Abstract
Background and aims
Independent component analysis (ICA) of the electroencephalogram (EEG) overcomes many of the classical problems in EEG analysis. We used ICA to determine the brain responses to painful stimulation of the oesophagus.
Methods
Twelve subjects with a median age of 41 years were included. With a nasal endoscope, two series of 35 electrical stimuli at the pain threshold were given to the distal oesophagus and the EEG was subjected to ICA. The sessions were separated by 30 minutes. For each component head models, event related images, spectral perturbation, coherence analysis, and dipoles were extracted. The most valid components were found according to time/frequency information and reliability in both experiments.
Results
Reliable components with the most valid dipoles were found in the thalamus, insula, cingulate gyrus, and sensory cortex. Time locked activities were consistent with upstream activation of these areas, and cross coherence analysis of the sources demonstrated dynamic links in the β(14–25 Hz) and γ(25–50 Hz) bands between the suggested networks of neurones. The thalamic components were time and phase locked intermittently, starting around 50 ms. In the cingulate gyrus, the posterior areas were always firstly activated, followed by the middle and anterior regions. Components with dipoles in the sensory cortex were localised in several regions of the somatosensory area.
Conclusions
The method gives new information relating to the localisation and dynamics between neuronal networks in the brain to pain evoked from the human oesophagus, and should be used to increase our understanding of clinical pain.
Keywords: oesophagus, experimental pain, electroencephalography, signal analysis, independent component analysis
Pain arising from the oesophagus is very common but the underlying neurophysiological mechanisms are poorly understood. In particular, little is known about the cerebral processing of pain from the upper gastrointestinal tract. Evidence for abnormal brain processing to external stimuli has been shown in patients with organic and functional disorders of the gut, and such neuroplastic changes may be of major importance to understand the symptoms in chronic gut pain.1,2,3 However, activation or deactivation of specific brain regions provides only limited understanding of the mechanisms working in the complex neuromatrix activated in gut pain. To obtain a better understanding of the brain in health and disease it is therefore crucial to identify the temporal activation and dynamic connections between the activated brain centres. Most studies addressing gut pain have been based on positron emission tomography or functional magnetic resonance imaging (fMRI).4 These methods do not measure neuronal activity itself but use indirect methods based on changes in metabolism or local blood flow circulation. The techniques have the advantage of high spatial resolution but their sensitivity to the temporal sequence of the cerebral events is rather low.5 As the brain centres specific for the exogenous component in visceral pain are activated within the first 50–150 ms after stimulation, methods that reflect neuronal activity directly are needed to describe the process.2
Magnetoencephalography (MEG) and electroencephalography (EEG) can monitor brain activity to external stimuli with a high time resolution. Brain mapping of event related potentials (ERPs) to time locked electrical stimuli has the advantage of high temporal resolution.6,7 ERPs mainly arise from synchronised extracellular currents in large groups of cells responding to an external stimulus, thus forming an “electrical dipole”. Local neuronal activities produce far field EEG signals that reach the scalp by volume conduction, and ERPs are a surface view of several such neuronal sources. The solution to the so‐called “inverse problem”(that is, modelling of the intracerebral dipoles underlying a certain scalp ERP distribution) uses a mathematical approach where a non‐physiological spherical three shell model is applied to the data.8,9 However, this method has several limitations, reducing the accuracy of its results.9
Recently, a novel approach to separate signal mixtures and hence bypass the inverse problem has been introduced by Makeig and colleagues.10 This method known as “independent component analysis (ICA)” separates multichannel data into a sum of component activities. These components are maximally temporally independent and minimise the influence of volume conduction without referring to an explicit head model. Furthermore, the model provides extensive neurophysiological information on the identified components with high temporal resolution, and cross coherence analysis allows detection of dynamic connections between different centres in the brain. The method has previously been used on large EEG datasets of, for example, visual stimuli, and was shown to fit near perfectly the dipolar projections of cortical sources.10
We hypothesised that ICA could be used to separate signal mixtures identifying the localisation and temporal sequence of brain activation to oesophageal pain, and to provide new information about the cross talk between the activated centres. The aims were:(1) to use ICA to increase the physiological information of the EEG response to oesophageal pain;(2) to study localisation and temporal activation of the activated brain regions by modelling the best fitting dipoles to the obtained components; and (3) to study the cross talk between brain regions with cross coherence analysis of selected components.
Materials and methods
Subjects
Twelve subjects, two females and 10 males, median age 41 years,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 were included. All were healthy and did not suffer from any symptoms suggestive of gastrointestinal diseases or disorders associated with pain. None used any form of drugs, and alcohol was not allowed 24 hours prior to the study. The study was conducted according to the Helsinki II declaration and the experiment was approved by the local ethics committee (No VN 2003/120 mch).
Stimulation and assessment
Subjects fasted for eight hours before the experiment. The experiment was performed with subjects in the supine position with the head tilted 30° upwards. Intubation was done with a 6 mm nasal endoscope (Ultra Slim Gastroscope (Pentax EG‐1840)), without any sedation. A specially designed stainless steel electrode for electrical stimulation was constructed from a biopsy forceps. The modified forceps had a stimulation tip 1 mm in diameter with an inter‐electrode interval of 5 mm. An electrical stimulator (JNi‐Biomedical, Aalborg, Denmark) was used to deliver the visceral stimuli. The intensity of the current was limited to 80 mA and voltage to 200 V. After transnasal insertion, the endoscope was advanced 5 cm above the gastro‐oesophageal junction. An anatomical landmark was identified to ensure the same position of the electrodes for the two stimulation sessions. The stimulator forceps was inserted and slightly pressed against the mucosa, resulting in an impression of 0.5 cm, which could not be felt. Inter‐electrode impedance was <2 KΩ throughout the experiment.
Thirty five stimuli were applied at 0.2 Hz. Each stimulus was a train of five rectangular constant current pulses with a duration of 1 ms delivered at 200 Hz. The intensity of the current was first increased in steps of 1 mA, starting at 0.5 mA. Dependent on the subject's reaction, the steps could be reduced to 0.1–0.5 mA. During stimulation, the sensation was rated on a visual analogue scale which was anchored at 0 = no sensation to 10 = unbearable pain. Hence subjects were instructed to rate the sensation as 1 on the scale when they could feel the stimulus and 5 when the sensation changed from unpleasantness to pain (pain detection threshold (PDT)).11 The subject rested quietly and relaxed with the eyes open and was asked to minimise blinking and focus on a fixed point. Thirty five stimuli were then given with an intensity corresponding to the PDT. During the initial electrical stimulation where the PDT was found, visual control was maintained. When the EEG was recorded, visual control was not possible due to noise from the equipment interfering with the recordings but the position of the forceps was controlled visually between the stimulation blocks. If inter‐electrode impedance increased, reflecting a change in scope position, visual control was re‐established and the electrodes placed in position. After this stimulation the subject was allowed to rest with the endoscope in situ for 30 minutes, and then the stimulation was repeated for reproducibility at the same anatomical position guided by the landmarks. During the stimulation subjects were observed by a physician, and the electrocardiogram was continuously monitored.
