Abstract
Background
Recent reports have shown that physical activity improves the outcome of patients with colorectal cancer as well as breast and prostate cancer. However, the mechanisms whereby physical activity reduces cancer mortality are not well established.
Methods
Incident cases of colorectal cancer were identified among participants of the Melbourne Collaborative Cohort Study, a prospective cohort study of 41 528 Australians recruited from 1990 to 1994. Information on tumour site and stage, treatments given, recurrences, and deaths were obtained from systematic review of the medical records. Baseline assessments of physical activity and body size were made, and cases with available plasma had pre‐diagnosis insulin‐like growth factor 1 (IGF‐1) and insulin‐like growth factor binding protein 3 (IGFBP‐3) levels measured. We assessed associations between these hormones and colorectal cancer specific deaths with respect to physical activity.
Results
A total of 526 cases of colorectal cancer were identified, of which 443 had IGF‐1/IGFBP‐3 levels measured. Median follow up among survivors was 5.6 years. For the physically active, increasing IGFBP‐3 by 26.2 nmol/l was associated with a 48% reduction in colorectal cancer specific deaths (adjusted hazard ratio (HR) 0.52 (0.33–0.83); p = 0.006). No association was seen for IGF‐1 (adjusted HR 0.90 (0.55–1.45); p = 0.65). For the physically inactive, neither IGF‐1 nor IGFBP‐3 was associated with disease specific survival.
Conclusions
This study supports the hypothesis that the beneficial effects of physical activity in reducing colorectal cancer mortality may occur through interactions with the insulin‐like growth factor axis and in particular IGFBP‐3.
Keywords: colorectal cancer, physical activity, insulin‐like growth factor 1, insulin‐like growth factor binding protein 3
There is now compelling evidence that physical activity is associated with a reduced risk of colorectal cancer (CRC).1 In fact, increasing physical activity may be the most important risk factor that is amenable to modification by individuals wishing to reduce their risk of this common malignancy. Most studies have demonstrated a reduction in the order of 40–50% for those at the highest levels of physical activity, with many demonstrating a dose‐response relationship.1 Benefits are seen for men and women, across all age groups studied, and for both occupational and recreational physical activity, making this finding highly unlikely to be the result of confounding.
Few studies have attempted to address the possible influence of physical activity on survival from CRC. There is however emerging evidence of a protective effect. We have reported findings from a cohort of 526 CRC cases that demonstrated a 31% reduction in cancer deaths for the physically active compared with the physically inactive across all stages.2 The benefit was greatest for stage II and III disease, with a hazard ratio (HR) for CRC specific survival of 0.49 (adjusting for age, sex, and stage) in this subgroup. Another study that reported outcomes for 816 stage III colon cancer patients receiving adjuvant chemotherapy found a 35% improvement in disease free survival for individuals in the highest quintile of regular physical activity compared with the lowest quintile.3
Some mechanisms have been suggested whereby physical activity may reduce CRC deaths, including reduced bowel transit time, alterations in prostaglandin levels/ratios, and through effects on the immune system.4,5 There is growing support for the idea that physical activity might exert its effect via reducing hyperinsulinaemia and via the insulin‐like growth factor (IGF) axis.6,7,8 In support of this hypothesis, cohort studies have shown an increased risk of CRC with higher levels of insulin‐like growth factor 1 (IGF‐1) and C peptide, and an inverse relationship with insulin‐like growth factor binding protein 3 (IGFBP‐3) levels,9,10,11,12 while other studies have failed to show a clear effect.13,14
We explored this hypothesis further, using the cohort of CRC cases in which we have previously demonstrated a prognostic benefit of physical activity. We analysed the associations between IGF‐1 and IGFBP‐3 levels and survival, assessing the effect on disease specific survival separately for those who were physically active and inactive.
Methods
Study population
The Melbourne Collaborative Cohort Study (MCCS) is a prospective cohort study of 41 528 people (17 049 men) aged between 27 and 75 years at baseline (99.3% were aged 40–69 years).15 Recruitment occurred between 1990 and 1994. Subjects were recruited via the electoral rolls (registration to vote is compulsory for Australian adults), advertisements, and community announcements. The Cancer Council Victoria's Human Research Ethics Committee approved the study protocol. Subjects gave written consent to participate and for the investigators to obtain access to their medical records.
