated livers revealed massive hepatocellular activation of caspase 3 in cells with CK intermediate filament breakdown and granular cytoplasmic condensation. In contrast, immunoreaction for activated caspase 3 was similar to controls in livers perfused with GCDCA and SSZ (magnification ×400). Representative images from six independent experiments are shown.
Is GCDCA induced cholestasis reversed by sulfasalazine?
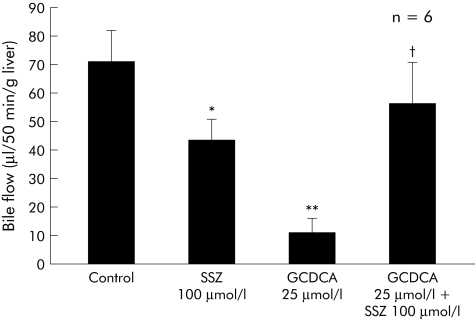
Bile flow was 1.31 (0.31) µl/min/g of liver (n = 6) after 20 minutes before bile acids or their carrier DMSO (0.1% v/v) were infused, indicating an adequate secretory capacity of the livers. GCDCA (25 µmol/l) reduced bile flow to 17% of controls (p<0.01 v control). Sulfasalazine alone (100 µmol/l) also reduced bile flow to 62% of controls (p<0.05 v control). In contrast, sulfasalazine markedly increased bile flow in GCDCA treated livers to 79% of controls (p<0.05 v GCDCA) (fig 7). Thus sulfasalazine partially reversed GCDCA induced cholestasis in the intact liver.
Figure 7 Sulfasalazine partially reversed glycochenodeoxycholic acid (GCDCA) induced cholestasis. Dimethyl sulfoxide (0.1% v/v), GCDCA, sulfasalazine (SSZ), and GCDCA in combination with SSZ were administered for 60 minutes (GCDCA) and 70 minutes (SSZ). GCDCA significantly reduced bile flow (p<0.01 v control). While SSZ alone decreased bile flow, SSZ significantly increased bile flow in GCDCA treated livers (*p<0.05, **p<0.01 v control; †p<0.05 v GCDCA). Results are expressed as mean (SD) of six experiments.
Does sulfasalazine reduce bile acid uptake in the intact organ?
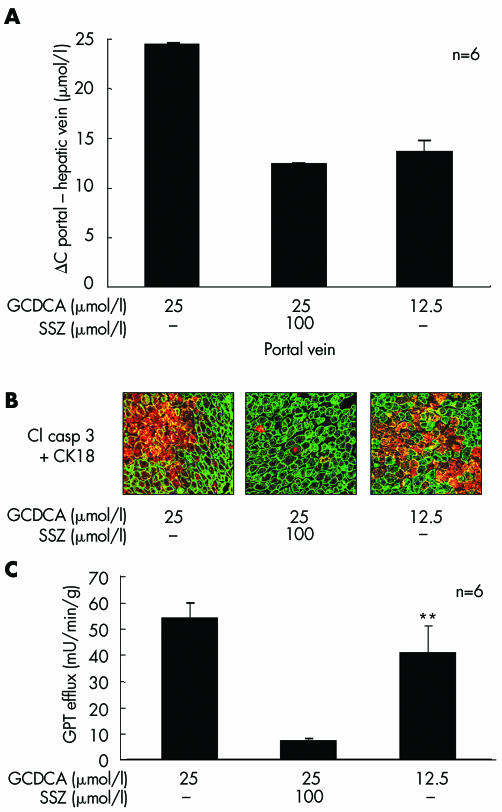
We investigated the possible role of sulfasalazine induced reduction of bile acid uptake as a major mechanism for its protective effect on GCDCA induced liver damage in the perfused liver. Bile acid uptake was calculated by subtracting GCDCA concentration in the hepatovenous effluate from the concentration in the portal vein because this difference likely reflects uptake of bile acids into the liver. Similar to the results observed in HepG2‐Ntcp cells, GCDCA uptake was reduced by 45 (5)% after 45 minutes of perfusion with sulfasalazine (n = 6), suggesting inhibition of bile acid uptake as a possible mechanism for the protective effect of sulfasalazine (fig 8A). Based on this experiment, we next evaluated if half the dose of GCDCA (12.5 µmol/l)—which is presumably taken up by the liver during concomitant administration of sulfasalazine and 25 µmol/l GCDCA—still causes liver damage and hepatocyte apoptosis. If inhibition of uptake is a major mechanism of protection, liver damage should not occur using 12.5 µmol/l GCDCA. However, when 12.5 µmol/l GCDCA was used, marked hepatocyte apoptosis (fig 8B) and liver injury (GPT 40.7 (9.5) mU/min/g) still occurred (fig 8C). A combination of 12.5 µmol/l GCDCA and 100 µmol/l sulfasalazine again significantly reduced liver damage and hepatocyte apoptosis (data not shown). Thus inhibition of bile acid uptake appears to play only a minor role in the protective effects of sulfasalazine in the intact liver.
