Abstract
Background and aims
Conservative therapy for patients with acute colonic pseudo obstruction (Ogilvie's syndrome) may be successful initially but relapses are common. The aim of the present study was to evaluate the effect of polyethylene glycol (PEG) electrolyte balanced solution on the relapse rate of the syndrome after initial resolution with neostigmine or endoscopic decompression.
Patients and methods
The study was performed on 30 consecutive patients who presented with abdominal distension and radiographic evidence of colonic dilation, with a caecal diameter ⩾10 cm, that resolved conservatively. Patients then were randomised to receive daily 29.5 g of PEG (n = 15) or similar placebo (n = 15). Patients were monitored daily for a seven day period for stool and flatus evacuations, and colonic diameter on abdominal radiographs. Administration of the test solutions and assessment of patient symptoms and x rays were performed in a blinded fashion. A caecal diameter ⩾8 cm with a concomitant ⩾10% increase after initial successful therapeutic intervention was considered as a relapse and these patients, after a second therapeutic intervention, were eligible to receive open label PEG.
Results
Twenty five patients received neostigmine as the initial therapeutic intervention which resulted in resolution of colonic dilation in 88% of cases. Eight patients had successful endoscopic decompression. Five (33.3%) patients in the placebo group had recurrent caecal dilation compared with none in the PEG group (p = 0.04). Therapy with PEG resulted in a significant increase in stool and flatus evacuations (p = 0.001 and 0.032, respectively) as well as in a significant decrease in the diameter of caecum, ascending and transverse colon, and abdominal circumference (p = 0.017, 0.018, 0.014, and 0.008, respectively).
Conclusions
Administration of PEG in patients with Ogilvie's syndrome after initial resolution of colonic dilation may increase the sustained response rate after initial therapeutic intervention.
Keywords: colonic pseudo obstruction, Ogilvie's syndrome, polyethylene glycol, constipation
Acute colonic pseudo obstruction (Ogilvie's syndrome) is a rare disorder in which massive dilation of the colon develops without any mechanical obstruction. It usually occurs in hospitalised or institutionalised patients with serious underlying medical or surgical conditions.1 Even though the pathogenesis of the syndrome is not completely understood, the most popular theory supports an imbalance in autonomic innervation of the colon which leads to excessive parasympathetic suppression or sympathetic stimulation.1,2 It has been proposed that transient impairment of the sacral plexus may cause atony of the distal large bowel and functional obstruction with proximal dilation.3 This is supported by the observation in many patients that a cut off at the splenic flexure is present on abdominal radiographs.3,4,5
Ischaemia and perforation are the feared complications of the syndrome. Spontaneous perforation has been reported in 3–15% of patients with a mortality rate of 50% or higher.6 The rate of ischaemia and perforation rapidly increases when the duration of distension exceeds six days3,7 while active intervention is required when caecal diameter exceeds 10 cm.8 In these cases neostigmine is considered to be the agent of choice.8 It has been shown that neostigmine induces rapid resolution in up to 90% of cases.9,10 If there is no improvement after neostigmine administration, urgent endoscopic decompression with tube placement is indicated.8,11 However, it is well documented that some patients experience early recurrence after initial resolution with neostigmine or colonoscopic decompression. Even though there are no prospective trials, the rate of recurrence is estimated to be approximately 6–39%.2,10 These patients are particularly difficult to manage as neostigmine may be less effective9 and continuous endoscopic intervention carries significant risks.
In routine clinical practice many physicians prescribe osmotic laxatives after resolution of colonic dilation in an effort to restore daily stool and flatus evacuations and to minimise the risks of early recurrence of the syndrome. To our knowledge, this approach has not yet been validated. Polyethylene glycol (PEG) electrolyte balanced solutions are osmotic laxatives frequently used for the management of chronic functional constipation.12,13,14 PEG opposes the dehydration of bowel contents, leading to modification of stool consistency and increased faecal bulk. This in turn stretches muscle fibres in the bowel wall and probably triggers myogenic peristalsis. Increased retention of water in the colon lubricates and softens stools, and allows comfortable bowel action. PEG passes virtually unchanged through the whole gastrointestinal tract, including the colon. It is not metabolised, and its effect is not dependent on the state of the colonic microflora.15 It may also induce acceleration of colonic transit through the left colon and rectum.14 These effects of PEG may be of particular importance in patients with Ogilvie's syndrome, after resolution of colonic dilation.