Recordings
The EEG was recorded from 64 surface electrodes using a standard EEG cap (Quick‐Cap International, Neuroscan, USA) following the extended international 10–20 system. Impedance was below 5 kΩ. In addition, two electrodes were placed at the right upper brow and the left external canthus to monitor eye movements. A linked ears reference was used. EEG signals were sampled at 1000 Hz and band pass filtered between 0.05 and 70 Hz (SynAmps, Neuroscan). ERPs were gathered separately and sampled from 100 ms before and 500 ms after the onset of the stimulus. The grand mean of the ERPs from all subjects obtained at baseline and at the reproducibility experiment after 30 minutes is shown in fig 1. ERPs of the separate runs were appended to one single file for further offline analysis. The data were corrected for linear trends. ERP images, which are two‐dimensional, colour coded potential variations of the event locked EEG waveforms, were generated for the individual channels, as shown in fig 2. A small picture of the conventional ERPs was shown below the ERP image. ERP images were used instead of conventional evoked brain potentials mainly as they are suitable for detecting and removing stereotyped eye, muscle, and noise artefacts.12
Figure 1 Grand mean of the evoked brain potentials from all 12 subjects following electrical stimulation of the distal oesophagus. The solid line represents the baseline vertex potential and the broken line the reproducibility experiment after 30 minutes. The stimulus was given at the vertical bar (time 0) and N1, N2, P1, and P2 denote the first and second negative and positive peaks, respectively.
Figure 2 Event related potential (ERP) image of the electroencephalography (EEG) in Cz in a selected subject. The image consists of 35 parallel lines representing individual trials stacked on top of each other, as shown on the y axis. The amplitude of the EEG is coded in colours corresponding to the vertical bar (µV). The traditional one dimensional ERP showing the mean deviation (in μV) is displayed under the ERP image. The activity power spectrum of the EEG is shown in the bottom picture.
Concept for EEG analysis and explanation of features
The concept of the analysis leading to the illustration of event related brain dynamics is illustrated step by step in fig 3. The main features are described below whereas more detailed accounts are given in the appendix.
Figure 3 Flow chart of the data processing. EEG, electroencephalography; ICA, independent component analysis; ERP, event related potentials. For details of the analysis, see methods section and appendix.
ERP image
ICA was applied to the single trial data resulting in separation of 64 maximal independent components. Each component is composed of a two dimensional image (ERP image)(similar to fig 2) where the potentials for each of the 35 recordings are sorted in order of time and then plotted as parallel lines where the colour code represents the amplitude of the EEG potential.13,14 Furthermore, a scalp map was displayed (see figs 4, 5).
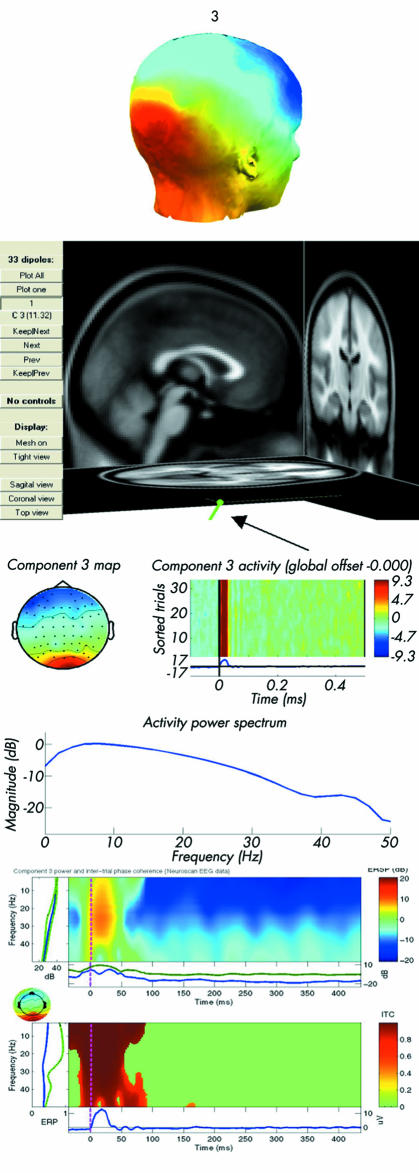
Figure 4 Dipole from an independent component representing a typical artefact outside the skull. The picture represents from the top:(1) surface scalp map showing the interpolated projection of the component to a head model;(2) equivalent current dipole projected into a standardised brain image;(3) event related potential image, the scalp map showing the projection of the component on the scalp electrodes and power spectrum, as described in fig 2, but where the EEG is now replaced with the independent component;(4) event related spectral perturbation (ERSP); and (5) inter‐trial coherence (ITC) images. ERSP is shown as a two dimensional (frequency by latency) image of mean change in spectral power (in dB, as shown on the vertical bar) from baseline. ITC show the strength (from 0 to 1, as shown in the vertical bar) of phase locking of the electroencephalography signals.
Figure 5 Independent electroencephalography (EEG) components localised in the thalamus, middle part of cingulate gyrus (MCC), insula, and sensory cortex of a selected subject (No 2 in table 1 and fig 6). The picture represents from the top:(1) surface scalp map showing the interpolated projection of the component to a head model;(2) equivalent current dipole projected into a standardised brain image;(3) event related potential image, the scalp map showing the projection of the component on the scalp electrodes and power spectrum;(4) event related spectral perturbation (ERSP); and (5) inter‐trial coherence (ITC) images. ERSP is shown as a two dimensional (frequency by latency) image of mean change in spectral power (in dB, as shown on the vertical bar) from baseline. ITC show the strength (from 0 to 1, as shown in the vertical bar) of phase locking of the EEG signals.
Artefacts
The ICA was also used for artefact rejection. ERP images from all components were investigated and a dipole analysis (see below) was performed. Artefacts identified with the two methods were then compared and components relating to stimulus artefacts were identified.
Event related spectral dynamics and inter‐trial coherence analysis
To examine stimulus and response induced changes in the EEG spectrum, we computed event related spectral perturbation (ERSP) transforms for each ICA component.12,15 ERSPs identify characteristically changes in spectral power (figs 4, 5). Inter‐trial phase coherence (ITC) analysis tests the degree of phase locking (that is, non‐random phase relationship) between EEG processes and was used to test the validity and degree of consistency of the experimental events (see figs 4, 5).16
Extraction of dipoles
The signal sources identified with ICA have been shown to be significantly more “dipole‐like” than the raw EEG, which is an a priori advantage for the dipole analysis.12 Dipoles were computed with software from the EEGLAB toolbox.12 For illustration of dipoles corresponding to the components, a four shell model simulating brain, dura, skull, and skin was used from the EEGLAB toolbox.
