Abstract
Background and aim
Stressful life events are known to modulate the development or relapse of disease in both inflammatory bowel disease and irritable bowel disease patients but underlying mechanisms remain unclear. Stress is known to effect mast cells, interferon γ (IFN‐γ), and myosin light chain phosphorylation to trigger colonic epithelial barrier dysfunction. The aim of this study was to investigate whether acute stress induced or chemical mast cell activation impaired expression and function of epithelial tight junctions, and altered colonocyte differentiation in mice.
Methods
Colonic paracellular permeability was assessed as the in vivo lumen to blood ratio of 51Cr‐EDTA in different groups of mice (controls, stressed, mast cell degranulator BrX‐537A treated), pretreated or not with the mast cell stabiliser doxantrazole. Involvement of mast cells and IFN‐γ was evaluated in wild‐type and IFN‐γ deficient mice. Tight junction alteration was assessed by histology, transmission electron microscopy, and real time reverse transcription‐polymerase chain reaction. Colonocyte differentiation was determined by protein kinase C ζ (PKCζ) immunofluorescence and western blotting, and alkaline phosphatase activity assay.
Results
Acute stress induced a three day delayed increase in colonic paracellular permeability which involved mast cell degranulation and overproduction of IFN‐γ. The colonic epithelial barrier was morphologically altered and expression of mRNA encoding tight junction proteins ZO‐2 and occludin was decreased. Moreover, three days after acute stress, colonocyte differentiation was reduced, as shown by decreased expression of both PKCζ isotype and alkaline phosphatase.
Conclusion
These data highlight new mechanisms whereby an acute stress acts on the gastrointestinal tract by inducing alterations in colonocyte differentiation and decreased expression of mRNA encoding tight junction proteins. Thus phenotypic changes in colonocytes could pave the way for stress related intestinal disorders.
Keywords: stress, colonocytes, inflammatory bowel disease, Crohn's disease, ulcerative colitis, differentiation, mast cell, interferon γ
Evidence that stress can modulate the course of inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS) comes from clinical observations. In ulcerative colitis (UC) patients, a long term perceived stress increases the risk of exacerbation over a period of months to years.1 Moreover, relapses in UC are observed more often in patients reporting stressful events in the preceding month.2 Similarly, in Crohn's disease (CD) patients, psychological stress seems to facilitate relapse.3 In both UC and CD, studies have shown that relapse was frequently preceded by increased paracellular permeability.4,5,6 Accordingly, downregulation of tight junction associated proteins has been observed in human tissues from CD and UC.7 Increased gut paracellular permeability is also observed in IBS patients.8,9 However, the specific mechanisms underlying such alterations in gut paracellular permeability in both IBS and IBD patients are poorly understood, as is the role of stress in such alterations.10,11,12
Animal models have provided new insights into the relationship between acute or repeated stress and intestinal permeability. In rats, an acute restraint stress impairs the intestinal barrier, involving enhancement of antigen uptake as early as two hours after the initial stimulus.13 Another effect of acute stress was documented by Saunders and colleagues,14 showing that in rats, ion secretion and permeability to ions and small inert probes was enhanced after stress. Moreover, chronic stress induces mucosal mast cell hyperplasia and impairs intestinal permeability.15,16 We have previously demonstrated that the effect of chronic stress on colonic paracellular permeability (CPP) involves both synthesis and release of interferon γ (IFN‐γ) associated with bacterial translocation.17 Consequently, we wished to know if the effects of stress on CPP seen in that study,17 72 hours after the first stress session, were linked to daily repetition of stress or simply the delay elapsed from the first stress session.
Three hypotheses may explain this delay. (i) Multiple stress sessions are required to give rise to prolonged recruitment of immunocytes and subsequent release of cytokines responsible for colonocyte tight junction (TJ) opening.17 (ii) Only one stress session is needed to affect cytokine expression that may produce phenotypic changes in colonocytes after a delay. Indeed, IFN‐γ increases paracellular permeability of monolayers by reducing mRNA levels of ZO‐1 and occludin, two proteins of the TJ complex, after a similar delay18,19. (iii) One stress session immediately alters intestinal stem cell differentiation and proliferation programming. These alterations in differentiation may be observed only when these cells have migrated from the bottom of the crypt to the surface. Two proinflammatory cytokines, tumour necrosis factor α (TNF‐α) and IFN‐γ are known to modulate the production of specific component of colonocytes, such as laminin, resulting in changes in cell differentiation/proliferation.20
In the present investigation, we aimed (i) to characterise the long term (three days) effects of a single, acute, short (two hours) stress session on CPP in mice, (ii) to evaluate if this delay was linked to a primary effect on colonocyte differentiation, and (iii) to observe the role of mast cells in triggering this effect by comparing these time related alterations induced by an acute stress with those induced by single administration of a mast cell degranulator.
Material and methods
Animals
Eight week old male Swiss 3T3 mice (Janvier, Le Genest Saint‐Isle, France) were used. Male B6‐Ifng−/− mice have been described previously,21 and were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA), bred, and maintained in the animal facility of the Institut Fédératif de Recherche Claude De Preval (Toulouse, France). Male C57BL/6J mice (Elevage Janvier, Le Genest Saint‐Isle, France) were used as the control strain. They were kept at a constant temperature (23 (1°C) in a pathogen free animal facility, maintained on a 12:12 hour light:dark cycle (lights on at 7 am). Food and water were available ad libitum. All experiments were approved by the local institutional animal care and use committee.