In the present study, we tried to evaluate prospectively the effect of PEG on patients with acute colonic pseudo obstruction after resolution of colonic dilation with neostigmine or colonoscopic decompression.
Patients and methods
Definitions and patient selection
During a three year period, patients were recruiting for the study from inpatient medical and surgical wards of Athens Naval and Veterans Hospital and “Evangelismos” General Hospital. Patients were initially considered as candidates for entrance into the study if they had a diagnosis of acute colonic pseudo obstruction, with a caecal diameter ⩾10 cm on abdominal radiographs that failed to improve within 24 hours of conservative management. Conservative treatment included administering nothing by mouth, nasogastric suction, intravenous fluid and electrolyte replacement, and discontinuation (when possible) of any drugs that could adversely affect colonic motility, such as narcotics and anticholinergic agents.
Acute colonic pseudo obstruction was defined as marked colonic distension without mechanical obstruction. Mechanical obstruction was ruled out by the finding of air throughout the colonic segments, including the rectosigmoid, on plain abdominal radiographs and abdominal computed tomography (CT) scans. When air was not demonstrable in the left colon, mechanical obstruction was ruled out by radiographic contrast enemas.
Resolution of the syndrome was defined as a ⩾10% reduction in abdominal distension with a ⩾20% concomitant reduction in caecal diameter on abdominal radiographs within three hours of neostigmine administration or immediately after colonoscopic decompression.
Relapse (treatment's failure) was defined as a caecal diameter ⩾8 cm with a concomitant ⩾10% increase on abdominal radiographs with respect to the value that each patient had after initial resolution of the syndrome.
Exclusion criteria included failure to induce a resolution in colonic dilation after neostigmine administration or endoscopic decompression, signs of bowel perforation with peritoneal signs on physical examination or free air on radiographs or abdominal CT scans, a history of colon cancer or partial colonic resection, pregnancy, or lactation.
The study protocol was approved by the local ethics committees and written informed consent forms were obtained from all subjects before their entrance into the study.
Neostigmine administration and endoscopic decompression
All patients who failed to improve within 24 hours of conservative therapy received neostigmine 2 mg intravenously over a period of 3–5 minutes unless they had: a baseline heart rate <60 beats per minute, systolic blood pressure <90 mm Hg, active bronchospasm requiring medication, or serum creatinine concentration of more than 3 mg/dl (265 μmol/l). All patients were monitored by electrocardiography, atropine was available at the bedside, and 1 mg was given intravenously as needed for symptomatic bradycardia. Patients were advised to remain supine for at least 60 minutes after the injection. Vital signs were recorded immediately before the injection, every five minutes for 30 minutes after the injection, and every three hours thereafter. Maximal abdominal circumference and diameter of the caecum, and ascending and transverse colon on plain abdominal radiographs were measured before and three hours after injection.
All patients who failed to respond within three hours of neostigmine administration or had any of the previously mentioned contraindications underwent colonoscopic decompression with tube placement over a guidewire, under fluoroscopic control. The procedure was considered successful if the ascending colon was reached and the tube was placed in the ascending colon or caecum.11 After endoscopy, decompression tubes were placed on low intermittent suction and flushed with 20–30 ml of normal saline solution every 2–4 hours to maintain patency. All endoscopies were performed by the same endoscopist (SS). Maximal abdominal circumference and diameter of the caecum and ascending and transverse colon on plain abdominal radiographs were measured before and immediately after colonoscopy.