Extraction of the most valid components
After the analysis the most valid components were selected according to the following criteria for their dipoles and time/frequency images:(1) residual variance of the dipole corresponding to the component in question should be less than 18 %;(2) components should have a clearly well defined pattern in the time and/or frequency domain (ERP and ERSP images);(3) based on the ITC the temporal evolution of the EPR images should be reliable throughout the 35 recording trials; and (4) dipoles of the components should be found in the same brain region in the reproducibility experiment.
Cross coherence analysis
Cross coherence analysis of the components was used to identify synchrony (the degree of phase locking) and thus dynamic links between different networks of neurones.17 To examine the degree of synchronisation between selected components, we computed the event related cross coherence.12 High cross coherence amplitude and zero phase difference at a given frequency indicate a high degree of synchronisation between the components. Cross coherence was performed for the components to dipoles in the thalamus, insula, posterior, middle, and anterior cingulate gyrus, and sensory cortex in the subject selected for fig 5.
Results
All subjects completed the experiment. Median electrical intensity used to evoke pain was 12 (5–28) mA. Only two females were recruited for the study and although the small number did not allow determination of any differences in sex, the frequency characteristics of the components, their dipole localisation, and the cross correlation did not differ from those in males. No autonomic symptoms or cardiac arrhythmias were observed, and no subjects had any complaints at the follow up visit. As expected, there was individual variation in the localisation of the estimated dipoles to the individual components. Hence fig 5 shows the dipoles and corresponding ICA for a typical subject (male aged 24 years—subject 2 in table 1, fig 6). In case this subject displayed more dipoles in a given anatomical area, the component with dipoles which mostly resembled that of the other subjects was selected.
Table 1 Residual variance (%, top) and latency (ms, bottom) for the first activation of the independent components with dipoles in the thalamus, anterior, middle, and posterior cingulate cortex (ACC, MCC, PCC), sensory cortex (Sensory), insula, and cerebellum.
| Subject No | Thalamus | ACC | MCC | PCC | Sensory | Insula | Cerebellum |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 13 | 10 | 11 | 6 | 15 | 11 |
| 50 | 150 | 100 | 60 | 125 | 125 | 250 | |
| 2 | 8 | 11 | 7 | 4 | 7 | 5 | 17 |
| 50 | 150 | 150 | 100 | 150 | 125 | 250 | |
| 3 | 8 | 13 | 5 | 7 | 6 | 17 | 17 |
| 50 | 75 | 70 | 50 | 150 | 150 | 240 | |
| 4 | 12 | 15 | 4 | 16 | 6 | 15 | 11 |
| 50 | 75 | 75 | 50 | 100 | 100 | 250 | |
| 5 | 10 | 11 | 8 | – | 13 | – | – |
| 50 | 125 | 100 | 100 | ||||
| 6 | – | 15 | 12 | – | 4 | – | – |
| 100 | 75 | 125 | |||||
| 7 | 2 | 11 | 3 | 5 | 10 | 2 | 11 |
| 50 | 100 | 75 | 50 | 125 | 100 | 300 | |
| 8 | 17 | 4 | 12 | 7 | 6 | 6 | 11 |
| 50 | 90 | 75 | 50 | 100 | 125 | 225 | |
| 9 | 4 | 9 | 14 | 12 | 6 | 10 | 15 |
| 50 | 100 | 60 | 50 | 150 | 125 | 275 | |
| 10 | 11 | 9 | 8 | 6 | 6 | 17 | 7 |
| 40 | 100 | 75 | 50 | 100 | 125 | 225 | |
| 11 | 13 | 7 | 6 | 9 | 4 | 12 | – |
| 40 | 110 | 85 | 50 | 100 | 125 | ||
| 12 | 11 | 8 | 4 | 12 | 5 | 6 | 12 |
| 50 | 90 | 75 | 50 | 110 | 120 | 300 |
−, Consistent dipoles not found according to inclusion criteria (see text).
Figure 6 Localisation and orientation in the brain in the saggital view (arrows) for the first activation of independent components with dipoles in the thalamus, anterior, middle, and posterior cingulate cortex (ACC, MCC, PCC), sensory cortex (Sensory), insula, and cerebellum. B, bilateral; R, right; L, left. Consistent dipoles not found according to inclusion criteria (see text).
Artefacts
Pilot experiments showed that, for example, eye movement artefacts located in the frontal area could easily be reproduced when ICA was applied to the data during the recordings before pain stimulation. The outcome of the artefact rejection procedure showed that it separated the artefacts and considered them as independent activities out of the cortex. A typical artefact identified with the rejection procedure is shown in fig 4. The location was clearly outside the skull, situated in the neck area with a latency of a few milliseconds. The artefact also clearly demonstrated time and phase locking in a short period relating to the stimulus, after which there was no coherence and total desynchronisation in the ERSP picture. Hence electrical activity had probably propagated through the chest tissue to reach the skin in the neck area.
Cortical activations
Cortical regions, residual variance, and latencies of the first time locked activity of the dipoles in the baseline experiment for all subjects are shown in table 1. In fig 6, orientation of the components are shown together with information about laterality. In three subjects the signal to noise ratio of the temporal electrodes was rather low, resulting in high residual variance for the dipoles. Thus components with dipoles fulfilling the criteria for inclusion were not seen in all brain areas. In the nine subjects with optimal quality of the EEG signals, components with dipoles fulfilling the criteria were most consistently identified in the thalamus, posterior‐middle and anterior cingulate cortex, insula, sensory cortex, and cerebellum. However, other areas were also reliably identified in most subjects, although there was major interindividual variation.
Components with thalamic dipoles
As shown in table 1, 11 subjects had clearly defined components with dipoles in the thalamus, and in four subjects the components were bilateral. An example from a selected subject is shown in fig 5. Typically, the components were time and phase locked intermittently, starting around 50 ms. In the frequency domain, the thalamic components had early synchronisation in the 5–30 Hz range and were followed by alternating synchronisation and desynchronisation in the same frequency range, as typically shown in fig 5.
Components with dipoles in the cingulate gyrus
Components with dipoles in the middle and anterior part of the cingulate gyrus were clearly identified in all subjects whereas 10 also had posterior activation. The component with a posterior cingulate dipole from the selected subject is shown in fig 5. The dipoles were mainly unilateral but due to limited spatial resolution of EEG processing and the close relation between the two cingulate gyri, bilateral activation could not be excluded. The time locked activities of the posterior areas were always firstly activated, followed by the middle and anterior regions (table 1). The direction of the dipoles varied considerably and activation was longstanding in most subjects. The frequency content of the components was predominantly synchronised in the 0–20 Hz range.
Components with insular dipoles
Ten subjects showed a clear insular activation (fig 5) whereas residual variance for the dipoles in the remaining two subjects was slightly above 18%. The components showed bilateral dipole activation with the time locked activation occurring simultaneously. Orientation of the dipoles was mainly radial. Time and phase locked activity was typically maximal after 125 ms. Based on the latency, the dipoles were probably activated shortly after the thalamic dipoles. Synchronisation in the frequency content was mainly seen in the 0–30 Hz range, simultaneous with the time locked activity.