Stress sessions
Acute stress was carried out as mixed restraint and acoustic stress sessions lasting two hours: animals were placed in individual cylinders and submitted to an acoustic stress using an ultrasound bath (operating frequency 46 kHz), placed 20 cm away from the animals (sound level 82 dB). Control animals (sham stress) were placed in cylinders for a few seconds but immediately replaced and left undisturbed in their cages (ambient sound level 65 dB).
Permeability measurements
Mice were anaesthetised by subcutaneous injection of 2 mg xylazine/2 mg ketamine in 0.1 ml of saline, and were disposed on their back. Then, a catheter (OD 1 mm; Portex, Hythe, UK) was inserted rectally 4 cm from the anus and attached to the animal's tail. Assessment of CPP was performed using 51Cr‐EDTA as a selective marker of paracellular permeation of TJs, as previously described.17 To determine CPP, 0.7 μCi of 51Cr‐EDTA (Perkin Elmer, Paris, France) in 0.5 ml saline were slowly administered into the colon (0.25 ml/h). After two hours, mice were sacrificed and the colon was removed. Radioactivity of the colon and the rest of the body were counted, and permeability was expressed as the ratio between the rest of the body and total (mice plus colon) radioactivity (expressed as a percentage).
IFN‐γ protein assay
Colons were excised, washed with saline, and the mucosa was scrapped off and recovered. Proteins were extracted with phosphate buffered saline (PBS; Invitrogen, Cergy Pontoise, France) containing complete EDTA free protease inhibitor cocktail (Roche, Meylan, France). Total protein concentration was assessed by BCA protein assay kit (Pierce, Brebières, France). IFN‐γ protein assay was performed with Duoset ELISA kit (R&D Systems, Lille, France) according to the manufacturer's instructions.
Real time reverse transcription‐polymerase chain reaction (RT‐PCR)
After extraction from distal colon tissue by the acid guanidinium thiocyanate‐phenol‐chloroform method, total RNA was converted to cDNA using random hexonucleotides and then used for PCR. We conducted reverse transcription with QuantiTect SYBR Green RT‐PCR Kit (Qiagen, Courtaboeuf, France). After amplification, we determined the threshold cycle (Ct) to obtain expression values of 2−ΔΔCt, as described previously.22
Histological studies
Colons were collected and fixed for 24 hours in Duboscq‐Brazil solution, embedded in paraffin, and then stained with hemalun‐eosin. Colons were observed on microscopic 7 μm cross sections. Histological changes were graded semiquantitatively from 0 to 4 according to previously described criteria.23
Transmission electron microscopy (TEM) study
All specimens were prepared according to standard protocols. Three animals per group (stressed, BrX‐537A treated, and controls) were used for structural observation of TJs. Ultrathin sections (70 nm) were prepared with an ultramicrotome system (Reichert OMU2) and collected on copper/palladium grids. Sections were examined with a Hitachi HU11C electron microscope. To evaluate changes in TJ morphology, the junctional regions of two random sections of longitudinally sectioned villi were examined for each specimen by an anatomopathologist. All sectioned TJs on the top half of villi were assessed. Examinations were performed on a total of approximately 50 TJs per specimen. TJs were considered open when the size of dilatation was more than 20 nm.24 Results were expressed as percentage of opened TJs.
Colonocyte differentiation
Immunofluorescence experiments
Colon were collected and immersed in 10% freshly depolymerised paraformaldehyde fixative for two hours at room temperature and cryoprotected in 30% sucrose/PBS. Sections of distal colon (10 μm) were incubated with a rabbit antimouse polyclonal antibody raised against a peptide mapping at the carboxy terminus of protein kinase C ζ (PKCζ), a marker of differentiation,25 diluted to 1:500 (Santa Cruz Biotechnology, Le Perray‐en‐Yvelines, France). After incubation for 24 hours at 4°C, sections were washed in PBS/milk 0.1% and incubated with FITC labelled donkey antirabbit IgG antibody (Molecular Probes, Leiden, the Netherlands) diluted to 1:1000 for 1.5 hours. Fluorescence was observed using a Nikon Eclipse E600 microscope equipped with appropriate optics and filter modules (Nikon France, Champigny‐sur‐Marne, France).
Western blotting analysis of PKCζ
Colons were excised and the mucosa was scraped off and recovered. Proteins were extracted with RIPA buffer and quantified. Equal amounts of each extract were subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis in 10% slabs and then electrotransferred onto 0.45 μm nitrocellulose membranes. Membrane was blocked with Tris buffered saline/milk 6% and then incubated for one hour at room temperature with the rabbit anti‐PKCζ (1/200). After washing, horseradish peroxidase conjugated goat antirabbit (Upstate, Mundolsheim, France) at a 1:5000 dilution was added for 45 minutes at room temperature. The membrane was then incubated for 1–5 minutes with visualiser detection reagent (Upstate).