We identified cases of adenocarcinoma of the colon or rectum diagnosed during follow up to 1 August 2002 by matching participants to the Victorian Cancer Registry. Cases were identified from notifications to the Victorian Cancer Registry of diagnoses of adenocarcinoma of the colon and rectum (International Classification of Diseases 9th revision rubric 153.0–153.4, 153.6–153.9, 154.0, 154.1, 154.8 or 10th revision rubric C18.0, C18.2–C18.9, C19, C20, C21.8). For this analysis, subjects were excluded if they had a diagnosis of CRC before baseline. Information on tumour characteristics (site, size, stage, and degree of differentiation), adjuvant treatments, recurrences, and deaths was obtained from medical records. Deaths were also identified by the Victorian Cancer Registry and by linkage to the National Death Index. Victorian deaths were complete to 1 July 2004 and in other states to the end of 2002. Only one participant diagnosed with CRC is known to have left Victoria after diagnosis.
Assessment of physical activity
All participants in the cohort study answered three questions relating to their level of non‐occupational physical activity at study entry (baseline). The first question was “On average (for example, over the last six months) how many times per week did you exercise vigorously for a period of at least 20 minutes?” The second question was “On average (for example, over the last six months) how many times per week did you engage in less vigorous exercise for recreation, sport, or health and fitness purposes, which did not make you sweat or feel out of breath?” Answers were recorded as “none at all”, “once or twice a week”, or “three or more times a week”. The answers to these two questions were combined to classify subjects with regard to their physical activity levels. Those who reported any regular exercise (either “once or twice a week” or “three or more times a week”) were classified as “physically active”. Those who answered “none at all” to both questions regarding exercise were classified as “physically inactive”.
Body composition
Height, weight, and waist circumference were measured directly at baseline clinic attendance according to written protocols that were based on standard procedures.16 Weight was measured to 100 g using digital electronic scales, height to 1 mm using a stadiometer, and waist and hip circumferences were measured to 1 mm using a two metre metal anthropometric tape. Body mass index (BMI) was calculated as weight in kg divided by the square of height in metres. Bioelectrical impedance analysis was performed with a single frequency (50 kHz) electrical current produced by a BIA‐101A RJL system analyser (RJL systems, Detroit, Michigan, USA). Resistance and reactance were measured with subjects in the supine position. Adipose mass, non‐adipose mass, and per cent body fat were calculated from these measurements using formulae that had been developed in Caucasian populations of similar age and BMI distribution to the MCCS participants.17
Measurement of plasma IGF‐1 and IGFBP‐3 levels
Each participant had a blood sample taken at baseline, from which 2 ml of plasma were extracted and stored in liquid nitrogen. We attempted to measure hormone levels for cases of colon cancer that were diagnosed within the cohort by 1 August 2002. Samples from subjects receiving hormone replacement therapy were not included in the analyses. Plasma samples were retrieved from storage and shipped in batches on dry ice to the laboratory of one of the authors (HM) where IGF‐1 and IGFBP‐3 were to be measured.
Samples were thawed in a warm water bath, vortexed rapidly for a few seconds, and centrifuged at 2000 rpm (210 g) for 10 minutes. IGF‐1 was measured by enzyme linked immunosorbent assay (ELISA) using reagents supplied by Diagnostic Systems Laboratories Inc (DSL‐10‐5600; Webster, Texas, USA). The assay utilises an ethanolic‐HCl extraction to separate IGF‐1 from the binding proteins in plasma before quantification by an enzymatically amplified immunoasay. Intra‐assay and interassay coefficients of variation for IGF‐1 at 27.2 nmol/l were 7% and 9%, respectively. IGFBP‐3 was measured by ELISA using commercial reagents (DSL‐10‐6600). Intra‐assay and interassay coefficients of variation at 88.3 nmol/l were 3% and 8%, respectively.
To ensure quality control, 10% of the samples in each batch were randomly placed samples from pooled plasma that were collected in 1991 and stored with the participants' samples in liquid nitrogen. The laboratory was blind to these samples. For the pooled plasma samples, overall coefficient of variation was 12% for IGF‐1 (9% within batches and 7% between batches) and 9% for IGFBP‐3 (8% and 3%).
In order to estimate the reliability of plasma measurements, some members of the cohort had two blood samples taken approximately one year apart. The two samples from 115 subjects (none were cases in this analysis) were each divided in two and hormone levels measured on two occasions one week apart. As a measure of reliability, we calculated the intraclass correlation (ICC), which is the ratio between the variance due to variation between subjects over the sum of variance components due to between subject variance, between time variance within subjects, and measurement error. ICC was 0.38 for IGF‐1 (95% confidence interval (CI) 0.27–0.51) and 0.78 for IGFBP‐3 (95% CI 0.73–0.84).
The quartiles of hormone levels used were calculated from the 443 cases. “Within batch” quartiles were also generated to test for measurement variations.