Components with dipoles in the sensory cortex
Components with dipoles in the sensory cortex had a mainly radial direction and were seen in all subjects, although tangential orientation was also occasionally seen (figs 5, 6). Components with dipoles that fulfilled the inclusion criteria were localised bilaterally throughout the more lateral part of the postcentral gyrus (the primary somatosensory area) in all subjects. In some subjects there was a close relation with the pharynx area (according to the somatosensory homunculus) but in others activations were closer to the opercular region. Ten of the subjects had more than one component with dipoles in the sensory cortex. Time and frequency locked latency for the activations varied between 100 and 300 ms and phase consistent EEG activity was very individual and spread throughout the 0–40 Hz range.
Components with dipoles in other areas
Components with reproducible dipoles were seen in the cerebellum, and frontal and motor cortex in many subjects. However, the location of the dipoles showed major interindividual variation. Components with dipoles in the cerebellum were found in nine subjects with a latency of approximately 250 ms, and components with dipoles in the motor cortex were found in seven subjects with activation after 150 ms. Other brain areas were also activated but only in single subjects and these are not reported.
Cross correlation analysis between components
In the subject used in fig 5, cross correlation of the independent components with dipoles in the above brain areas was performed to identify the degree of phase synchronisation between the neuronal centres. The latency and frequency content of the first synchronous activations are shown in table 2. Phase synchronisation in the beta (14–25 Hz) and gamma (25–50 Hz) bands was mostly seen although the synchronisation between the posterior and middle cingulate cortex was in the alpha (8–14 Hz) range. Synchronisation between the thalamus and insula/sensory cortex was rather early whereas the suggested communication between the insula and cingulate cortex took place after 150–350 ms. The findings suggest upstream activation from the thalamus to the insula and sensory cortex, mainly in the beta band. In the cingulate cortex, integration between the posterior, middle, and anterior areas took place in all three frequency bands. Cross correlations were also performed for the reproducibility experiment in this subject and showed similar results with respect to latency and frequency content (data not shown). Analyses were also done for other subjects and similar early correlations in the beta and gamma bands were mainly found (data not shown).
Table 2 Source coherence between the independent components shown for the selected subject in fig 5.
| Thalamus | Insula | PCC | MCC | ACC | |
|---|---|---|---|---|---|
| Insula | 75, β | ||||
| PCC | 75, β | 150, γ | |||
| MCC | 100, β | 350, γ | 120, β | ||
| ACC | 200, γ | 150, β | 100, α | 150, γ | |
| Sensory | 60, γ | 50, β | 130, γ | 150, β | 100, γ |
Cross correlation was performed to identify the degree of phase synchronisation between the components. The latency (ms) of the first synchronous correlation with a correlation coefficient above 0.45 is shown together with the dominant frequency content of the first synchronic activity.
ACC, MCC, and PCC, anterior, middle, and posterior cingulate cortex; Sensory, sensory cortex
α = 8–14 Hz;β = 14–25 Hz;γ = 25–50 Hz.
Discussion
This is the first time independent component analysis has been used to describe time and phase locked EEG activity to pain stimuli. The method overcomes many of the classical problems in EEG analysis while preserving temporal resolution. Active brain sources were seen in the thalamus, insula, posterior, medial, and anterior cingulate gyrus, and sensory cortex. The method not only provides anatomical information but increases the information by demonstration of synchronous neural oscillations, especially in the beta and gamma bands between different brain regions.
Methodological considerations
Subjects were intubated via the nasal route with an ultra thin endoscope. Compared with other invasive methods,18 the endoscope was tolerated for several hours, and when subjects became accustomed to the procedure it did not evoke any form of unpleasantness. Other methods with blinded stimulation of the viscera (such as electrodes mounted on catheters) may carry the risk of variable quality of the stimulation.11 The current method is rather invasive compared with smaller catheters but when the nasal route can be used it is our experience that the size of the device does not matter. We used electrical stimulation, which in the gut stimulates a mixed population of afferent fibres consisting mostly of small myelinated A‐delta fibres together with a few non‐myelinated C‐fibres.19 Mechanical stimulation can also be used for recording of ERPs but electrical stimuli are more reliable and both stimuli are thought to activate a similar network of cortical neurones.20 The earliest thalamic response seen after approximately 50 ms reflects the conduction velocity in A‐delta fibres (9–14 m/s),21,22 which is consistent with animal data showing that 40% of visceral afferents responsible for nociceptive information are myelinated.23
Several methods are available to model the brain centres responding to painful stimuli. Methods based on blood flow changes such as positron emission tomography (PET) and fMRI have been applied to visceral pain stimuli of the oesophagus.24,25 However, activation and deactivation of multiple areas in the brain takes place shortly after stimulation, and many of these processes are common for other sensory events not relating specifically to pain. Such complexity causes major difficulties in the interpretation of the evoked pain response when slowly changing alterations in blood flow are used for monitoring.5,6,26 In contrast with these methods, MEG and EEG based experiments detect neuronal activity with a very high temporal resolution.6,9,27 The major problem with EEG is the varying effect of volume conduction leading to poor spatial resolution of far field scalp sources. ICA minimises the effect of volume conduction and identifies the activity of the individual cortical sources with high accuracy.10 In contrast with most other models for dipole analysis, the ICA algorithm is more objective, relying only on statistics, and does not implement any assumptions on the biophysics or geometry of the head. The analyses were performed for individual subjects as the averaging process may cancel out the activity of many brain sources. This is especially the case with ICA where averaging may cause instability and carry the risk to assign more sources into a single component.15
Activation in different brain regions to oesophageal pain
Previous source analysis of early brain activation to stimulation of the oesophagus has been modelled based on MEG or EEG signals. The first MEG based studies found evidence for bilateral activation of the primary and secondary sensory cortex, insula, cingulate, and prefrontal cortex.28,29,30,31 Previous EEG studies using brain mapping and dipole source modelling of ERPs to mechanical stimulation of the oesophagus found evidence of dipoles close to the cingulate gyrus and insula.32,33,34 We have modelled the electrical dipoles to painful electrical stimulation of the sigmoid colon.8 The earliest dipolar activities to the stimulation were observed in the bilateral insula and in the anterior cingulate cortex, while the secondary sensory cortex on both hemispheres was activated later. However, brain activation of the upper and lower gut may differ35 and these results cannot be directly compared with those after oesophageal stimulation. Recently, Hobson et al recorded both EEG and MEG responses to painful electrical stimulation of the distal oesophagus.36 The method of MEG analysis used an adaptive beam forming technique, allowing estimation of virtual depth electrodes overcoming the usual weakness in MEG to identify deep sources.37 In this study the earliest cortical activity was recorded in the sensory cortex and posterior insula followed by the anterior insula and cingulate cortex. In our study, ERP images showed that the thalamus was activated first, followed by activation of the posterior cingulate. The more anterior parts of the cingulate were then activated simultaneously with the insula. Our latency readings in the ERP images were validated with respect to amplitude and phase by the ITC and ERSP diagrams. However, temporal resolution may not be as detailed as that used in, for example, MEG studies where recordable neuronal activity is more short lasting and well defined. On the other hand, cross correlations analysis showed that upstream activation from the thalamus to the insula and from the insula to the sensory cortex was very early (∼ 75 ms), and probably the early insular activation in these networks are of more importance to explain the exogenous brain activation than a simple description of active brain areas.