Randomisation
After initial resolution of colonic dilation, according to the previously described criteria, all patients were blindly randomised, using the closed envelope draw method, to receive daily administration of either 29.5 g of PEG (sach Klean‐Prep; Pirex Ltd, Norgin, Ireland) in 500 ml of water, in two doses, or a similar placebo. The placebo consisted of flour, sugar, and vanilla powder, manufactured by the Greek Naval Pharmacy. Each preparation was provided in identical sachets. Administration was per mouth or via a nasogastric tube. Patients were monitored daily immediately after initial resolution and for a seven day period, for stool and flatus evacuations, maximal abdominal circumference, and diameter of the caecum, and ascending and transverse colon on plain abdominal radiographs. Administration of the test solutions and assessment of the patient's symptoms and x rays were performed in a blinded fashion.
In patients who had a relapse, treatment failure was established and neostigmine was administered as previously described. If the patient had failed to improve after neostigmine administration, endoscopic decompression was performed. After resolution of colonic dilation, these patients were eligible to receive open label PEG.
Definitions of end points
The primary end point of the study was relapse rate of acute colonic pseudo obstruction after initial resolution with neostigmine or endoscopic decompression.
Secondary end points were: (i) efficacy and safety of neostigmine administration in patients with acute colonic pseudo obstruction, (ii) feasibility, safety, and efficacy of colonoscopic decompression with tube placement in patients with acute colonic pseudo obstruction, not responding to neostigmine administration, and (iii) safety of PEG administration in patients with acute colonic pseudo obstruction after resolution of colonic dilation.
All adverse events were coded according to the World Health Organisation dictionary. For each adverse event, the causal relationship with the study drug and the event's severity were noted.
Statistical analysis
The sample size calculation was based on a previous report10 showing that the incidence of recurrent colonic dilation after neostigmine administration is approximately 40%. Assuming that the risk would be reduced to 10% by administration of a PEG based laxative, approximately 30 patients would be required for each group with a two tailed test to achieve a β value of 0.2 and an α error of 5%. An interim analysis was performed after recruitment of 50% of patients.
Quantitative variables were expressed as mean (SEM) and qualitative variables as number (%). Each continuous parameter between the two treatment groups was analysed using the two sample Student's t test. Categorical data were examined using the χ2 test with Yates' correction or Fisher's exact test, as appropriate. A p value ⩽0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS 10.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
Results
Primary end point
During the study period 32 consecutive patients presented with Ogilvie's syndrome. In two patients neostigmine administration and endoscopic decompression failed to induce resolution of colonic dilation and, as per the protocol, were excluded. These patients were found at laparotomy to have ischaemic colonic necrosis that required bowel resection.
The remaining 30 patients were randomised equally to one of the two groups. Fourteen patients (47%) had undergone recent surgery (three total hip replacement, three total knee replacement, three prostatectomy, two hysteretomy, one amputation of a leg, one lumbar laminectomy, and one femoral fracture with internal fixation) while the rest had Parkinson's disease (three patients), previous cerebrovascular accident (three patients), multiple sclerosis (two patients), previous spinal cord injury with paralysis (two patients), acute respiratory failure (two patients) with chronic obstructive pulmonary disease and respiratory infection, myocardial infarction (one patient), Alzheimer's disease (one patient), acute pancreatitis (one patient), and metastatic lung cancer (one patient). The characteristics of the patients at diagnosis are presented in table 1. The two groups were similar in age, sex, history of constipation (defined as less than three evacuations per week during the last six months), previous use of narcotics and anticholinergics medications or laxatives, mechanical ventilation, history of recent surgical procedures, white blood cell count, abdominal circumference, colonic diameters on plain abdominal radiographs, and type of initial therapeutic intervention.