Colorectal cancer characteristics
We reviewed the medical records of all CRC cases to obtain a range of clinical information. Stage was categorised into four groups based on the American Joint Committee on Cancer (AJCC) staging system: stage I (T1–2, N0, M0), stage II (T3–4, N0, M0), stage III (Tany, N1–2, M0), and stage IV (Tany, Nany, M1). In the case of synchronous cancers, the characteristics of the tumour with the higher stage (or the larger tumour if they were the same stage) were used. The site of the primary tumour was classified as “right colon” if it was located proximal to the splenic flexure. “Left colon” tumours were located between the splenic flexure and the peritoneal reflection, and rectal cancers below the peritoneal reflection. Degree of differentiation (well, moderate, or poorly) was determined from the histopathological report. Deaths due to CRC, or as a direct result of treatment for it, were termed “disease specific deaths”.
Statistical analysis
Cox's proportional hazard regression models, with time since diagnosis of CRC as the time axis, were used to estimate the HRs for disease specific survival associated with each measure at baseline. Follow up ended at death or 1 July 2004 (the date that ascertainment of deaths by the Victorian Cancer Registry was complete), whichever came first. All models were adjusted for age at diagnosis (as a continuous variable), sex, stage of disease (as a categorical variable), degree of differentiation (as a categorical variable), per cent body fat, and BMI. A separate category was used for cases with missing stage or degree of differentiation. HRs were calculated per increase of one standard deviation (SD) as well as for each quartile of hormone level. Further adjustment for waist circumference or height made virtually no difference to any of the results and therefore these models are not presented. Interactions were also fitted to test for differences in associations by physical activity category. Tests based on Schoenfeld residuals and graphical methods using Kaplan‐Meier curves18 showed no evidence that proportional hazard assumptions were violated for any analyses. All statistical analyses were performed using SPSS (version 12.0 Chicago, Illinois, USA).
Results
We identified 526 cases of CRC from within the cohort of 41 528 who were diagnosed following enrolment up until the end of July 2002. Of these cases, plasma IGF‐1 and IGFBP‐3 levels were not available for 83 due mainly to an insufficient/missing sample (n = 50) or because they were using hormone replacement therapy (n = 28), leaving 443 cases of CRC with IGF‐1 and IGFBP‐3 values. All of the results shown are for these 443 cases. Median time from cohort study entry until diagnosis was 5.4 years and median duration of follow up (for survivors) from CRC diagnosis was 5.6 years. Patient characteristics are shown in table 1. AJCC stage and tumour site was unknown for 11 cases, while in 54 cases the degree of differentiation was not stated in the histopathology report. Mean age at diagnosis was 66.3 years (range 42–79).
Table 1 Insulin‐like growth factor 1 (IGF‐1) and insulin‐like growth factor binding protein 3 (IGFBP‐3) levels by patient characteristics.
| No of patients | Mean IGF‐1 (nmol/l) | Mean IGFBP‐3 (nmol/l) | |
|---|---|---|---|
| All cases | 443 | 22.5 | 111.2 |
| Male | 231 | 23.5 | 108.6 |
| Female | 212 | 21.4 | 114.0 |
| AJCC stage | |||
| I | 110 | 22.8 | 112.1 |
| II | 125 | 22.0 | 110.4 |
| III | 125 | 22.7 | 110.9 |
| IV | 72 | 22.7 | 112.4 |
| Unknown | 11 | ||
| Primary tumour site | |||
| Right colon | 138 | 22.6 | 109.3 |
| Splenic flexure | 1 | ||
| Left colon | 143 | 23.0 | 114.3 |
| Rectum | 150 | 21.9 | 109.7 |
| Unknown | 11 | ||
| Degree of differentiation | |||
| Well | 37 | 24.5 | 110.3 |
| Moderate | 254 | 22.7 | 112.7 |
| Poor | 98 | 20.8 | 107.6 |
| Unknown | 54 | ||
| Physically active | 194 | 22.5 | 112.8 |
| Physically inactive | 249 | 22.4 | 109.9 |
A total of 249 cases reported doing no exercise at baseline, 64 cases participated in regular vigorous exercise, and 165 exercised less vigorously. Some (35 cases) did both vigorous and less vigorous exercise, resulting in 194 that reported participating in some form of regular exercise (that is, “physically active”). As previously reported, we found no significant differences in the tumour characteristics between the physically active and the physically inactive.2 Mean IGF‐1 level was 22.5 (SD 8.5) nmol/l while the mean IGFBP‐3 level was 111.2 (SD 26.2) nmol/l. IGF‐1 and IGFBP‐3 levels did not differ significantly by sex, tumour factors (stage, site, or degree of differentiation), or physical activity. There was however a strong correlation between IGF‐1 and IGFBP‐3 levels (r = 0.55).