Alkaline phosphatase activity
Alkaline phosphatase (AP) activity was determined according to the method of Garen and Levinthal (1960). Briefly, mucosa was homogenised in 50 mM Tris buffer (pH 7.4) by several passages through a 26 gauge needle fitted to a 2 ml syringe. The amount of p‐nitrophenol released was determined by a spectrophotometer at 410 nm. Enzyme activity was expressed as mU per mg of total protein (1 unit being 1 μmol of substrate hydrolysed per minute).26
Experimental design
To investigate impairment of colonic barrier function, we assessed permeability to 51Cr‐EDTA in 20 groups of eight Swiss male mice (20–25 g) and two groups of male IFN‐γ deficient C57BL/6J mice (20–25 g) and their controls. Four groups were controls (two sham stressed and two treated with DMSO, the vehicle for BrX‐537A), and were sacrificed either at T+2 hours or T+3 days. On day 1, eight groups were subjected to a single acute stress session (two hours) and eight other groups were treated with the mast cell activator BrX‐537A. Two groups (one stress group, one BrX‐537A group) were sacrificed each day from day 0 to day 6. Two groups received pretreatment with doxantrazole (10 mg/kg) 30 minutes before stress or BrX‐537A and were sacrificed at day 3. Six groups of eight B6‐Ifng−/− and wild‐type C57Bl/6J mice were subjected either to one stress session or BrX‐537A, or to sham treatments.
Six groups (including one control group) of eight Swiss mice were used to evaluate the kinetics (from T0 to T+4 days) of colonic IFN‐γ protein expression after BrX‐537A treatment.
Six groups of three mice were used for TEM and histological studies, two hours and three days after acute stress stimulation or after chemical mast cell degranulation (BrX‐537A).
Eight groups of eight mice were used for quantification of occludin, ZO‐1, and ZO‐2 mRNA expression by real time RT‐PCR, from T0 to T+6 days.
Colonic AP activity was assayed in 10 groups (including two control groups) of eight mice subjected to either acute stress or BrX‐537A. Animals subjected to acute stress were sacrificed at 12:30h; BrX‐537A‐treated animals were sacrificed at 16:00h.
Three groups of five mice were used to evaluate PKCζ expression.
Drugs
Bromolasalocid ethanolate (BrX‐537A) was injected intraperitoneally in mice at 2 mg/kg, diluted in DMSO. Doxantrazole was injected intraperitoneally to stabilise the mast cell membrane at a dose of 10 mg/kg diluted in NaHCO3 5%.
Statistical analysis
All values are expressed as mean (SD) for permeability measures and mean (SEM) for other experiments. Data were analysed by the Student's t test or by one way analysis of variance followed by a post hoc Tukey's test or the non‐parametric Mann‐Whitney test where appropriate.
Results
Kinetics of acute stress effect on CPP
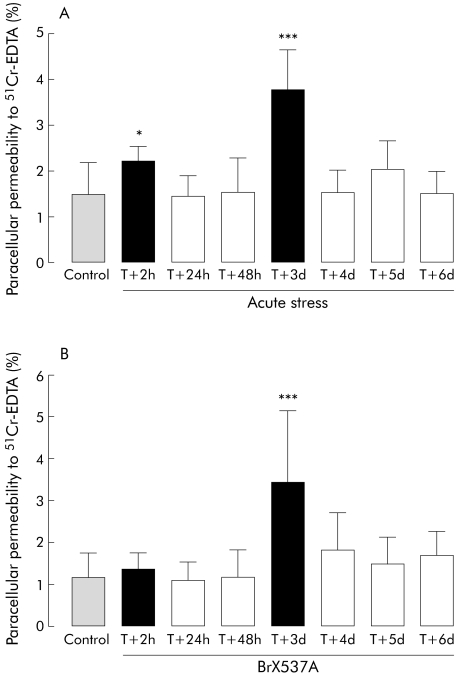
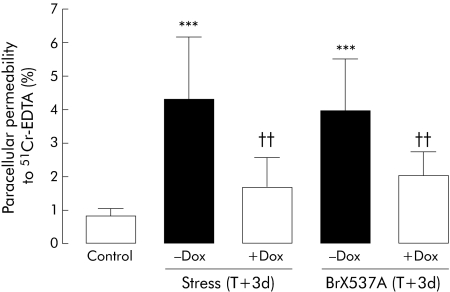
CPP was measured daily for six days after acute stress or sham stress (control) (fig 1A). A short term increase (46%) was observed from two to four hours after the end of the two hour stress session (2.2 (0.4)% v 1.5 (0.7)%; p<0.05), while 24 and 48 hour values did not differ significantly from controls (p>0.05). However, 72 hours after the stress session, 51Cr‐EDTA paracellular permeability was again significantly increased (153%) compared with the control (sham) group (3.8 (0.8)% v 1.5 (0.7)%, respectively; p<0.001). From four to six days after acute stress, 51Cr‐EDTA permeability was similar to that observed in sham animals (fig 1A). Sham stressed mice did not have permeability changes at day 3 (1.7 (0.7)%). Doxantrazole pretreatment inhibited the effects of stress observed after 72 hours (fig 2) (1.7 (0.8)% for doxantrazole+stress v 4.3 (1.8)% for stress).