The latency of the activations in the current study was compatible with upstream activation of the thalamus, followed by the insula and then the sensory and cingulate gyrus. The thalamus is believed to be a relay area, receiving afferent information from the spinothalamic tracts and the activation pattern is consistent with this function.26 However, in most subjects long lasting activation and deactivation in the ERSP was shown from 50 ms to the end of the time window (500 ms). This may reflect the fact that the thalamus is not only a relay but may also orchestrate activation of different perceptions, emotions, and actions during processing of pain.
Activation of the insula was bilateral. Previous EEG studies also found evidence for early bilateral insular activation.8,32 In common with the MEG studies of the oesophagus,30,31,36 we found insular activation shortly before or simultaneously with activation of the sensory cortex in most subjects. This is in agreement with animal studies of anatomical data, suggesting that the insula has an important function for integrating visceral sensory and motor activity together with limbic integration.38 Similar to the thalamus, insular activation sequentially covered most of the selected time window and is probably also a candidate for coordination of dynamic links between different brain areas.
Independent components with early activation were also localised in the cingulate gyrus. Activation started in the posterior part, but according to the latency of the time and frequency locked activation, it progressed to the medial and anterior parts. Activation of the cingulate cortex was long lasting with alternating synchronisation and desynchronisation of the frequency content. The cingulate gyrus was also activated in EEG studies,8,32 and in most PET and fMRI studies to stimuli of the oesophagus, stomach, and rectum.1,25,39,40 Activation of the cingulate gyrus was observed in most studies of somatic pain, especially when associated with a strong emotional response to the stimulus.9,41,42,43 Hence it has been proposed that cingulate activation is associated with, for example, attention, anticipation, and affective/cognitive responses to pain.7,42 The cingulate gyrus has direct connections to a variety of brain areas, such as the limbic system, autonomic effector areas (vagal motor nucleus, amygdala, hypothalamus), and centres of arousal and pain modulation (periaqueductal grey, locus coeruleus). Sequential cingulate activation observed in the current study is in line with such complex activation and illustrates the many functions of this brain area during processing of painful visceral stimuli. Different functions of the posterior and anterior cingulate cortex were previously demonstrated44 as well as abnormal cortical activation in the anterior cingulate cortex in patients with visceral pain due to the irritable bowel syndrome.3,45 Future studies should aim at using the current method to evaluate cingular activation in patients with diseases of the oesophagus.
Components with dipoles in the sensory cortex were bilateral and localised in several regions of the postcentral gyrus, representing the somatosensory area. The primary somatosensory area was also activated in MEG studies of the oesophagus,28,29,36 where the dipole to oesophageal stimulation was close to the cortical representation of the pharynx and trunk regions. Components were individually spread in the somatosensory cortex and in some subjects the dipoles were close to the temporal gyrus and operculum, representing the secondary somatosensory area. Bilateral activation of this area has been suggested to be involved in attention and rating of the strength and quality of pain by comparing hurting and non‐hurting sites.43 In previous MEG studies to stimulation of the oesophagus, the secondary somatosensory area was consistently activated.28,29,30,31,36 Direct projections from the thalamus to the secondary sensory cortex have been demonstrated in anatomical studies,46 and the rather early activation suggests that this area is an important projection for gut afferents.
Frequency analysis and synchronous neural oscillations activated to oesophageal pain
The spectral perturbation analysis gives indirect information about functions in the brain areas identified by the ICA. Previous studies have used EEG to demonstrate tonic somatic pain related increases in beta (14–25 Hz) and gamma (32–100 Hz) activity together with a decrease in alpha (8–12 Hz) activity.47,48,49 These experiments were however based on different pain stimulations and surface EEG recordings, thus subjected to major uncertainties with respect to the localisation of the active neuronal centres. ERSP for the independent components (as shown for the selected subject in fig 5) gave some indication of the mean changes in spectral power and hence dominant EEG activity. According to these activations, the dominating EEG was spread in the range from 0 to 40 Hz, and the lower frequency bands in particular were also activated. Consistent with these results, an increase in the lower frequency bands has been observed during pain stimuli of the skin and muscle.50
Observations of the frequency content in scalp EEG or individual dipoles are however of less relevance compared with dynamic connections between different centres in the brain during the processing of pain. Thus local networks of neurone assemblies are thought to be correlated when there is phase locking between the EEG rhythms.17,50 Recently, models have linked synchronous EEG activity of the brain with the dynamics of fundamental processing in a network of neuronal centres. Cognitive processing such as memory and attention seems to be coupled to synchronised activity in the theta/gamma and alpha/gamma rhythms.51,52 Hence local and large scale integration of synchronous activity between regional neuronal assemblies may represent a tool to understand pain processing from a dynamic point of view, as opposed to the more traditional static models. Studies using conventional EEG have typically applied spectral analysis to assess the degree of synchrony between scalp electrodes. However, such an approach is ambiguous as the surface EEG is produced by various amplitude changes in several processes at different brain locations.15 The current data obtained from the cross correlation of ICA overcome the signal mixture problems with surface EEG and indicate that large scale integration between neuronal centres involved in oesophageal pain mainly takes place in the beta and gamma bands. The time sequence of the phase synchrony was consistent with upstream from the thalamus to the insula and sensory cortex. Large scale synchrony in the gamma band has previously been shown during cognitive processing, memory, and attention.17,51,53 In table 2, only the first significant correlation was shown, but later in the time window correlations in other frequency bands were also found. However, a detailed and comprehensive analysis of these data should be reserved for a study specifically designed for this purpose.
The relationship between different centres may be of major importance in our understanding of abnormal processing of gut pain. Most neuroimaging studies are based on a priori assumptions of correlations between active centres, and only rarely experiments describe causation relating to the information of flow between brain areas. It has been suggested that patients with functional diseases of the gut suffer from decreased descending inhibition of the afferent activity from brainstem structures. Recently, Mayer et al challenged this hypothesis using connectivity analysis in a PET study to evaluate the covariation of different brain regions activated to rectal distension. They found evidence for a lack of activation of inhibitory corticolimbic systems in patients with irritable bowel syndrome whereas healthy controls and patients with ulcerative colitis (with quiescent disease) showed the expected brainstem (dorsal pons and periaquaductal grey) inhibition by networks between the ventrolateral frontal cortex and the medial prefrontal cortex.54 Such analysis of the “brainweb” could also be done with the current method which has much better temporal resolution, targeting the direct electrical responses in the active sources. As there is evidence of abnormal pain processing in the central nervous system in patients with, for example, non‐cardiac chest pain,2 comparative studies using ICA at the EEG following stimulation of the oesophagus may increase our understanding of symptoms thought to arise from the gut.