Survival
By 31 July 2004 we had identified 170 deaths, and of these 148 were classified as CRC specific deaths (six were “treatment related” without recurrent disease), while 22 (13%) were due to “other” causes, 11 in the physically active and 11 in the physically inactive.
Table 2 shows the associations between IGF‐1 and IGFBP‐3 levels and CRC specific survival for all cases, for the physically inactive, and for the physically active. When all cases were analysed together, IGF‐1 levels were not associated with CRC specific survival (HR for IGF‐1 0.95 (95% CI 0.80–1.14)) while for IGFBP‐3 the HR for CRC specific survival was 0.87 (95% CI 0.74–1.03). We next analysed the associations with CRC specific survival separately for the physically inactive and the physically active. In the physically inactive, neither IGF‐1 (HR 1.13 (95% CI 0.92–1.39)) nor IGFBP‐3 (HR 1.08 (95% CI 0.88–1.33)) were associated with CRC specific survival.
Table 2 Hazard ratios (HR) and 95% confidence interval (95% CI) for colorectal cancer specific survival for all cases, the physically inactive, and the physically active, adjusted for stage (categorical), degree of differentiation (categorical), age and sex, per cent fat, and body mass index.
| HR (95% CI) comparing quartile of plasma level with quartile 1 | HR per increase of 1 SD in hormone level | |||
|---|---|---|---|---|
| Q2 | Q3 | Q4 | ||
| All cases | ||||
| IGF‐1 per SD | 1.29 (0.80–2.08) | 1.17 (0.72–1.89) | 0.95 (0.58–1.55) | 0.95 (0.80–1.14) p = 0.59 |
| IGFBP‐3 per SD | 0.86 (0.53–1.39) | 0.97 (0.61–1.56) | 0.70 (0.43–1.14) | 0.87 (0.74–1.03) p = 0.11 |
| Physically inactive | ||||
| IGF‐1per SD | 1.34 (0.74–2.45) | 1.06 (0.57–1.95) | 1.57 (0.86–2.84) | 1.13 (0.92–1.39) p = 0.24 |
| IGFBP‐3 per SD | 1.07 (0.59–2.00) | 1.30 (0.71–2.36) | 1.26 (0.68–2.35) | 1.08 (0.88–1.33) p = 0.45 |
| Physically active | ||||
| IGF‐1per SD | 0.69 (0.28–1.68) | 0.89 (0.35–2.16) | 0.30 (0.11–0.81) | 0.59 (0.41–0.86) p = 0.006 |
| IGFBP‐3 per SD | 0.42 (0.18–1.00) | 0.43 (0.18–1.04) | 0.17 (0.07–0.44) | 0.49 (0.33–0.77) p<0.001 |
Standard deviation (SD) for insulin‐like growth factor 1 (IGF‐1) is 8.5 nmol/l; SD for insulin‐like growth factor binding protein 3 (IGFBP‐3) is 26.2 nmol/l.
Quartiles are based on all 443 cases.
On the other hand, when the physically active were analyzed separately, a strong association between IGF‐1/IGFBP‐3 levels and disease specific survival was observed. For those who enjoyed regular physical activity, increasing IGFBP‐3 by 26.2 nmol/l was associated with a 51% reduction in the likelihood of a cancer death, after adjustment for age, sex, stage, degree of differentiation, adiposity, and BMI (HR 0.49; p<0.001). Similarly, an increase in IGF‐1 of 8.5 nmol/l resulted in a 41% risk reduction (HR 0.59; p = 0.006). A test for interaction demonstrated a significant difference between the HRs with respect to IGFBP‐3 for the physically active compared with the physically inactive (p = 0.012). The same interaction for IGF‐1 was of borderline significance (p = 0.051). An association between IGF‐1/IGFBP‐3 levels and overall survival was also seen in exercisers; however, the magnitude of this effect was not as large. Increasing IGFBP‐3 resulted in a 32% reduction in all cause mortality (HR 0.68 (0.51–0.92); p = 0.01), the corresponding result for IGF‐1 was a 33% reduction in deaths (HR 0.67 (0.48–0.93); p = 0.02). Again, no association was seen for non‐exercisers with respect to overall survival.
As we found a significant relationship between IGF‐1 and IGFBP‐3 levels within the whole group, we repeated our original analyses with IGF‐1 and IGFBP‐3 levels included in the models (table 3). In other words, the effect of IGF‐1 was adjusted for IGFBP‐3 (in addition to the original variables) and vice versa. As before, we found no association between survival and either hormone level for all cases combined or when the physically inactive were assessed separately. The association of IGF‐1 with survival for the physically active was lost when adjusted for IGFBP‐3 (in addition to age, sex, stage, degree of differentiation, adiposity, and BMI) (HR 0.90 (0.55–1.45); p = 0.65) but the association with IGFBP‐3 remained largely unchanged when adjusted for IGF‐1 levels (HR 0.52 (0.33–0.83); p = 0.006).