Figure 1 (A) Acute stress: colonic paracellular permeability (CPP) from two hours to six days after an acute stress session to Swiss mice. Control group was sham stressed mice killed at T+2 h. (B) BrX‐537A: CPP from two hours to six days after BrX‐537A injection (2 mg/kg intraperitoneally) to Swiss mice. Control group was BrX‐537A vehicle (DMSO) injected mice, killed at T+2 h. Values are mean (SD) per cent 51Cr‐EDTA crossing the colonic wall. *p<0.05, ***p<0.001 compared with control value; n = 8 per group. T = time after single stress session or BrX‐537A perfusion.
Figure 2 Colonic paracellular permeability 72 hours after an acute stress session or BrX‐537A injection (2 mg/kg intraperitoneally) and after pretreatment with the mast cell stabiliser doxantrazole (Dox 10 mg/kg intraperitoneally). Doxantrazole was administered 30 minutes before the stress session or BrX‐537A injection. Values are mean (SD) per cent 51Cr‐EDTA crossing the epithelial barrier. ***p<0.001 compared with controls; ††p<0.01 compared with doxantrazole vehicle; n = 8 per group. T = time after single stress session or BrX‐537A perfusion.
Effects of mast cell degranulation on CPP
CPP was not affected 2, 24, or 48 hours after BrX‐537A compared with vehicle but CPP was increased (183%) 72 hours after BrX‐537A treatment (fig 1B) (3.4 (1.7)% v 1.2 (0.6)% for control; p<0.001). Permeability recovered to normal by day 4 (1.8 (0.8)% v 1.2 (0.6)%). DMSO treated animals sacrificed at day 3 showed normal permeability (1.6 (0.5)%). Doxantrazole pretreatment inhibited the delayed effects of BrX‐537A seen on day 3 (fig 2).
IFN‐γ in stress and BrX‐537A induced increase in CPP
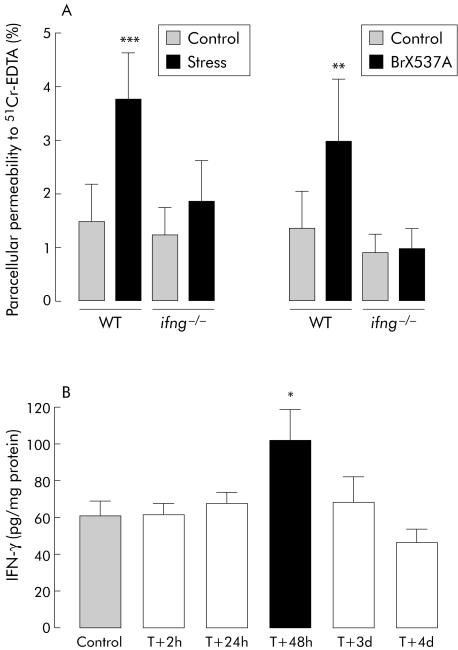
In IFN‐γ deficient mice (fig 3A), stress did not modify colonic permeability after 72 hours compared with sham stressed animals (1.8 (0.8)% v 1.3 (0.5)%, respectively; NS). Similarly, 72 hours after BrX‐537A administration, CPP was not affected whereas an increase was noted in wild‐type mice (1.4 (0.7)% v 3.0 (1.2)%; p<0.01). In Swiss mice, BrX‐537A increased IFN‐γ protein expression significantly in colonic mucosa 48 hours after treatment (101.9 (16.7) v 60.9 (7.6) pg/mg of total proteins; p<0.05); values returned to normal by day 4 (fig 3B).
Figure 3 (A) Colonic paracellular permeability measured 72 hours after an acute stress session or BrX‐537A injection (2 mg/kg intraperitoneally) in B6‐Ifng‐−/− mice and their wild‐type (WT) control C57BL/6 mice. Values are mean (SD) per cent 51Cr‐EDTA crossing the epithelial barrier. **p<0.01 and ***p<0.001 compared with control value; n = 8 per group. (B) Time dependent effects of BrX‐537A injection (2 mg/kg intraperitoneally) on interferon γ (IFN‐γ) expression (pg of IFN‐γ per mg of total protein). Values are mean (SEM). *p<0.05 compared with vehicle values; n = 8 per group. T = time after BrX‐537A perfusion.
Colonic morphological changes after mast cell degranulation
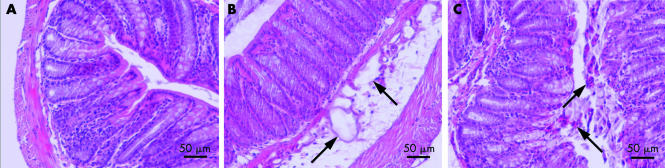
Twenty hours after BrX‐537A administration, several typical colonic epithelial alterations were observed, such as the presence of submucosal oedema (fig 4B), disorganisation of the epithelium with an increase in immunocyte presence and lymphoid follicles (fig 4C), and an increase in luminal mucus (data not shown). A semiquantitative histological damage score was 1–2 at two hours after BrX‐537A but was 3–4 at T+72 hours.
Figure 4 Colon section after BrX‐537A injection (2 mg/kg intraperitoneally). Samples were collected 24, 48, and 72 hours after treatment, fixed with Duboscq‐Brazil solution, and paraffin included. Control section (A) showed a normal colon. However, 72 hours after BrX‐537A injection, alterations appeared on several samples, such as the presence of submucosal oedema (B), disorganisation of the epithelium with an increased presence of immunocytes and lymphoid follicles (C), and an increase in luminal mucus (data not shown). A semiquantitative histological damage score after BrX‐537A was 1–2 at two hours but reached 3–4 at T+72 hours. n = 5 per group.