Table 1 Characteristics of the patients in the polyethylene glycol (PEG) and placebo groups at presentation.
| Characteristic | PEG (n = 15) | Placebo (n = 15) | p Value |
|---|---|---|---|
| Sex (M/F) | 8/7 | 8/7 | 0.99 |
| Age (y) | 75.8 (2.0) | 77.5 (2.4) | 0.59 |
| Neostigmine (n) | 12 | 13 | 0.99 |
| Endoscopic decompression (n) | 5 | 3 | 0.68 |
| Mechanical ventilation (n) | 2 | 3 | 0.99 |
| Narcotics, anticholinergics (n) | 10 | 11 | 0.99 |
| Recent surgery (n) | 8 | 6 | 0.71 |
| History of constipation (n) | 4 | 3 | 0.99 |
| Previous use of laxatives (n) | 10 | 12 | 0.68 |
| Caecal diameter (cm) | 12.5 (0.4) | 13.3 (0.3) | 0.18 |
| Ascending colon diameter (cm) | 9.8 (0.4) | 10.4 (0.3) | 0.29 |
| Transverse colon diameter (cm) | 8.5 (0.3) | 9.0 (0.2) | 0.25 |
| Abdominal circumference (cm) | 115.5 (2.3) | 116.6 (2.2) | 0.73 |
| White blood cells (×103/mm3) | 17.1 (0.7) | 16.2 (0.8) | 0.45 |
| Contrast enema (n) | 5 | 6 | 0.99 |
Values are mean (SEM).
The two groups were also comparable after initial therapeutic intervention in terms of abdominal circumference and colonic diameters on plain abdominal radiographs (table 2).
Table 2 Characteristics of the patients in the polyethylene glycol (PEG) and placebo groups after initial therapeutic intervention.
| Characteristic | PEG | Placebo | p Value |
|---|---|---|---|
| Caecal diameter (cm) | 6.8 (0.6) | 7.1 (0.6) | 0.75 |
| Ascending colon diameter (cm) | 5.6 (0.4) | 5.8 (0.5) | 0.78 |
| Transverse colon diameter (cm) | 5.1 (0.4) | 5.0 (0.4) | 0.89 |
| Abdominal circumference (cm) | 99.8 (1.8) | 100.8 (1.4) | 0.69 |
| Neostigmine non‐responders (n) | 3 | 2 | 0.99 |
| Atropine (n) | 1 | 1 | 0.99 |
Values are mean (SEM).
Characteristics of the patients at the end of the treatment period (seven days after randomisation) are presented in table 3. Therapy with PEG resulted in a significant increase in stool and flatus evacuations (p = 0.001 and 0.032, respectively) as well as in a significant decrease in the diameter of the caecum, ascending and transverse colon, and abdominal circumference (p = 0.017, 0.018, 0.014, and 0.008, respectively). During the follow up period, five (33.3%) patients in the placebo group, initially treated with neostigmine, relapsed compared with none in the PEG group (p = 0.04). Mean (SEM) caecal diameter on recurrence was 10 (0.7) cm (range 9–11) while mean (SEM) time until establishment of recurrent colonic dilation was 1.8 (0.8) days.
Table 3 Characteristics of the patients in the polyethylene glycol (PEG) and placebo groups at the end of the treatment period.
| Characteristic | PEG | placebo | p Value |
|---|---|---|---|
| Caecal diameter (cm) | 3.4 (0.2) | 5.6 (0.8) | 0.017 |
| Ascending colon diameter (cm) | 3.1 (0.1) | 4.6 (0.5) | 0.018 |
| Transverse colon diameter (cm) | 3.0 (0.06) | 4.2 (0.4) | 0.014 |
| Abdominal circumference (cm) | 92.2 (1.1) | 101.3 (2.9) | 0.008 |
| Stool evacuations (n/day) | 1.4 (0.1) | 0.6 (0.1) | 0.001 |
| Flatus evacuations (n/day) | 2.4 (0.1) | 1.5 (0.3) | 0.032 |
| Relapse (n) | 0 | 5 | 0.04 |
Values are mean (SEM).
All five patients with recurrent colonic dilation received neostigmine. It was successful in two cases (2/5, 40%) while three patients had endoscopic decompression. All five patients, after resolution of colonic dilation, received PEG in the open label arm of the study. There were no further recurrences after seven days of treatment.