Table 3 Hazard ratios (HR) and 95% confidence interval (95% CI) for colorectal cancer (CRC) specific survival for all cases, the physically inactive, and the physically active, adjusted for stage (categorical), degree of differentiation (categorical), age and sex, per cent fat, BMI, and insulin‐like growth factor 1/insulin‐like growth factor binding protein 3 (IGF‐1/IGFBP‐3).
| HR (95% CI) comparing quartile of plasma level with quartile 1 | HR per increase of 1 SD in hormone level | |||
|---|---|---|---|---|
| Q2 | Q3 | Q4 | ||
| All cases | ||||
| IGF‐1 per 8.5 nmol/l | 1.29 (0.80–2.08) | 1.16 (0.71–1.90) | 1.10 (0.62–1.94) | 1.01 (0.89–1.14) p = 0.90 |
| IGFBP‐3 per 26.2 nmol/l | 0.87 (0.53–1.42) | 0.99 (0.60–1.64) | 0.72 (0.41–1.24) | 0.87 (0.71–1.06) p = 0.16 |
| Physically inactive | ||||
| IGF‐1per 8.5 nmol/l | 1.28 (0.70–2.34) | 0.93 (0.49–1.75) | 1.36 (0.67–2.75) | 1.08 (0.85–1.37); p = 0.54 |
| IGFBP‐3 per 26.2 nmol/l | 1.04 (0.56–1.92) | 1.22 (0.64–2.33) | 1.17 (0.59–2.32) | 1.04 (0.82–1.32); p = 0.73 |
| Physically active | ||||
| IGF‐1per 8.5 nmol/l | 0.84 (0.35–2.02) | 1.36 (0.52–3.53) | 0.90 (0.28–2.85) | 0.90 (0.55–1.45); p = 0.65 |
| IGFBP‐3 per 26.2 nmol/l | 0.48 (0.19–1.19) | 0.50 (0.20–1.28) | 0.23 (0.08–0.73) | 0.52 (0.33–0.83); p = 0.006 |
Standard deviation (SD) for IGF‐1 is 8.5 nmol/l; SD for IGFBP‐3 is 26.2 nmol/l.
Quartiles are based on all 443 cases.
For the physically active, the adjusted HR for CRC specific survival, comparing the highest quartile with the lowest quartile of IGFBP‐3, was 0.24 (0.08–0.73). The corresponding HR for the physically inactive was 1.17 (0.59–2.32). There were no significant associations with CRC specific survival seen for any of the quartiles of IGF‐1 for either the physically inactive or the physically active.
Again, the test for interaction demonstrated a significant difference between the HRs with respect to IGFBP‐3 for the physically active compared with the physically inactive (p = 0.012). However, the same interaction for IGF‐1 was now not statistically significant (p = 0.08).
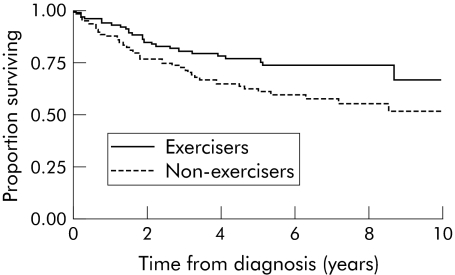
We also observed a differential effect of exercise based on IGFBP‐3 levels. For cases with high IGFBP‐3 levels (levels greater than the median value) “exercisers” had a clear CRC specific survival advantage over “non‐exercisers” (fig 1). In this group with “high” IGFBP‐3 levels, “exercisers” were approximately half as likely to die of CRC than “non‐exercisers” (adjusted HR 0.44 (95% CI 0.25–0.76); p = 0.003). On the other hand, CRC specific survival for those cases with “low” IGFBP‐3 levels (less than the median) was not influenced by physical activity (adjusted HR 1.03 (95% CI 0.62–1.71); p = 0.92). A similar analysis dividing cases by IGF‐1 levels revealed no interaction, with similar beneficial effects of exercise being seen in both cases with either “high” (HR 0.65) or “low” (HR 0.62) IGF‐1 levels.
Figure 1 Kaplan‐Meier curve for colorectal cancer (CRC) specific survival for cases with insulin‐like growth factor binding protein 3 (IGFBP‐3) levels above the median by exercise group.
Analyses using within batch quartiles to adjust for laboratory error made only a trivial difference to any of the results. Similarly, excluding cases diagnosed within one year of recruitment did not alter our findings. We did not have enough cases to analyse diabetics separately as only 24 cases were diabetic at baseline, and excluding known diabetics did not change our results.