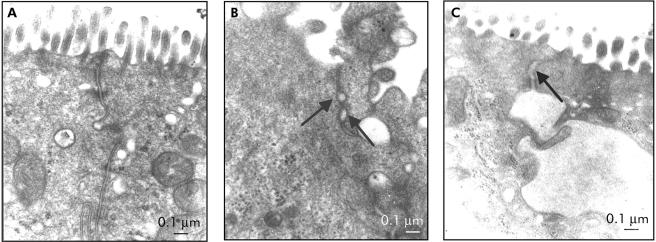
TEM observation of TJ opening state after stress or BrX‐537A
Structural observations of TJs were performed 72 hours after stress or BrX‐537A administration. In most of the samples, we observed alterations not seen in control conditions (fig 5A). In fig 5B and 5C, black arrows show the opening of TJs (>20 nm). Below these dilatations, highly dilated intercellular spaces corresponding to intermediary intercellular junctions are observed. The percentage of opened TJs reached 14.3% and 13.2% 72 hours after BrX‐537A or stress session, respectively, this percentage being more than doubled compared with vehicle (6.4%).
Figure 5 Opening state of tight junctions (TJs) observed by transmission electronic microscopy (TEM). All specimens were prepared according to standard protocols. Seventy two hours after (B) stress or (C) BrX‐537A injection (2 mg/kg intraperitoneally), TEM showed opening of TJs (>20 nm, indicated by the black arrows); below the black arrows, other dilatations do not correspond to TJs.
TJ protein expression
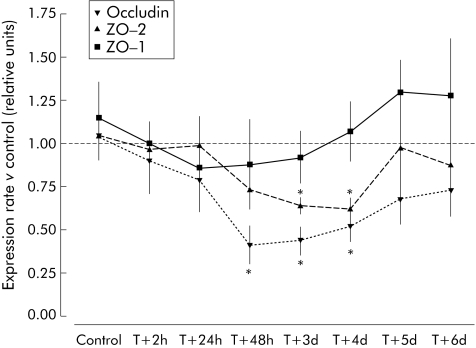
At three day after a single stress session, expression levels of TJ proteins were affected. Indeed, expression of mRNAs encoding occludin and ZO‐2 were decreased by 60% (0.44 (0.08) v 1.04 (0.11) arbitrary units; p<0.05) and 43% (0.62 (0.06) v 1.05 (0.14); p<0.05), respectively, compared with controls. Expression of ZO‐1 mRNA was unchanged (fig 6).
Figure 6 Time related changes in mRNA expression of ZO‐1, ZO‐2, and occludin after acute stress in mice. Values are mean (SEM) and expressed as arbitrary units (normally expression is 1 arbitrary unit relative to glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene expression). *p<0.05 compared with GAPDH mRNA expression; n = 8 per group. T = time after single stress session.
Alteration of colonocyte differentiation
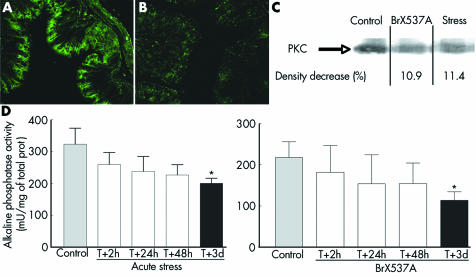
Under control conditions, PKCζ was present in the surface mucosa of colonic epithelium (fig 7A) but 72 hours after BrX‐537A treatment PKCζ immunoreactivity was decreased in the upper crypt region (fig 7B). Similarly, western blotting analysis of PKCζ (fig 7C) showed lower expression 72 hours after the acute stress session or BrX‐537A (11.4% and 10.9% of density decrease compared with controls).
Figure 7 (A, B) Altered differentiation of colonocytes 72 hours after BrX‐537A injection (2 mg/kg intraperitoneally). Under control conditions, proliferating cells were confined to the lower two thirds of the crypt (not shown) while differentiating and mature cells were found in the upper crypt region and on the flat surface mucosa (A). Treatment with BrX‐537A markedly decreased protein kinase C ζ (PKCζ) labelling in the upper crypt region (B). Western blot (C) performed on colons from sham stressed, stressed, and BrX‐537A treated (2 mg/kg intraperitoneally) mice. PKCζ levels were lower in stressed animals and BrX‐537A treated mice, showing that PKCζ differentiation is reduced after stress exposure. The experiments are representative of three independent analyses (n = 3 for each group). Alkaline phosphatase activity (mean (SEM), n = 8) was significantly reduced 72 hours after stress (D, left panel) or BrX‐537A (D, right panel). *p<0.05 v control.
AP activity decreased continuously from the first day after BrX‐537A treatment (fig 7D), with maximal significant reduction after 72 hours (113.7 (20.8) v 217.8 (38.0) mU/mg protein for controls; p<0.05). A similar decrease in AP activity was seen three days after an acute stress session.