Secondary end points
Neostigmine was administered to 25 patients at presentation and in five patients on recurrence of colonic dilation. It resulted in resolution of colonic dilation in 22 (88%) and two (40%) cases, respectively (fig 1). The most frequent adverse effect was cramping abdominal pain which was noted by 17 (56.6%) patients. It was described as mild by six (20%) patients and as moderate to severe by 11 (36.6%) patients. Four (13.3%) patients vomited and 10 (33.3%) experienced excessive salivation. Two patients developed symptomatic bradycardia and atropine was administered as per protocol.
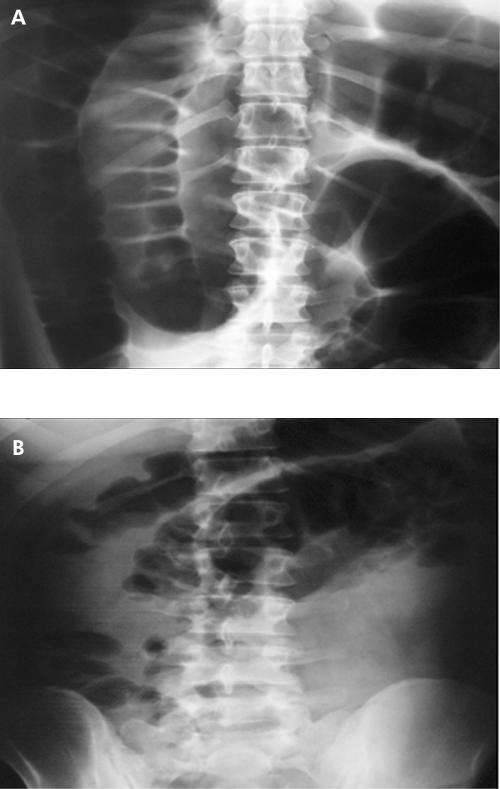
Figure 1 Abdominal radiographs in a patient with acute colonic pseudo obstruction before (A) and three hours after (B) neostigmine administration.
Urgent endoscopic decompression was performed in eight patients at presentation (five from the PEG group and three from the placebo group). These patients were non‐responders to neostigmine (n = 3) or had a contraindication to neostigmine administration (n = 5). It was successful and uneventful in all cases. Three patients also had successful and uneventful colonoscopic decompression on recurrence of colonic dilation due to non‐response to neostigmine. Colonic dilation did not recur in any of the patients who had endoscopic decompression.
Therapy with PEG did not result in any serious adverse events and none of the patients stopped therapy. Four patients in the PEG group and one patient in the placebo group developed nausea and one patient in the PEG group vomited after a single dose. Three patients in the PEG group developed mild abdominal colicky pain compared with one patient in the placebo group.
Discussion
Acute colonic pseudo obstruction is a common clinical scenario for which gastroenterologists are consulted. During the past decade, management options have changed, especially with the advent of neostigmine. Currently, it is generally accepted that neostigmine induces an initial response rate in approximately 90% of cases.9,10 However, rates of sustained response appear to be significantly lower. The only prospective placebo controlled clinical trial thus far9 reported a sustained response rate of 89%, while Loftus and colleagues,10 in a retrospective analysis of 18 patients, suggested that the sustained response rate might be as low as 61%. In patients who present with recurrent colonic dilation after neostigmine administration, a second trial of the drug is indicated.9 In non‐responders to neostigmine, urgent colonoscopic decompression with tube placement in the ascending colon or distally is indicated.8,11
Ours is the largest prospective study in patients with acute colonic pseudo obstruction reported to date. In our series, neostigmine resulted in an initial response in up to 88% of cases within three hours of administration. The three hour period was chosen because of the short half life of neostigmine. Our results confirm previous studies showing that approximately 90% of patients initially respond to neostigmine.9,16,17 However, the rate of sustained response to neostigmine appears to be significantly lower. Five of 13 patients (38.9%) who were initially randomised to the placebo group and received neostigmine as firstline therapy had recurrent colonic dilation within seven days after drug administration. A second trial of neostigmine was attempted but its efficacy was far lower (40%). The seven day period was chosen because patients usually have recurrent colonic dilation within the first four days after initial therapeutic intervention.11