Discussion
We have shown in a previous report2 that those who are physically active prior to their diagnosis of CRC enjoy superior survival compared with those who are relatively inactive, and that this advantage remains after adjustment for standard prognostic indicators. This observation has contributed to the interest that has grown concerning possible links between cancer survival and both physical activity and adiposity. Associations have been observed in this regard not only for CRC 2,3 but also for breast19,20 and prostate21 cancer with the improvement in survival being a result of decreased cancer deaths rather than reduced deaths from cardiovascular disease.
In this report we have built on our previous finding by measuring IGF‐1 and IGFBP‐3 in plasma taken at recruitment to our cohort study some years before the subjects' diagnosis with CRC. We have shown that for people who are physically active, but not for those who are physically inactive, increasing plasma levels of IGFBP‐3, but not IGF‐1, are associated with improved survival from CRC. In addition, the previously reported benefit of regular physical activity was observed in cases with high but not low levels of IGFBP‐3 but was seen across all levels of IGF‐1. Together these results suggest a link between the improvement in cancer specific survival from exercise and circulating IGFBP‐3 levels.
The strengths of our study are its reasonable size, availability of plasma samples collected prior to diagnosis, standardised collection of epidemiological and clinical information, and completeness of follow up. Its limitations relate mainly to the fact that the assessment of both physical activity and IGF‐1/IGFBP‐3 levels occurred at a single point in time prior to the diagnosis of CRC. It is possible that the stronger associations we observed with IGFBP‐3 are because it was measured with less error, partly because it had less within person variability. We did not measure physical activity or plasma hormone levels following diagnosis and, therefore, cannot assess possible variations in either of these over the follow up period. We have however previously reported that the improved survival for the physically active was observed predominantly for stage II and III disease and was related to fewer recurrences, raising the possibility that increased physical activity may influence tumour behaviour and, in particular, metastatic potential.
One other study has attempted to assess the impact of the IGF axis on survival in patients diagnosed with CRC.22 This study assessed baseline IGF‐1 and IGFBP‐3 levels in 527 cases with metastatic CRC receiving palliative chemotherapy. Higher levels of IGFBP‐3 were associated with an increased response to chemotherapy, a longer time to progression, and improved overall survival. IGF‐1 was not related either to response, time to progression, or overall survival. This study supports our finding that IGFBP‐3 is more important than IGF‐1 to the prediction of outcome in CRC and may have prognostic significance.
Our current knowledge of the mechanism by which physical activity may positively influence the behaviour of cancers is poorly understood. IGF‐1 is central to the regulation of growth processes. In the circulation, over 90% of IGF‐1 is bound to IGFBP‐3 both of which are produced mainly in the liver. The main stimulus for IGF‐1 production comes from growth hormone.23 This stimulatory effect of growth hormone is modulated by insulin, which increases growth hormone receptor levels.24 In addition to increasing circulating IGF‐1, hyperinsulinaemia inhibits the synthesis of other IGF binding proteins.25 Studies have shown that, after binding to its receptors which are found on normal colonic mucosal cells as well as colon cancer cells, IGF‐1 can stimulate cell proliferation, inhibit apoptosis,26 and promote angiogenesis.27,28 On the other hand, IGFBP‐3 inhibits the action of IGF‐1 by limiting the availability of free hormone. IGFBP‐3 is also known to have actions independent of IGF‐1, including the ability to induce apoptosis (possibly via the tumour suppressor gene p‐53),29,30,31 and inhibit growth via the transforming growth factor β pathway.32 Our data suggest that these later factors are likely to be the most important because of the lack of an effect of IGF‐1 levels when corrected for IGFBP‐3 levels.
While it is well known that regular physical activity can decrease insulin resistance and, hence, serum insulin levels33 in addition to reducing adiposity, several studies have tried to assess the impact physical activity might have on the IGF axis, with mixed results. Some have shown little or no effect,34,35,36 while others have demonstrated reductions in IGF‐1 levels.37,38 Similarly, a clear relationship has not been demonstrated between physical activity and IGFBP‐3, with some studies showing elevated levels26,36,37 and others observing no change in levels.38,39 It is important to note that energy balance may play an important role in determining the response of the IGF axis to physical activity.40 Equally important is the timing of hormone measurement with respect to physical activity, with significant variations seen between measurement immediately following exercise and two or 48 hours later.36,40
In conclusion, we have shown an association between CRC specific survival and IGFBP‐3 levels in those who were physically active prior to diagnosis, with higher pre‐diagnosis levels of IGFBP‐3 resulting in a significantly lower risk of death. This association was not seen for those who were physically inactive prior to diagnosis nor was there any association seen between IGF‐1 and outcome, irrespective of physical activity. This study supports the hypothesis that the beneficial effects of physical activity in reducing CRC mortality may occur through interactions with the IGF axis and in particular IGFBP‐3.