Discussion
In this study, we demonstrated that a single acute stress session had a similar delayed (three days) increase in CPP as that observed with repeated daily stress. This indicates that chronicity is not a prerequisite for the long term influence of stress on the colonic epithelial barrier, as previously suggested.17 We also demonstrated that mast cells have a “priming early” role in this delayed effect by initiating alterations in colonocytes differentiation. Indeed, we found that these alterations mainly consisted of decreased expression of mRNAs encoding for TJ proteins such as ZO‐2 and occludin and we observed decreased expression of two markers of differentiation (PKCζ and AP) 72 hours after acute stress or mast cell degranulation. Our studies also extend previous finding by showing that incomplete or delayed differentiation of colonocytes caused by inflammation leads to an increase in intercellular space and CPP.27
Role of mast cells and IFN‐γ
Defective epithelial barrier function may allow uptake of luminal antigens that stimulate an immune/inflammatory response. Previous studies have shown that stress induces long term alterations in gut permeability but this effect was thought to be linked to the 4–7 days of repeated stressful stimulus.15 Indeed, repetitive exposure to water avoidance stress induces sustained abnormalities in colonic epithelial barrier function in rats.28 We have previously demonstrated that repeated stress exposure leads to an increase in CPP and identified IFN‐γ as a major mediator of this effect.17 We report here that delayed effects are seen 72 hours after the end of an acute stress session and that a single treatment with a mast cell degranulator (BrX‐537A) reproduces this delayed effect. Historically, classification of rodent mast cell subtypes has been based on phenotypic differences between connective tissue mast cells, particularly of the skin and peritoneal cavity, and mucosal mast cells, particularly of the intestinal lamina propria.29 The two types of mast cells also differ in their responsiveness to secretagogues Indeed, mucosal mast cells are refractory to stimulation by the compound 48/80 whereas BrX‐537A can act on both types of mast cells to release histamine.30,31 An early and short lasting effect (2–4 hours) was seen at the end of an acute stress session but this effect was not reproduced with BrX‐537A, suggesting that mast cells are not involved in this early effect of stress, contrasting with the delayed effect of stress reproduced by BrX‐537A.
Mast cells are considered to be key cells in the effect of stress on colonic mucosal activation. Indeed, in mast cell deficient Ws/Ws rats subjected to water avoidance stress (one hour/day) for five days, none of the ultrastructural abnormalities of the epithelium seen in normal rats were present.15 Mast cell degranulation is responsible for this CPP increase and several hypotheses may explain this delayed effect. Firstly, tryptase constitutes the major protein released on mucosal mast cell degranulation and can activate PAR‐2, a receptor highly expressed throughout the gastrointestinal tract. Tryptase is released in the setting of inflammation and allergic response into the intestinal lumen and vasculature. In rats, intracolonic infusion of PAR‐2 activating peptide leads to intestinal inflammation and impairment of intestinal permeability.32 Moreover, we also showed that CD4+/CD8+ T cells are involved in stress induced impairment of the colonic mucosal barrier as stress did not induce any modification of CPP in SCID mice.17 Thus our results suggest a specific link between mast cell activation and IFN‐γ secretion by CD4+/CD8+ T cells, as described in vitro.33 Mast cells also release nerve growth factor which can play a role in the stress induced increase in CPP.34
A 2–3 day delay has already been described in vitro for the effects of IFN‐γ on T84 cells to decrease levels of ZO‐1 and to alter apical actin organisation, which leads to disorganisation of TJs and increased permeability.18 In the present study, we observed a decrease in ZO‐2 and occludin mRNA expression after stress, occurring at days 3 and 4. Despite the fact that we did not observe CPP modification at day 4, we suggest that expression of other TJ proteins, such as claudins or ZO‐1, may be modulated in order to recover the integrity of TJs. In the present study, we observed a slight, but not significant, increase in ZO‐1 expression at day 4.
The CPP increase occurred 72 hours after the stimulus (stress or BrX‐537A) whereas IFN‐γ protein expression was highest at T+48 hours. This agrees with in vitro studies showing a deleterious effect of IFN‐γ on barrier integrity several days after exposure to the cytokine.35 Mast cells do not affect permeability in IFN‐γ deficient mice, confirming that IFN‐γ acts distally to mast cell stimulation to affect CPP.17 In our study, IFN‐γ protein expression increased 48 hours after BrX‐537A injection. A membrane permeant inhibitor of myosin‐like chain kinase (PIK) can reverse the increase in permeability induced by IFN‐γ and TNF‐α.36 This observation suggests that IFN‐γ and TNF‐α decrease intestinal epithelial barrier function through activation of myosin‐like chain kinase.17 Therefore, IFN‐γ receptor could be the activator of the intracellular pathway leading to perijunctional actinomyosin ring contraction and TJ opening.37
Alterations in epithelial cell differentiation
Whatever the method used to induce stress, the delay in CPP effect may be linked to alterations in colonocyte differentiation. PKCζ, a differentiation marker of colonocyte, is expressed more at the upper part of the colon crypt.25 Other widely used markers such as lactase‐phloridzin hydrolase or sucrase‐isomaltase were not used in the present study as they are more specific for the small intestine and cell lines. After mast cell degranulation by BrX‐537A, PKCζ is expressed less 72 hours after treatment, indicating alteration of the colonocyte differentiation process occurring concomitantly with downregulation of ZO‐2 and occludin, at the time of the critical increase in CPP. AP shows similar results—that is, a decrease in activity after an acute stress session or BrX‐537A treatment. These two markers have been validated as describing the proliferation/differentiation process in colonocytes.26,38 This three day delay between the stimulus and observed alterations in colonocyte differentiation is in agreement with all published data, showing that it corresponds to the time of epithelial cell turnover.39 However, IFN‐γ is unlikely to exert a direct effect on colonocyte differentiation as its secretion appears 48 hours after the stress, while colonocytes present a three day turnover.