Figure 8 Reduced bile acid uptake is not sufficient to explain the effects of sulfasalazine in the intact liver. Rat livers were perfused with glycochenodeoxycholic acid (GCDCA 12.5 or 25 µmol/l) or the carrier dimethyl sulfoxide only, in the absence or presence of 100 µmol/l sulfasalazine (SSZ) for 60 minutes. (A) After 15 minutes of bile acid administration, chenodeoxycholic acid concentrations were determined in the hepatovenous effluate. The difference between portal vein concentration (concentration in the perfusate) and hepatic vein concentration (concentration in the effluate) as an indirect measure of bile acid uptake into the liver is mapped on the y axis. GCDCA at both concentrations was almost completely taken up into the liver because it could only be detected in trace amounts in the hepatovenous effluate. In contrast, 11 (1) µmol/l of chenodeoxycholic acid were detected in the hepatovenous effluate when GCDCA (25 µmol/l) and SSZ (100 µmol/l) were administered, indicating that nearly 50% of GCDCA was not taken up. Bile acid uptake into the liver was almost identical when GCDCA was administered at 12.5 µmol/l or was coadministered with SSZ (100 µmol/l) at 25 µmol/l. (B) Hepatocyte apoptosis was determined by immunohistochemistry of activated caspase 3 and cytokeratin (CK) 18 after 60 minutes of bile acid administration (magnification ×400). Hepatocyte apoptosis was markedly more pronounced with GCDCA 12.5 µmol/l compared with GCDCA 25 µmol/l in combination with 100 µmol/l SSZ, although bile acid uptake into the liver was similar under both conditions. Representative images from six independent experiments are shown. (C) After 55 minutes of bile acid administration, glutamate‐pyruvate transaminase (GPT) activities in the hepatovenous effluate were determined photometrically. Comparable to (B), GPT efflux as a marker of liver damage was significantly increased with GCDCA 12.5 µmol/l compared with GCDCA 25 µmol/l in combination with 100 µmol/l SSZ (**p<0.01). Results in (A) and (C) are mean (SD) of six independent experiments.
Discussion
The principle findings of this study indicate that sulfasalazine is a potent inhibitor of bile acid mediated hepatocyte apoptosis in vitro and in the intact liver. In addition, sulfasalazine ameliorates GCDCA induced cholestasis in perfused rat livers. These results suggest that sulfasalazine may be of potential benefit in the treatment of cholestatic liver diseases by reducing liver injury and cholestasis.
