Abstract
Background and aims
Fatigue is the commonest symptom described by patients in most populations with the autoimmune liver disease primary biliary cirrhosis (PBC), and appears to be unrelated to liver disease severity. At present, it is unclear how the fatigue experienced by patients (only characterised to date in cross sectional studies) evolves over time. In this study, we set out to address how fatigue had changed over four years of follow up in a geographically defined cohort of PBC patients who participated in an earlier cross sectional study of fatigue impact.
Methods
Participants in the original 2000 study who were still alive in 2004 were asked to complete the same fatigue assessment tool (fatigue impact score, FIS). In those who had died between 2000 and 2004, medical notes, death certificates, and primary care records were reviewed.
Results
A total of 108 of the original cohort of 136 patients were alive at the time of the follow up study, 99 of whom (92%) participated in the follow up study. With the exception of four patients who underwent transplantation between 2000 and 2004, all of whom showed significant improvement in fatigue severity as assessed by FIS, fatigue severity was unchanged over four years of follow up. Among the 28 patients who died during the follow up period, survival was significantly lower in patients who were fatigued at the 2000 baseline (FIS above the median for the whole PBC population (40/160); log rank test, p = 0.006 v non‐fatigued patients at baseline). Increased fatigue severity was independently associated with decreased survival on multivariate analysis. Fatigued PBC subjects were significantly more likely to have suffered a cardiac death than non‐fatigued patients.
Conclusions
The fatigue phenotype appears to be highly stable in PBC. The presence of fatigue in PBC is independently associated with a significantly increased risk of death in general, and cardiac death in particular. Factors underpinning fatigue in PBC, and the mechanisms whereby fatigue is associated with increased mortality, warrant further study.
Keywords: liver cirrhosis, biliary, epidemiology, fatigue, health related quality of life
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease with an autoimmune aetiology.1,2 Studies performed in UK3,4 Canadian,5,6 USA,7,8 and French9 populations have suggested that the condition is frequently characterised by profound fatigue which can often dramatically reduce patient quality of life.10 The severity of the fatigue experienced by UK PBC patients is significantly greater than that experienced by age and sex matched chronic disease and community controls.3,4 A recent small study based on a principally Scandinavian cohort did not, in contrast, identify significant fatigue in PBC patients, suggesting the possibility of population variation in symptom penetrance.11 In contrast with PBC associated pruritus, where significant recent progress has been made in understanding symptom pathogenesis and in optimising treatment,12 PBC associated fatigue remains largely resistant to treatment,13 although this is an area of active ongoing investigation.14,15 The limited studies performed to date in PBC and in animal models of cholestasis regarding fatigue pathogenesis have suggested a significant role for centrally mediated processes.16,17,18,19 Studies of the disease associations of fatigue in PBC have shown universal agreement that, in contrast with pruritus, the severity of fatigue experienced by PBC patients shows no relationship to any conventional parameter of liver disease severity, suggesting that fatigue may be less a direct result of liver pathology and more an independent manifestation of PBC as a systemic disease.3,5,6
All studies addressing fatigue in PBC published to date have been cross sectional in nature. It is therefore unclear how the impact of fatigue on individual patients changes over time. Indeed, it is not even clear whether the subgroup of patients experiencing fatigue (which ranges from 30% to 80% of all patients, depending on the population studied and the quantification approach used) is static or whether all patients experience fatigue some of the time. Furthermore, there is little information as to the relationship between the symptom of fatigue and prognosis. The aim of this study was to address the question of how fatigue changes over time in PBC, and to study the effects on clinical outcome of high and low fatigue states by performing follow up assessment of a representative group of PBC patients originally studied in 2000.4 This group of patients constituted a comprehensive unselected cohort of PBC patients defined by geography, and was identified specifically to allow us to study fatigue severity in a meaningful patient cohort not biased by clinic attendance frequency.
Subjects and methods
The study cohort consisted of 136 patients defined by residence within the geographical area NE1–NE25 (Newcastle‐upon‐Tyne and surrounding suburbs) who participated in a previous study of fatigue and its impact in PBC.4 Median fatigue impact score (FIS) for this population was 40 (maximum possible 160). For the purposes of this follow up study, patients with an FIS ⩾40 at the study outset were defined as the high fatigue group and FIS <40 the low fatigue group. The rationale for studying this patient population, and the methods used to identify the study population, have previously been described.4 At the time of the initial study, all patients were asked to complete a symptom assessment tool that determined self perceived health status and also contained the FIS, previously validated as a fatigue assessment tool for self completion use in English speaking PBC patients.3,6 Patients also completed a detailed questionnaire addressing any other health problems that they were experiencing, and medications that they were receiving. Pruritus severity was assessed using a visual analogue scale (VAS). For the follow up study, patients were sent the same symptom and health assessment tool. Where patients had died, comprehensive additional information regarding clinical events prior to death was gathered from hospital records, primary health care records, and death certificates to supplement the study documentation. Using a previously adopted approach for cause of death assignation,20 two of the investigators (JN and NB) examined all case documentation for each patient who died and independently ascribed the principal cause of death. Agreement regarding cause was present for all cases. The investigators who ascribed principal cause of death from the case documentation were blinded at the time as to the fatigue status at study enrolment of the patients who died. The design adopted for the control arm of the original cohort study, which included full anonymisation of controls subjects, precluded us from performing a follow up study of control subjects. Ethics approval for this study was granted by the local research ethics committee.