The increase in CPP may be responsible for bacterial translocation which in turn stimulates the colonic mucosal immune system.17 Repeated neonatal maternal deprivation in rats also promotes bacterial translocation into the liver, spleen, and mesenteric lymph nodes, associated with an increase in CPP in adult life.34
Increased CPP is a common event in gastrointestinal diseases. Previous studies have described the exacerbating effect of stress for intestinal diseases. Intestinal permeability is increased in patients with CD and in a significant subset of their first degree relatives.4,5 A prospective study on UC patients found that long term psychological stress increases the risk of exacerbation and we have provided evidence that stress is associated with a delayed increase in CPP.1 In IBS, auditory stress modulates visceral perception and autonomic responses associated with emotional responses, including anxiety and anger.40 Moreover, increase in intestinal and/or CPP has been described in IBS patients.8,41 Although the effect of stress in IBD or IBS evolution is largely suspected, the activated pathways and their respective contribution towards altering the immune balance of the mucosal flora remain largely unknown. Recently, colonic mucosal immune stimulation has been largely confirmed to biopsies from IBS patients. This may contribute to the genesis of visceral hypersensitivity.8,42
In summary, our results provide original data showing that an acute stress has a delayed effect on CPP, primarily through mast cell activation and IFN‐γ production by altering TJ protein expression and colonocyte differentiation. This suggests a key role for mast cells and IFN‐γ in induction of transient phenotypic changes of colonocyte with possible implications in the genesis of functional bowel disorders.
Acknowledgements
The authors thank Institut Fédératif de Recherche Claude De Preval (INSERM IFR 30, Toulouse, France) for the B6‐Ifng−/− mice, and Bernard Joseph for technical assistance. Bromolasalocid ethanolate (BrX‐537A) was a gift from Hoffmann‐La Roche (Basel, Switzerland). Doxantrazole was obtained from Wellcome (Beckenham, UK). This work was supported by an institutional grant from INRA.
Abbreviations
TJs - tight junctions
IBD - inflammatory bowel disease
CD - Crohn's disease
UC - ulcerative colitis
IFN‐γ - interferon γ
AP - alkaline phosphatase
PKCζ - protein kinase C ζ
CPP - colonic paracellular permeability
IBS - irritable bowel syndrome
TNF‐α - tumour necrosis factor α
PBS - phosphate buffered saline
RT‐PCR - reverse transcription‐polymerase chain reaction
TEM - transmission electron microscopy
Footnotes
Conflict of interest: None declared.
References
- 1.Levenstein S, Prantera C, Varvo V.et al Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol 2000951213–1220. [DOI] [PubMed] [Google Scholar]
- 2.Bitton A, Sewitch M J, Peppercorn M A.et al Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am J Gastroenterol 2003982203–2208. [DOI] [PubMed] [Google Scholar]
- 3.Mittermaier C, Dejaco C, Waldhoer T.et al Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18‐month follow‐up study. Psychosom Med 20046679–84. [DOI] [PubMed] [Google Scholar]
- 4.May G R, Sutherland L R, Meddings J B. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology 19931041627–1632. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt J, Vogelsang H, Hubl W.et al Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 19933411437–1439. [DOI] [PubMed] [Google Scholar]
- 6.Arslan G, Atasever T, Cindoruk M.et al (51)CrEDTA colonic permeability and therapy response in patients with ulcerative colitis. Nucl Med Commun 200122997–1001. [DOI] [PubMed] [Google Scholar]
- 7.Gassler N, Rohr C, Schneider A.et al Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 2001281G216–G228. [DOI] [PubMed] [Google Scholar]
- 8.Spiller R. C, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post‐dysenteric irritable bowel syndrome. Gut 200047804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ait‐Belgnaoui A, Bradesi S, Fioramonti J.et al Acute stress‐induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 2005113141–147. [DOI] [PubMed] [Google Scholar]
- 10.Saunders P R, Hanssen N P, Perdue M H. Cholinergic nerves mediate stress‐induced intestinal transport abnormalities in Wistar‐Kyoto rats. Am J Physiol 1997273G486–G490. [DOI] [PubMed] [Google Scholar]
- 11.Meddings J B, Swain M G. Environmental stress‐induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 20001191019–1028. [DOI] [PubMed] [Google Scholar]
- 12.Qiu B S, Vallance B A, Blennerhassett P A.et al The role of CD4+ lymphocytes in the susceptibility of mice to stress‐induced reactivation of experimental colitis. Nat Med 199951178–1182. [DOI] [PubMed] [Google Scholar]
- 13.Kiliaan A J, Saunders P R, Bijlsma P B.et al Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol 1998275G1037–G1044. [DOI] [PubMed] [Google Scholar]
- 14.Saunders P R, Kosecka U, McKay D M.et al Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol 1994267G794–G799. [DOI] [PubMed] [Google Scholar]