Acknowledgements
This study was made possible by the contribution of many people, including the original investigators and the diligent team who recruited participants and who continue working on follow up. We would like to express our gratitude to the many thousands of Melbourne residents who continue to participate in the study. We also acknowledge the contribution of Ms Sonia Dunn for assistance with plasma IGF‐1 and IGFBP‐3 analyses.
Abbreviations
CRC - colorectal cancer
BMI - body mass index
AJCC - American Joint Committee on Cancer
IGF‐1 - insulin‐like growth factor 1
IGFBP‐3 - insulin‐like growth factor binding protein 3
MCCS - Melbourne Collaborative Cohort Study
ELISA - enzyme linked immunosorbent assay
ICC - intraclass correlation
Footnotes
Cohort recruitment was funded by VicHealth and the Cancer Council Victoria. This study was funded by a grant from the National Health and Medical Research Council (209057) and was further supported by infrastructure provided by the Cancer Council Victoria.
Conflict of interest: None declared.
References
- 1.Friedenreich C M, Orenstein M R. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr 2002132(suppl 11)3456–64S. [DOI] [PubMed] [Google Scholar]
- 2.Haydon A M, MacInnis R J, English D R.et al The effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 20065562–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerhardt J A, Heseltine D, Niedzwiecki D.et al The impact of physical activity on patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. Proc Am Soc Clin Oncol 200524abstract 3534. [DOI] [PubMed] [Google Scholar]
- 4.Quadrilatero J, Hoffman‐Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J Sports Med Phys Fitness 200343121–138. [PubMed] [Google Scholar]
- 5.Westerlind K C. Physical activity and cancer prevention—mechanisms. Med Sci Sports Exerc 2003351834–1840. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E. Insulin, insulin‐like growth factors and colon cancer: a review of the evidence. J Nutr 2001131(suppl 11)3109–20S. [DOI] [PubMed] [Google Scholar]
- 7.Kaaks R, Toniolo P, Akhmedkhanov A.et al Serum C‐peptide, insulin‐like growth factor (IGF)‐I, IGF‐binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 2000921592–1600. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A, Ulrich C, Slate S.et al Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control 19989487–509. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Pollak M N, Giovannucci E.et al Prospective study of colorectal cancer risk in men and plasma levels of insulin‐like growth factor (IGF)‐I and IGF‐binding protein‐3. J Natl Cancer Inst 199991620–625. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Pollak M N, Platz E A.et al A prospective study of plasma insulin‐like growth factor‐1 and binding protein‐3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 20009345–349. [PubMed] [Google Scholar]
- 11.Nomura A M, Stemmermann G N, Lee J.et al Serum insulin‐like growth factor I and subsequent risk of colorectal cancer among Japanese‐American men. Am J Epidemiol 2003158424–431. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Giovannucci E, Pollak M.et al A prospective study of plasma C‐peptide and colorectal cancer risk in men. J Natl Cancer Inst 200496546–553. [DOI] [PubMed] [Google Scholar]
- 13.Probst‐Hensch N M, Yuan J M, Stanczyk F Z.et al IGF‐1, IGF‐2 and IGFBP‐3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer 2001851695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmqvist R, Hallmans G, Rinaldi S.et al Plasma insulin‐like growth factor 1, insulin‐like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut 200250642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles G G, English D R. The Melbourne Collaborative Cohort Study. IARC Sci Publ 200215669–70. [PubMed] [Google Scholar]
- 16.Lohman T, Roche A, Martorell R.Anthropometric standardization reference manual. Champaign: Kinetics Books, 1988
- 17.Roubenoff R, Baumgartner R N, Harris T B.et al Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci 199752M129–M136. [DOI] [PubMed] [Google Scholar]
- 18.Collett D.Modelling survival data in medical research. Boca Raton: Chapman and Hall/CRC, 1999
- 19.Holmes M D, Chen W Y, Feskanich D.et al Physical activity and survival after breast cancer diagnosis. JAMA 20052932479–2486. [DOI] [PubMed] [Google Scholar]