To our knowledge, this is the first trial showing that daily administration of PEG electrolyte balanced solution in patients with acute colonic pseudo obstruction after initial successful therapeutic intervention might increase the sustained response rate. There were several lines of evidence that led us to investigate a possible therapeutic role of PEG in patients with acute colonic pseudo obstruction. Firstly, PEG based laxatives induce acceleration in colonic transit, predominantly through the distal colon,18,19 which is principally affected in Ogilvie's syndrome. This effect is attributed either to increased faecal bulk in the distal colon that triggers myogenic peristalsis or to a direct effect on colonic motility,20 although the latter has not been demonstrated unanimously.21 Additionally, previous studies22,23 indicate that the volatile short lived gas nitric oxide (NO), one of the major inhibitory neurotransmitters released by enteric neurones, may by responsible for gut dysmotility and dilation. In vitro1 and in vivo24 addition of the NO synthase inhibitor nitro‐L‐arginine methyl ester was followed by strong phasic contractions in colonic muscular circular strips of a patient with megacolon and resolution of dilation, respectively. Recent evidence suggests that PEG may reduce the rate of NO production acting either as a storage molecule25 or by decreasing NO synthase.26
We decided to adopt a strict and well standardised protocol for evaluation and follow up of patients with acute colonic pseudo obstruction. The initial response to neostigmine was defined as a ⩾10% reduction in abdominal distension with a ⩾20% concomitant reduction in caecal diameter on abdominal radiographs within three hours of neostigmine administration. These values were based on the previously published results by Ponec and colleagues.9 All “responders”, according to the previously described criteria, also had prompt evacuation of flatus or stool within three hours after neostigmine administration. During follow up, for ethical reasons, we arbitrarily defined relapse as a caecal diameter ⩾8 cm with a concomitant ⩾10% increase on abdominal radiographs with respect to the value that each patient had after initial resolution of the syndrome. Mean (SEM) caecal diameter on recurrence was 10 (0.7) cm (range 9–11) and this proves that all patients had clinically significant colonic dilation on recurrence.
Our results should be interpreted with caution because we did not enrol the desired number of patients and thus a type I error cannot be excluded. However, in the largest prospective study in patients with acute colonic pseudo obstruction reported so far, we found that PEG was clearly superior to placebo and we believe that it would be inappropriate to deprive other patients of such an effective therapy.
In conclusion, our study confirms previously published studies showing that patients with acute colonic pseudo obstruction usually have an increased initial response rate to neostigmine administration. However, the sustained response rate appears to be significantly lower. Administration of PEG electrolyte balanced solution after resolution of colonic dilation increases the sustained response rate after initial therapeutic intervention.
Acknowledgements
During the preparation of the manuscript Professor A Avgerinos passed away. We would like to express our gratitude for being a great teacher and mentor to many young gastroenterologists.
Abbreviations
PEG - polyethylene glycol
CT - computed tomography
NO - nitric oxide
Footnotes
Conflict of interest: None declared
References
- 1.De Giorgio R, Barbara G, Stanghellini V.et al Review article: the pharmacological treatment of acute colonic pseudo‐obstruction. Aliment Pharmacol Ther 2001151717–1727. [DOI] [PubMed] [Google Scholar]
- 2.Saunders M D. Acute intestinal pseudoobstruction. Curr Gastroenterol Rep 20046410–416. [DOI] [PubMed] [Google Scholar]
- 3.Vanek V W, Al‐Salti M. Acute pseudo‐obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum 198629203–210. [DOI] [PubMed] [Google Scholar]
- 4.Nivatvongs S, Vermeulen F D, Fang D T. Colonoscopic decompression of acute pseudo‐obstruction of the colon. Ann Surg 1982196598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spira I A, Rodrigues R, Wolff W I. Pseudo‐obstruction of the colon. Am J Gastroenterol 197665397–408. [PubMed] [Google Scholar]