- 15.Santos J, Yang P C, Soderholm J D.et al Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 200148630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soderholm J D, Yang P C, Ceponis P.et al Chronic stress induces mast cell‐dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 20021231099–1108. [DOI] [PubMed] [Google Scholar]
- 17.Ferrier L, Mazelin L, Cenac N.et al Stress‐induced disruption of colonic epithelial barrier: role of interferon‐gamma and myosin light chain kinase in mice. Gastroenterology 2003125795–804. [DOI] [PubMed] [Google Scholar]
- 18.Youakim A, Ahdieh M. Interferon‐gamma decreases barrier function in T84 cells by reducing ZO‐1 levels and disrupting apical actin. Am J Physiol 1999276G1279–G1288. [DOI] [PubMed] [Google Scholar]
- 19.Mankertz J, Tavalali S, Schmitz H.et al Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci 20001132085–2090. [DOI] [PubMed] [Google Scholar]
- 20.Francoeur C, Escaffit F, Vachon P H.et al Proinflammatory cytokines TNF‐alpha and IFN‐gamma alter laminin expression under an apoptosis‐independent mechanism in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2004287G592–G598. [DOI] [PubMed] [Google Scholar]
- 21.Dalton D K, Pitts‐Meek S, Keshav S.et al Multiple defects of immune cell function in mice with disrupted interferon‐gamma genes. Science 19932591739–1742. [DOI] [PubMed] [Google Scholar]
- 22.Livak K J, Schmittgen T D. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)). Methods 200125402–408. [DOI] [PubMed] [Google Scholar]
- 23.Neurath M F, Fuss I, Kelsall B L.et al Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 19951821281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soderholm J D, Olaison G, Peterson K H.et al Augmented increase in tight junction permeability by luminal stimuli in the non‐inflamed ileum of Crohn's disease. Gut 200250307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verstovsek G, Byrd A, Frey M R.et al Colonocyte differentiation is associated with increased expression and altered distribution of protein kinase C isozymes. Gastroenterology 199811575–85. [DOI] [PubMed] [Google Scholar]
- 26.Gamet L, Daviaud D, Denis‐Pouxviel C.et al Effects of short‐chain fatty acids on growth and differentiation of the human colon‐cancer cell line HT29. Int J Cancer 199252286–289. [DOI] [PubMed] [Google Scholar]
- 27.Ramage J K, Hunt R H, Perdue M H. Changes in intestinal permeability and epithelial differentiation during inflammation in the rat. Gut 19882957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders P R, Santos J, Hanssen N P.et al Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci 200247208–215. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe D D, Baram D, Mekori Y A. Mast cells. Physiol Rev 1997771033–1079. [DOI] [PubMed] [Google Scholar]
- 30.Fargeas M J, Theodourou V, Fioramonti J.et al Relationship between mast cell degranulation and jejunal myoelectric alterations in intestinal anaphylaxis in rats. Gastroenterology 1992102157–162. [DOI] [PubMed] [Google Scholar]
- 31.Pearce F L, Befus A D, Gauldie J.et al Mucosal mast cells. II. Effects of anti‐allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol 19821282481–2486. [PubMed] [Google Scholar]
- 32.Cenac N, Coelho A M, Nguyen C.et al Induction of intestinal inflammation in mouse by activation of proteinase‐activated receptor‐2. Am J Pathol 20021611903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Pater‐Huijsen F L, de Riemer M J, Reijneke R M.et al Products from human mast cell line cells enhance the production of interferon‐gamma by CD8+ and CD4+ T cells. Immunology 200210611–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreau F, Cartier C, Ferrier L.et al Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 2004127524–534. [DOI] [PubMed] [Google Scholar]
- 35.Nusrat A, Turner J R, Madara J L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells, Am J Physiol Gastrointest Liver Physiol 2000279G851–G857. [DOI] [PubMed] [Google Scholar]
- 36.Zolotarevsky Y, Hecht G, Koutsouris A.et al A membrane‐permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 2002123163–172. [DOI] [PubMed] [Google Scholar]
- 37.Bruewer M, Luegering A, Kucharzik T.et al Proinflammatory cytokines disrupt epithelial barrier function by apoptosis‐independent mechanisms. J Immunol 20031716164–6172. [DOI] [PubMed] [Google Scholar]
- 38.Orchel A, Dzierzewicz Z, Parfiniewicz B.et al Butyrate‐induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci 200550490–498. [DOI] [PubMed] [Google Scholar]
- 39.Fonti R, Latella G, Bises G.et al Human colonocytes in primary culture: a model to study epithelial growth, metabolism and differentiation. Int J Colorectal Dis 1994913–22. [DOI] [PubMed] [Google Scholar]
- 40.Dickhaus B, Mayer E A, Firooz N.et al Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol 200398135–143. [DOI] [PubMed] [Google Scholar]
- 41.Marshall J K, Thabane M, Garg A X.et al Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther 2004201317–1322. [DOI] [PubMed] [Google Scholar]
- 42.Barbara G, Stanghellini V, De Giorgio R.et al Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004126693–702. [DOI] [PubMed] [Google Scholar]