Conclusion
The independent component analysis used in the present study demonstrated sequential activation of the thalamus, insula, cingulate, and somatosensory cortex to stimulation of the oesophagus, with large scale synchrony between the components in the beta and gamma bands. The study provides new information about brain activation and dynamic processing of visceral pain. The method should be used in future studies to increase our understanding of the supraspinal processing of visceral pain in health and disease.
Acknowledgements
“Det Obelske Familiefond”, “Spar Nord Fonden”, the Danish Technical Research Council, and NeuralPRO for Research, awarded by the Commission of the European Union (Contract No HPRN‐CT‐2000‐00030), are acknowledged for financial support.
Abbreviations
EEG - electroencephalography
ERP - event related potentials
ERSP - event related spectral perturbation
ICA - independent component analysis
ITC - inter‐trial phase coherence
fMRI - functional magnetic resonance imaging
MEG - magnetoencephalography
PDT - pain detection threshold
PET - positron emission tomography
Appendix
ADVANTAGES OF THE INDEPENDENT COMPONENT ANALYSIS
Traditionally, methods based on signal form and analyses are used with the aim of studying pain related cortical activity. EEG activity directly reflects sources of cortical and subcortical signals where local field activity is similarly oriented by cortical geometry and partially synchronised.55 However, peaks in multichannel ERPs may be composed of activity in several neuronal sources and the recorded scalp maps can be generated by a large number of multisource models.56 Thus each electrode on the scalp surface records signals from oscillatory activity in a localised brain region. Furthermore, tangential current flow may not be detected even though the electrode located above the active brain region is selected57 and even focal brain activity when deeply located generates very widespread EEG patterns. Therefore, a major obstacle to using EEG data is the underdetermined nature of the “inverse problem”: given an EEG distribution from any number of scalp channels, any number of theoretical distributions of brain sources can be found that would produce it.57 The problem of finding the locations of many active dipoles simultaneously is, for practical purposes, unsolvable. The inverse problem is further complicated by the finding that early signalling may not only be “bottom up”, but concurrent “top down” activity also takes place.57,58 For this reason, inverse modellers have tended to focus their attention on cases in which a single dipolar scalp map is observed and expected. Other attempt to fit multiple dipoles to longer portions of ERP waveforms were based on “prior knowledge”(or guess) as to where the sources should be located and thus subject to bias.9
To bypass these problems, we applied ICA to our data. The independent sources have been shown to be significantly more “dipole‐like” than the surface based raw EEG, even though neither the locations of the electrodes nor the biophysics of volume propagation figure into the ICA algorithm.15 This finding is consistent with their generation through partial synchrony of local field potential processes in a connected patch or domain of cortex. The success of ICA applied to EEG data is strictly determined by the degree to which EEG dynamics fit the ICA model. The first requirement that the underlying sources mix linearly in the electrode recordings is assured by the biophysics of volume conduction at EEG frequencies.59 The assumption of relative spatial stationarity of EEG sources is compatible, at least, with evidence of brain modularity from anatomy and functional imaging. Finally, the assumption of relative independence of the source signals is in line with physiological models that emphasise local intracortical and thalamocortical coupling in the generation of local electrical synchronies.60
METHODS USED IN THE ANALYSIS
Independent component analysis (ICA) and event related potential (ERP) images
Infomax ICA61,62 is one of a family of algorithms that exploit temporal independence to perform blind separation of underlying data sources. In the current experiment the runica algorithm (available with the EEGLAB toolbox of Delorme and Makeig12) was applied to the 35 single trials with pain stimulation of the oesophagus. The trials were time locked from −100 ms before to 500 ms after the onsets of the electrical mucosa stimuli. The natural gradient approach was used for the ICA. The analysis was applied to single trial data resulting in separation of 64 components having maximal temporal independency. Each component is comprised of a time course of its activity in every trial and a scalp map giving the strength of the volume conducted component at each scalp electrode (see fig 2).10 A two dimensional image (ERP image), similar to that in fig 2, was displayed for the independent components where the potentials for each component are sorted in order of time and then plotted as parallel coloured lines, forming a coloured image.14
Artefact rejection
ICA analysis was used to optimise the artefact rejection procedure. Artefact rejection was done based on the location, time, and spectral content of the components. Commonly in ERP research neural activity expressed in ocular data channels is ignored for fear of mislabelling eye activity artefacts as brain activity. Some ICA components of EEG recordings can be clearly identified as accounting primarily for eye movements, line or muscle noise, or other artefacts through their characteristic scalp maps and activity time courses.14,62 The total of 64×12 = 768 component maps and mean activity spectra from the 12 subjects were investigated with the ERP images. Furthermore, a dipole analysis (see below) was also performed. The artefacts identified with the two methods were then compared and components relating to stimulus artefact, eye movements, temporal muscle activity, and alpha ringing were removed. The artefacts identified with the two methods were mostly identical although the dipole analysis clarified the nature of the artefacts to a higher degree.
Event related spectral dynamics (ERSP) and inter‐trial coherence (ITC) analysis
Although time and frequency methods are complementary, ERPs carry the risk of discarding much of the dynamic information contained in the original data. Adding power spectrum analysis can be of value but is confounded by positive and negative potentials from different sources, cancelling each other.62 ERP images, ERSP, and ITC applied to the independent components reveal trial to trial stimulus and/or response locked features otherwise hidden in the total EEG variability.12,63 We computed event related spectral perturbation (ERSP) transforms for each ICA component using the EEGLAB toolbox.12,64 ERSPs identify characteristic changes in spectral power (in dB) from baseline across a frequency range of 3–50 Hz (see figs 4, 5). ERSP can be used to reflect the mean changes in spectral power for the extracted components and thus their contribution to large scale integration of the pain response.