- 20.Malin A, Matthews C E, Shu X O.et al Energy balance and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2005141496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannucci E L, Liu Y, Leitzmann M F.et al A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med 20051651005–1010. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs C, Pollak M, Sargent D J.et al Insulin‐like growth factor‐1 (IGF‐1), IGF binding protein‐3 (IGFBP‐3), and outcome in metastatic colorectal cancer (CRC): Results from Intergroup Trial N9741. Proc Am Soc Clin Oncol 200423abstract 3521 [Google Scholar]
- 23.Kaaks R, Lukanova A. Effects of weight control and physical activity in cancer prevention: role of endogenous hormone metabolism. Ann N Y Acad Sci 2002963268–281. [DOI] [PubMed] [Google Scholar]
- 24.Baxter R C, Turtle J R. Regulation of hepatic growth hormone receptors by insulin. Biochem Biophys Res Commun 197884350–357. [DOI] [PubMed] [Google Scholar]
- 25.Suikkari A M, Koivisto V A, Rutanen E M.et al Insulin regulates the serum levels of low molecular weight insulin‐like growth factor‐binding protein. J Clin Endocrinol Metab 198866266–272. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Rohan T. Role of the insulin‐like growth factor family in cancer development and progression. J Natl Cancer Inst 2000921472–1489. [DOI] [PubMed] [Google Scholar]
- 27.Warren R S, Yuan H, Matli M R.et al Induction of vascular endothelial growth factor by insulin‐like growth factor 1 in colorectal carcinoma. J Biol Chem 199627129483–29488. [DOI] [PubMed] [Google Scholar]
- 28.Freier S, Weiss O, Eran M.et al Expression of the insulin‐like growth factors and their receptors in adenocarcinoma of the colon. Gut 199944704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong J, Zhang G, Dong F.et al Insulin‐like growth factor (IGF)‐binding protein‐3 mutants that do not bind IGF‐I or IGF‐II stimulate apoptosis in human prostate cancer cells. J Biol Chem 200227710489–10497. [DOI] [PubMed] [Google Scholar]
- 30.Valentinis B, Bhala A, DeAngelis T.et al The human insulin‐like growth factor (IGF) binding protein‐3 inhibits the growth of fibroblasts with a targeted disruption of the IGF‐I receptor gene. Mol Endocrinol 19959361–367. [DOI] [PubMed] [Google Scholar]
- 31.Williams A C, Collard T J, Perks C M.et al Increased p53‐dependent apoptosis by the insulin‐like growth factor binding protein IGFBP‐3 in human colonic adenoma‐derived cells. Cancer Res 20006022–27. [PubMed] [Google Scholar]
- 32.Rajah R, Valentinis B, Cohen P. Insulin‐like growth factor (IGF)‐binding protein‐3 induces apoptosis and mediates the effects of transforming growth factor‐beta1 on programmed cell death through a p53‐and IGF‐independent mechanism. J Biol Chem 199727212181–12188. [DOI] [PubMed] [Google Scholar]
- 33.Regensteiner J G, Mayer E J, Shetterly S M.et al Relationship between habitual physical activity and insulin levels among nondiabetic men and women. San Luis Valley Diabetes Study. Diabetes Care 1991141066–1074. [DOI] [PubMed] [Google Scholar]
- 34.Nicklas B J, Ryan A J, Treuth M M.et al Testosterone, growth hormone and IGF‐I responses to acute and chronic resistive exercise in men aged 55–70 years. Int J Sports Med 199516445–450. [DOI] [PubMed] [Google Scholar]
- 35.Pyka G, Taaffe D R, Marcus R. Effect of a sustained program of resistance training on the acute growth hormone response to resistance exercise in older adults. Horm Metab Res 199426330–333. [DOI] [PubMed] [Google Scholar]
- 36.Kemmler W, Wildt L, Engelke K.et al Acute hormonal responses of a high impact physical exercise session in early postmenopausal women. Eur J Appl Physiol 200390199–209. [DOI] [PubMed] [Google Scholar]
- 37.Fairey A S, Courneya K S, Field C J.et al Effects of exercise training on fasting insulin, insulin resistance, insulin‐like growth factors, and insulin‐like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 200312721–727. [PubMed] [Google Scholar]
- 38.Schmitz K H, Ahmed R L, Yee D. Effects of a 9‐month strength training intervention on insulin, insulin‐like growth factor (IGF)‐I, IGF‐binding protein (IGFBP)‐1, and IGFBP‐3 in 30–50‐year‐old women. Cancer Epidemiol Biomarkers Prev 2002111597–1604. [PubMed] [Google Scholar]
- 39.Ngo T H, Barnard R J, Tymchuk C N.et al Effect of diet and exercise on serum insulin, IGF‐I, and IGFBP‐1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control 200213929–935. [DOI] [PubMed] [Google Scholar]
- 40.Nemet D, Connolly P H, Pontello‐Pescatello A M.et al Negative energy balance plays a major role in the IGF‐I response to exercise training. J Appl Physiol 200496276–282. [DOI] [PubMed] [Google Scholar]