- 6.Rex D K. Colonoscopy and acute colonic pseudo‐obstruction. Gastrointest Endosc Clin N Am 19977499–508. [PubMed] [Google Scholar]
- 7.Johnson C, Rice R, Kelvin F.et al The radiographic evaluation of gross cecal distension: emphasis on cecal ileus. AJR Am J Roentgenol 19861451211–1217. [DOI] [PubMed] [Google Scholar]
- 8.Eisen G M, Baron T H, Dominitz J A.et al Acute colonic pseudo‐obstruction. Gastrointest Endosc 200256789–792. [DOI] [PubMed] [Google Scholar]
- 9.Ponec R J, Saunders M D, Kimmey M B. Neostigmine for the treatment of acute colonic pseudo‐obstruction. N Engl J Med 1999341137–141. [DOI] [PubMed] [Google Scholar]
- 10.Loftus C G, Harewood G C, Baron T H. Assessment of predictors of response to neostigmine for acute colonic pseudo‐obstruction. Am J Gastroenterol 2002973118–3122. [DOI] [PubMed] [Google Scholar]
- 11.Geller A, Petersen B T, Gostout C J. Endoscopic decompression for acute colonic pseudo‐obstruction. Gastrointest Endosc 199644144–150. [DOI] [PubMed] [Google Scholar]
- 12.Corazziari E, Badiali D, Bazzocchi G.et al Long term efficacy, safety and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF‐100) in the treatment of functional chronic constipation. Gut 200046522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attar A, Lemann M, Ferguson A.et al Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 199944226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corazziari E, Bandiali D, Habib F I.et al Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF‐100) in treatment of chronic non‐organic constipation. Dig Dis Sci 1996411636–1642. [DOI] [PubMed] [Google Scholar]
- 15.Bouhnik Y, Neut C, Raskine L.et al Prospective, randomized, parallel‐group trial to evaluate the effects of lactulose and polyethylene glycol‐4000 on colonic flora in chronic idiopathic constipation. Aliment Pharmacol Ther 200419889–899. [DOI] [PubMed] [Google Scholar]
- 16.Stepherson B M, Morgan A R, Salaman J R.et al Ogilvie's syndrome: a new approach to an old problem. Dis Colon Rectum 199538424–427. [DOI] [PubMed] [Google Scholar]
- 17.Trevisani G T, Hyman N H, Church J M. Neostigmine: safe and effective treatment for acute colonic pseudoobstruction. Dis Colon Rectum 200043599–603. [DOI] [PubMed] [Google Scholar]
- 18.Fritz E, Hammer H F, Lipp R W.et al Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther 200521259–268. [DOI] [PubMed] [Google Scholar]
- 19.Hammer J, Pruckmayer M, Bergmann H.et al The distal colon provides reserve storage capacity during colonic fluid overload. Gut 199741658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemann M, Flourie B, Picon L.et al Motor activity recorded in the unprepared colon of healthy humans. Gut 199537649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herve S, Leroi A M, Mathiex‐Fortunet H.et al Effects of polyethylene glycol 4000 on 24h manometric recordings of left colonic motor activity. Eur J Gastroenterol Hepatol 200113647–654. [DOI] [PubMed] [Google Scholar]
- 22.Mourelle M, Casellas F, Guarner F.et al Induction of nitric oxide synthase in colonic smooth muscles from patients with toxic megacolon. Gastroenterology 19951091497–1502. [DOI] [PubMed] [Google Scholar]
- 23.Mourelle M, Vilaseca J, Guarner F.et al Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase. Am J Physiol 1996270G425–G430. [DOI] [PubMed] [Google Scholar]
- 24.Schwörer H, Bohn M, Waezsada S Y.et al Successful treatment of megacolon associated with colitis with a nitric oxide synthase inhibitor. Am J Gastroenterol 2001962273. [DOI] [PubMed] [Google Scholar]
- 25.Shishido S M, Ganzarolli de Oliveira M. Polyethylene glycol matrix reduces the rates of photochemical and thermal release of nitric oxide from S‐nitroso‐N‐acetylcysteine. Photochem Photobiol 200071273–280. [DOI] [PubMed] [Google Scholar]
- 26.De Winter B Y, Van Nassauw L, De Man J G.et al Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil 200517251–261. [DOI] [PubMed] [Google Scholar]