To test the validity and degree of consistency of the experimental events, we used inter‐trial phase coherence (ITC) analysis. ITC tests the degree of phase locking (that is, non‐random phase relationship) between EEG processes and the occurrence of experimental events across trials (see figs 4, 5).16 All changes shown are significant at p<0.05, according to the bootstrap statistics. ITC measure takes values between 0 and 1. A value of 0 represents no degree of synchronisation between EEG data and the time locking events; a value near 1 indicates their perfect degree of synchronisation. From the frequency‐domain point of view, ERPs are partly produced by this phase locking thus providing additional information about the relationship between the ERP of the independent component and whole EEG data.10
Extraction of dipoles
ICA identifies temporally independent signal sources in multichannel EEG data, and their projections to the scalp surface. These have been shown to be significantly more “dipole‐like”(or dipolar) than the raw EEG, even though neither the locations of the electrodes nor the biophysics of volume propagation figure in the infomax algorithm.12 For extraction of dipoles, the default is a four shell model simulating brain, dura, skull, and skin. These parameters correspond to typical parameters, which were also implemented as defaults in programs such as BESA 3.0 (Megis Software; MEGIS Software GmbH, Gräfelfing, Germany). The program models the conductance values for each of these shells. The residual variance of the dipole fits in the current study varied from 4% to 17%. Even higher residual variances up to 40% have been found in other recent studies using physiological stimuli65 but in other studies where relatively simple stimuli were analysed lower residual variances have been found.10 Thus in our laboratory we have found residual variances as low as 0.5% to tactile stimuli (unpublished data). However, pain processing is more complex than many other sensory modalities and involves many brain regions reflecting, for example, intensity, unpleasantness, cognition, and affective responses. Therefore, we applied three more criteria for selection of the dipoles and accepted higher residual variance values to allow more components to fulfil the inclusion criteria.
Cross coherence analysis
Cross coherence analysis of the components is used to identify synchrony between temporal structures of the signals. Previous research has provided evidence that oscillations in different frequency bands may reflect large scale integration of brain activity within behaviour, cognition, memory, and pain.17,51,52,53 The method determines the degree of phase locking between two events and coherent activation of the frequencies have been considered the most plausible candidate for dynamic links between different networks of neurones.17 In conventional EEG analysis interpretations of electrode coherences are ambiguous as they may be produced by various amplitude changes in several processes at different brain locations. The source analysis overcomes the problem of the overlapping sensor sensitivity profiles, making it possible to obtain more conclusive evidence for the origins of the observed activity. The interactions between cortical areas may then be analysed by estimating the phase locking from one source to another.12 To examine the degree of synchronisation between the components, we computed the event related cross coherence using the EEGLAB toolbox.12 The analysis gives the amplitude (index the amount of synchronisation at a given frequency) and phase (which of the two component activities that lead at the frequency in question). High cross coherence amplitude and zero phase difference at a given frequency indicate a high degree of synchronisation.
Footnotes
Conflict of interest: None declared.
References
- 1.Silverman D H, Munakata J A, Ennes H.et al Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology 199711264–72. [DOI] [PubMed] [Google Scholar]
- 2.Hobson A R, Aziz Q. Brain imaging and functional gastrointestinal disorders: Has it helped our understanding? Gut 2004531198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drewes A M, Rossel P, Le Pera D.et al Cortical neuroplastic changes to painful colon stimulation in patients with irritable bowel syndrome. Neurosci Lett 2005375157–161. [DOI] [PubMed] [Google Scholar]
- 4.Derbyshire S W G. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol 20039812–20. [DOI] [PubMed] [Google Scholar]
- 5.Davis K D. Neurophysiological and anatomical considerations in functional imaging of pain. Pain 20031051–3. [DOI] [PubMed] [Google Scholar]
- 6.Aziz Q, Thompson D G. Brain‐gut axis in health and disease. Gastroenterology 1998114559–578. [DOI] [PubMed] [Google Scholar]
- 7.Chen A C. New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. Int J Psychophysiol 200142147–159. [DOI] [PubMed] [Google Scholar]
- 8.Drewes A M, Rossel P, Le Pera D.et al Dipolar source modelling of brain potentials evoked by painful electrical stimulation of the human sigmoid colon. Neuroscience Lett 200435845–48. [DOI] [PubMed] [Google Scholar]
- 9.Valeriani M, Le Pera D, Tonali P. Characterizing somatosensory evoked potential sources with dipole models: Advantages and limitations. Muscle Nerve 200124325–339. [DOI] [PubMed] [Google Scholar]
- 10.Makeig S, Debener S, Onton J.et al Mining event‐related brain dynamics. Trends Cogn Sci 20048204–210. [DOI] [PubMed] [Google Scholar]
- 11.Drewes A M, Gregersen H, Arendt‐Nielsen L. Experimental pain in gastroenterology: A reappraisal of human studies. Scand J Gastroenterol 2003381115–1130. [DOI] [PubMed] [Google Scholar]
- 12.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 20041349–21. [DOI] [PubMed] [Google Scholar]
- 13.Jung T P, Makeig S, Westerfield M.et al Analysis and visualization of single‐trial event‐related potentials. Hum Brain Mapp 200114166–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung T P, Makeig S, Mckeown M J.et al Imaging brain dynamics using independent component analysis. Proc IEEE 2001891107–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delorme A, Makeig S, Fabre‐Thorpe M.et al From single‐trial EEG to brain area dynamics. J Neurocomput 200244–461057–1064. [Google Scholar]
- 16.Makeig S, Westerfield M, Jung T P.et al Dynamic brain sources of visual evoked responses. Science 2002295690–694. [DOI] [PubMed] [Google Scholar]
- 17.Varela F, Lachaux J P, Rodriguez E.et al The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 20012229–239. [DOI] [PubMed] [Google Scholar]
- 18.Drewes A M, Arendt‐Nielsen L, Jensen J H.et al Experimental pain in the stomach: a model based on electrical stimulation guided by gastroscopy. Gut 199741753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tougas G, Hudoba P, Fitzpatrick D.et al Cerebral‐evoked potential responses following direct vagal and esophageal electrical‐stimulation in humans. Am J Physiol 1993264G486–G491. [DOI] [PubMed] [Google Scholar]
- 20.Hobday D I, Hobson A, Furlong P L.et al Comparison of cortical potentials evoked by mechanical and electrical stimulation of the rectum. Neurogastroenterol Motil 200012547–554. [DOI] [PubMed] [Google Scholar]
- 21.Bromm B, Treede R D. Pain related cerebral potentials: late and ultralate components. Int J Neurosci 19879514–23. [DOI] [PubMed] [Google Scholar]
- 22.Kakigi R, Endo C, Neshige R.et al Estimation of conduction‐velocity of A‐delta fibers in humans. Muscle Nerve 1991141193–1196. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta J N, Gebhart G F. Gastrointestinal afferent fibers and sensation. In: Johnson L, ed. Physiology of the gastrointestinal tract. New York: Raven Press, 1994484–519.
- 24.Kern M K, Birn R M, Jaradeh S.et al Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology 19981151353–1362. [DOI] [PubMed] [Google Scholar]
- 25.Aziz Q, Andersson J L, Valind S.et al Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology 199711350–59. [DOI] [PubMed] [Google Scholar]
- 26.Coghill R C. Brain mechanisms supporting the pain experience: a distributed processing system. In: Max M, ed. Pain 1999—an updated review. Seattle: IASP Press, 199967–76.