At present, there are no data concerning the effects of sulfasalazine on apoptosis in hepatocytes. In T lymphocytes, sulfasalazine has been shown to induce apoptosis by caspase independent mechanisms.24 In a recent study, sulfasalazine promoted hepatic stellate cell apoptosis in vitro and in vivo.25 In human glioma cells and colon carcinoma cell lines, sulfasalazine inhibited Fas mediated apoptosis but simultaneously sensitised the cells to TRAIL mediated apoptosis.26 However, apoptosis could not be induced by sulfasalazine in SW620 colon carcinoma cells or primary human synoviocytes.24 Thus there appears to be a cell type specific sensitivity to sulfasalazine. In the present study, sulfasalazine inhibited Fas dependent apoptosis because GCDCA as well as TNF‐α/actinomycin D induced apoptosis are considered Fas dependent.4,27 Sulfasalazine also inhibited staurosporine induced apoptosis, suggesting that its protective effect is not limited to reduced formation of the death inducing signalling complex.
In contrast with studies in colon epithelial cells, hepatic stellate cells, and T lymphocytes,13,25,28 sulfasalazine induced NFκB activity in our model. However, this NFκB activation does not appear to be responsible for the observed beneficial effects of sulfasalazine because GCDCA induced apoptosis was unchanged when sulfasalazine was administered together with the NFκB inhibitor MG132. Interestingly, sulfasalazine modulated apoptosis in a NFκB independent manner in human glioma cells although sulfasalazine also significantly altered NFκB activity in this model.26 However, sulfasalazine appears to have a protective effect on mitochondria, as suggested by its capacity to reduce GCDCA induced ROS generation and caspase 9 activation, and by its reduction of staurosporine induced cytotoxicity, a model of cell death known to be mediated by mitochondrial dysfunction.29 Thus inhibition of the mitochondrial pathway might be partly responsible for the protective effect of sulfasalazine in our model. Interestingly, sulfasalazine has been described as a ROS scavenger in the past.30,31
Sulfasalazine reduced bile acid uptake in hepatocytes at higher concentrations. This reduction is due to competitive inhibition of the transfected bile acid transporter Ntcp. As uptake of GCDCA is necessary to induce apoptosis, it could be speculated that this is the major mechanism of action in our model. However, at a concentration of 100 µmol/l sulfasalazine, 70% of bile acids were still taken up by HepG2‐Ntcp cells and 50 µmol/l GCDCA (equivalent to 75 µmol/l GCDCA in combination with 100 µmol/l sulfasalazine) readily induces hepatocyte apoptosis. In addition, sulfasalazine also inhibited staurosporine induced apoptosis in HepG2‐Ntcp cells. Thus a direct effect on apoptosis signalling appears more important than the reduction in bile acid uptake because staurosporine does not require active transport into cells.22 This conclusion is further supported by perfusion studies. Sulfasalazine inhibited GCDCA induced apoptosis almost to control levels although bile acid uptake was only reduced by approximately 50%. In addition, when GCDCA was used at half the concentration (12.5 v 25 µmol/l), which is presumably still taken up by hepatocytes during sulfasalazine administration, significantly more liver damage and hepatocyte apoptosis occurred compared with the combination of sulfasalazine and GCDCA 25 µmol/l. This strongly suggests that the major mechanism of action of sulfasalazine on reduction of GCDCA induced liver damage is inhibition of apoptosis signalling. Reduction of bile acid uptake appears to be only a minor contributing mechanism.
Sulfasalazine partly reversed GCDCA induced impairment of bile flow in our model. How can this observation be explained? GCDCA is a toxic bile acid that significantly decreases bile flow, in part due to hepatocyte injury. Sulfasalazine protects the liver against GCDCA induced injury thereby restoring the capacity of the liver to generate bile. In addition, GCDCA might contribute to bile acid dependent bile flow when its cytotoxic effects are inhibited by sulfasalazine.32
The two metabolites of sulfasalazine, 5‐ASA and sulfapyridine, had no effect on GCDCA induced apoptosis. Similar observations have also been made in other models. In colon epithelial cells, sulfasalazine but not its metabolites suppressed NFκB activity12,13 and likewise, in T lymphocytes, only sulfasalazine induced apoptosis.28 Thus only the intact molecule seems to modulate intracellular signalling. This observation appears to be of clinical significance because only sulfasalazine, and not 5‐ASA, has beneficial effects in rheumatoid arthritis as a disease modifying agent.33
The protective effects of sulfasalazine observed in the present study were most pronounced at concentrations of 100 and 1000 µmol/l. Early studies have shown that peak serum levels of approximately 100 µmol/l sulfasalazine are reached in the serum of patients after oral administration.34 The concentration of sulfasalazine in the portal vein, which is crucial for the concentration reached in hepatocytes, is presumably even higher, because sulfasalazine undergoes enterohepatic circulation.35 Thus our results may have therapeutic implications for patients with chronic cholestasis.