- 27.Hollerbach S. Cerebral evoked responses to gastrointestinal stimulation in humans. Crit Rev Biomed Eng 199725203–242. [PubMed] [Google Scholar]
- 28.Furlong P L, Aziz Q, Singh K D.et al Cortical localisation of magnetic fields evoked by oesophageal distension. Evoked Potent Electroencephalogr Clin Neurophysiol 1998108234–243. [DOI] [PubMed] [Google Scholar]
- 29.Hecht M, Kober H, Claus D.et al The electrical and magnetical cerebral responses evoked by electrical stimulation of the esophagus and the location of their cerebral sources. Clin Neurophysiol 19991101435–1444. [DOI] [PubMed] [Google Scholar]
- 30.Loose R, Schnitzler A, Sarkar S.et al Cortical activation during oesophageal stimulation: a neuromagnetic study. Neurogastroenterol Motil 199911163–171. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzler A, Volkmann J, Enck P.et al Different cortical organization of visceral and somatic sensation in humans. Eur J Neurosci 199911305–315. [DOI] [PubMed] [Google Scholar]
- 32.Franssen H, Weusten B L, Wieneke G H.et al Source modeling of esophageal evoked potentials. Electroencephalogr Clin Neurophysiol 199610085–95. [DOI] [PubMed] [Google Scholar]
- 33.Weusten B L, Franssen H, Wieneke G H.et al Multichannel recording of cerebral potentials evoked by esophageal balloon distension in humans. Dig Dis Sci 1994392074–2083. [DOI] [PubMed] [Google Scholar]
- 34.Aziz Q, Furlong P L, Barlow J.et al Topographic mapping of cortical potentials evoked by distension of the human proximal and distal oesophagus. Electroencephalogr Clin Neurophysiol 199596219–228. [DOI] [PubMed] [Google Scholar]
- 35.Hobday D I, Hobson A R, Sarkar S.et al Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol 2002283G335–G339. [DOI] [PubMed] [Google Scholar]
- 36.Hobson A R, Furlong P L, Worthen S F.et al Real‐time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology 2005128610–619. [DOI] [PubMed] [Google Scholar]
- 37.Hillebrand A, Singh K D, Holliday I E.et al A new approach to neuroimaging with magnetoencephalography. Human Brain Mapp 200525199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Augustine J R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 199622229–244. [DOI] [PubMed] [Google Scholar]
- 39.Mertz H, Morgan V, Tanner G.et al Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000118842–848. [DOI] [PubMed] [Google Scholar]
- 40.Ladabaum U, Minoshima S, Hasler W L.et al Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 2001120369–376. [DOI] [PubMed] [Google Scholar]
- 41.Casey K L, Minoshima S. Can pain be imaged. In: Jensen TS, Turner JA, Wiesenfeld‐Hallin Z, eds. Proceedings of the 8th World Congress of Pain, Progress in Pain Research and Management. Seattle: IASP Press, 1997855–866.
- 42.Ladabaum U, Minoshima S, Owyang C. Pathobiology of visceral pain: Molecular mechanisms and therapeutic implications v. central nervous system processing of somatic and visceral sensory signals. Am J Physiol Gastrointest Liver Physiol 2000279G1–G6. [DOI] [PubMed] [Google Scholar]
- 43.Bromm B, Scharein E, Vahle‐Hinz C. Cortex areas involved in the processing of normal and altered pain. Prog Brain Res 2000129289–302. [DOI] [PubMed] [Google Scholar]
- 44.Phillips M L, Gregory L J, Cullen S.et al The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain 20031261248. [DOI] [PubMed] [Google Scholar]
- 45.Verne G N, Himes N C, Robinson M E.et al Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 200310399–110. [DOI] [PubMed] [Google Scholar]
- 46.Burton H. Corticothalamic connections from the second somatosensory area and neighboring regions in the lateral sulcus of macaque monkeys. Brain Res 1984309368–372. [PubMed] [Google Scholar]
- 47.Backonja M, Howland E W, Wang J.et al Tonic changes in alpha‐power during immersion of the hand in cold water. Electroencephalogr Clin Neurophysiol 199179192–203. [DOI] [PubMed] [Google Scholar]
- 48.Drewes A M, Nielsen K N, Stengaard‐Pedersen K.et al Electroencephalographic reactions during experimental superficial and deep pain stimuli. J Musculoskel Pain 1999729–44. [Google Scholar]
- 49.Chang P F, Arendt‐Nielsen L, Graven‐Nielsen T.et al Different EEG topographic effects of painful and non‐painful intramuscular stimulation in man. Exp Brain Res 2001141195–203. [DOI] [PubMed] [Google Scholar]
- 50.Le Pera D, Svensson P, Valeriani M.et al Long‐lasting effect evoked by tonic muscle pain on parietal EEG activity in humans. Clin Neurophysiol 20001112130–2137. [DOI] [PubMed] [Google Scholar]
- 51.Ward L M. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci 20037553–559. [DOI] [PubMed] [Google Scholar]
- 52.Croft R J, Williams J D, Haenschel C.et al Pain perception, hypnosis and 40 Hz oscillations. Int J Psychophysiol 200246101–108. [DOI] [PubMed] [Google Scholar]
- 53.Sederberg P B, Kahana M J, Howard M W.et al Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 20032310809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer E A, Berman S, Suyenobu B.et al Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 2005115398–409. [DOI] [PubMed] [Google Scholar]
- 55.Bagshaw A P, Liston A D, Bayford R H.et al Electrical impedance tomography of human brain function using reconstruction algorithms based on the finite element method. Neuroimage 200320752–764. [DOI] [PubMed] [Google Scholar]
- 56.Hupe J M, James A C, Girard P.et al Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol 200185134–145. [DOI] [PubMed] [Google Scholar]
- 57.Hoechstetter K, Bornfleth H, Weckesser D.et al BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 200416233–238. [DOI] [PubMed] [Google Scholar]
- 58.Engel A K, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top‐down processing. Nat Rev Neurosci 20012704–716. [DOI] [PubMed] [Google Scholar]
- 59.Nunez P L.Electric fields of the brain. The Neurophysics of EEG. New York: Oxford University Press, 1981
- 60.Salinas E, Sejnowski T J. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 20012539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bell A J, Sejnowski T J. An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 199571129–1159. [DOI] [PubMed] [Google Scholar]
- 62.Makeig S, Bell A J, Jung T P.et al Independent component analysis of electroencephalographic data. In: Touretzky D, Mozer M, Hasselmo M, eds. Advances in neural information processing systems. Cambridge, Massachusetts: MIT Press, 1996145–151.
- 63.Stone J V. Independent component analysis: an introduction. Trends Cogn Sci 2002659–64. [DOI] [PubMed] [Google Scholar]
- 64.Makeig S. Auditory event‐related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol 199386283–293. [DOI] [PubMed] [Google Scholar]
- 65.Marco‐Pallares J, Grau C, Ruffini G. Combined ICA‐LORETA analysis of mismatch negativity. Neuroimage 200525471–477. [DOI] [PubMed] [Google Scholar]