In summary, the data presented in the current study suggest that bile acid mediated apoptosis can be effectively inhibited by sulfasalazine at concentrations that are reached during therapeutic application of this drug in patients. Together with recent findings that sulfasalazine reduces hepatic fibrosis by promoting hepatic stellate cell apoptosis,25 our results have potential implications for reducing liver injury during cholestasis. This novel therapeutic strategy deserves further study, for example in patients with primary sclerosing cholangitis and ulcerative colitis which are concurrently treated with sulfasalazine.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft grant Ru 743/3‐1 (to CR) and grant Be 1242/5‐5 (to UB). The authors thank Ralf Wimmer for expert technical assistance. Parts of the data were presented at the Annual Meeting of the American Association for the Study of Liver Disease, Boston, MA, USA, 2002 (Hepatology 2002;36:324A).
Abbreviations
5‐ASA - 5‐aminosalicylic acid
DMSO - dimethyl sulfoxide
EMSA - electrophoretic mobility shift assay
GCDCA - glycochenodeoxycholic acid
GPT - glutamate‐pyruvate transaminase
LDH - lactate dehydrogenase
Ntcp - sodium taurocholate cotransporting polypeptide
NFκB - nuclear factor κB
ROS - reactive oxygen species
TCA - taurocholic acid
TNF‐α - tumour necrosis factor α
Footnotes
Conflict of interest: None declared.
References
- 1.Hofmann A F. Cholestatic liver disease: pathophysiology and therapeutic options. Liver 200222(suppl 2)14–19. [DOI] [PubMed] [Google Scholar]
- 2.Yoon J H, Gores G J. Death receptor‐mediated apoptosis and the liver. J Hepatol 200237400–410. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues C M, Fan G, Ma X.et al A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest 19981012790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinehr R, Graf D, Haussinger D. Bile salt‐induced hepatocyte apoptosis involves epidermal growth factor receptor‐dependent CD95 tyrosine phosphorylation. Gastroenterology 2003125839–853. [DOI] [PubMed] [Google Scholar]
- 5.Schmucker D L, Ohta M, Kanai S.et al Hepatic injury induced by bile salts: correlation between biochemical and morphological events. Hepatology 1990121216–1221. [DOI] [PubMed] [Google Scholar]
- 6.Faubion W, Guicciardi M, Miyoshi H.et al Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest 1999103137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodeman T, Bronk S F, Roberts P J.et al Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol 2000278G992–G999. [DOI] [PubMed] [Google Scholar]
- 8.Rust C, Karnitz L M, Paya C V.et al The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3‐kinase‐dependent survival signaling cascade. J Biol Chem 200027520210–20216. [DOI] [PubMed] [Google Scholar]
- 9.Iimuro Y, Nishiura T, Hellerbrand C.et al NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest 1998101802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svartz N. Sulfasalazine: II. Some notes on the discovery and development of salazopyrin. Am J Gastroenterol 198883497–503. [PubMed] [Google Scholar]
- 11.Gaginella T S, Walsh R E. Sulfasalazine: multiplicity of action. Dig Dis Sci 199237801–812. [DOI] [PubMed] [Google Scholar]
- 12.Wahl C, Liptay S, Adler G.et al Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 19981011163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber C K, Liptay S, Wirth T.et al Suppression of NF‐kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 20001191209–1218. [DOI] [PubMed] [Google Scholar]
- 14.Glasova H, Berghaus T M, Kullak‐Ublick G A.et al Tauroursodeoxycholic acid mobilizes alpha‐PKC after uptake in human HepG2 hepatoma cells. Eur J Clin Invest 200232437–442. [DOI] [PubMed] [Google Scholar]
- 15.Beuers U, Denk G U, Soroka C J.et al Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol 3‐kinase‐dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem 200327817810–17818. [DOI] [PubMed] [Google Scholar]
- 16.Bergmeyer H, Bernt E. In: Methods of enzymatic analysis. In: Bergmeyer H, Orlando FL, eds. New York: Academic Press, Inc, 1983574–579.
- 17.Fickert P, Zollner G, Fuchsbichler A.et al Ursodeoxycholic acid aggravates bile infarcts in bile duct‐ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 20021231238–1251. [DOI] [PubMed] [Google Scholar]
- 18.Stellaard F, Sauerbruch T, Paumgartner G. Simultaneous determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates in human serum using 13C‐labeled bile acids. J Lipid Res 1984251313–1319. [PubMed] [Google Scholar]
- 19.Scaffidi C, Fulda S, Srinivasan A.et al Two CD95 (APO‐1/Fas) signaling pathways. EMBO J 1998171675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganten T M, Koschny R, Haas T L.et al Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 200542588–597. [DOI] [PubMed] [Google Scholar]
- 21.Kuwana T, Mackey M R, Perkins G.et al Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002111331–342. [DOI] [PubMed] [Google Scholar]
- 22.Wei M C, Zong W X, Cheng E H.et al Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001292727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rust C, Bauchmuller K, Fickert P.et al Phosphatidylinositol 3‐kinase‐dependent signaling modulates taurochenodeoxycholic acid‐induced liver injury and cholestasis in perfused rat livers. Am J Physiol Gastrointest Liver Physiol 2005289G88–G94. [DOI] [PubMed] [Google Scholar]
- 24.Liptay S, Fulda S, Schanbacher M.et al Molecular mechanisms of sulfasalazine‐induced T‐cell apoptosis. Br J Pharmacol 2002137608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakley F, Meso M, Iredale J P.et al Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 2005128108–120. [DOI] [PubMed] [Google Scholar]
- 26.Hermisson M, Weller M. NF‐kappaB‐independent actions of sulfasalazine dissociate the CD95L‐ and Apo2L/TRAIL‐dependent death signaling pathways in human malignant glioma cells. Cell Death Differ 2003101078–1089. [DOI] [PubMed] [Google Scholar]
- 27.Faubion W, Gores G. Death receptors in liver biology and pathobiology. Hepatology 1999291–4. [DOI] [PubMed] [Google Scholar]
- 28.Liptay S, Bachem M, Hacker G.et al Inhibition of nuclear factor kappa B and induction of apoptosis in T‐lymphocytes by sulfasalazine. Br J Pharmacol 19991281361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamad N, Gutierrez A, Nunez M.et al Mitochondrial apoptotic pathways. Biocell 200529149–161. [PubMed] [Google Scholar]
- 30.Aruom O L, Wasil M, Halliwell B.et al The scavenging of oxidants by sulfasalazine and its metabolites. A possible contribution to their anti‐inflammatory effects? Biochem Pharmacol 1987363739–3742. [DOI] [PubMed] [Google Scholar]
- 31.Miyachi Y, Yoshioka A, Imamura S.et al Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut 198728190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann A F. Current concepts of biliary secretion. Dig Dis Sci 19893416–20S. [DOI] [PubMed] [Google Scholar]
- 33.Jones G, Halbert J, Crotty M.et al The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo‐controlled trials. Rheumatology (Oxford) 2003426–13. [DOI] [PubMed] [Google Scholar]
- 34.Schroder H, Campbell D E. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clin Pharmacol Ther 197213539–551. [DOI] [PubMed] [Google Scholar]
- 35.Klotz U. Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5‐aminosalicylic acid. Clin Pharmacokinet 198510285–302. [DOI] [PubMed] [Google Scholar]