Statistical analysis
Survival following the original cohort study was compared in fatigued and non‐fatigued patients using the log rank test. Proportions of fatigued and non‐fatigued patients suffering cardiac death were compared using Fisher's exact test. Cox proportional hazards regression was performed using SPSS to assess whether any association between fatigue scores and survival was independent of other clinical variables. Comparisons of biological parameters at enrolment between fatigued and non‐fatigued patients who subsequently died on follow up, and of FIS values at enrolment and follow up were by parametric and non‐parametric t test, as appropriate for the nature of the population value distributions. Proportions of patients in relevant subgroups were compared using Fisher's exact test.
Results
Study cohort
Of the original cohort of 136 patients, 28 (21%) died between the initial and follow up assessments. Detailed clinical records were available for all of these patients. Of the 108 survivors from the original cohort, 99 (92%) participated in the follow up study. Patient demographic, clinical, and laboratory data are given in table 1. Four of the surviving patients and none of the non‐survivors had undergone liver transplantation between the initial assessment and follow up.
Table 1 Demographic data for patients at study outset.4 Among the patients from the original cohort who were alive at follow up (n = 108), data are only given for those who agreed to participate in the follow up element of the study (n = 99, 92%).
| Baseline variable | All patients | Patients alive at follow up | Patients dead at follow up |
|---|---|---|---|
| Age (y) | 65 (21–88) | 62 (21–88) | 77 (53–86) |
| Bilirubin (μmol/l) | 8 (3–131) | 8 (3–131) | 10 (4–69) |
| Albumin (g/l) | 42 (28–51) | 42 (28–51) | 40 (35.5–45) |
| Prothrombin time (s) | 13 (11–24) | 13 (11–24) | 13 (12–18) |
| UDCA therapy (n (%)) | 39 (31) | 28 (28) | 11 (39) |
| Definite disease (n (%)) | 81 (64) | 62 (63) | 19 (68) |
| Probable disease (n (%)) | 46 (36) | 37 (37) | 9 (32) |
| Histologically confirmed advanced disease (n (%)) | 14 (11) | 9 (9) | 5 (18) |
| Pruritus present at enrolment (n (%)) | 84 (62) | 64 (59) | 20 (71) |
| FIS at enrolment | 40.5 (0–138) | 35.5 (0–136) | 54.5 (2–138) |
| FIS at follow up | na | 37.5 (0–142) | na |
| Female (n (%)) | 116 (91) | 90 (91) | 26 (93) |
Unless otherwise indicated, data are presented as median (range).
UDCA, ursodeoxycholic acid; FIS, fatigue impact score; na, not applicable
Subjects surviving to follow up
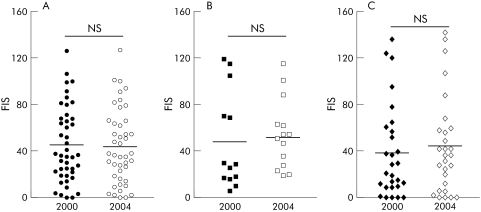
Among the 95 surviving non‐transplanted patients, fatigue severity, as assessed by the FIS, was unchanged after the four year follow up period (fig 1A, B). Significant change in fatigue score status among individual patients over the follow up period was limited to the four patients who underwent transplantation, all of whom reported significant improvements in their FIS score (fig 1B, C). Ursodeoxycholic acid (UDCA) usage appeared to have no detectable effect on fatigue severity (within the constraints of the study methodology) as no significant change in FIS was seen between 2000 and 2004 in patients not receiving UDCA at any point during the study period (fig 2A), in patients who commenced UDCA at standard doses (12–15 g/kg/day) between 2000 and 2004 (fig 2B), and in patients receiving UDCA throughout the study period (fig 2C).
Figure 1 (A) Distribution of fatigue impact score (FIS) values in 2000 and 2004 in patients surviving to follow up who were not transplanted during the follow up period. Mean value is denoted. (B) Correlation between individual FIS values in patients in 2000 and 2004. Non‐transplanted patients are denoted by closed circles, patients transplanted during follow up by open circles. Correlation data (with 95% confidence interval) are for the non‐transplanted patient group. (C) Distribution of FIS values in 2000 and 2004 in patients surviving to follow up who were transplanted during the follow up period. Mean value is shown.
Figure 2 Fatigue impact score (FIS) values in 2000 and 2004 for (A) patients not receiving ursodeoxycholic acid (UDCA) at any stage of the study, (B) patients who commenced UDCA at a dose of 10–12 mg/kg between 2000 and 2004 and who were still receiving this medication at the time of the follow up study, and (C) patients receiving UDCA at a dose of 10–12 mg/kg throughout the study period.
Subjects not surviving to follow up
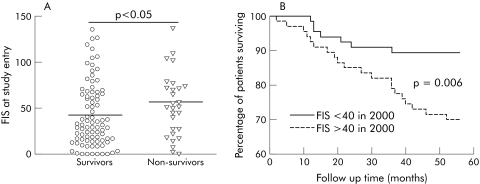
Patients who died during follow up had higher FIS values at the study outset (median FIS 54.5 (range 2–138)) than patients who survived to follow up and who participated in the follow up arm of the study (median FIS 35.5 (0–136); p<0.05) (fig 3A). Twenty of 28 (71%) patients who died during follow up had high fatigue scores at study outset (defined as FIS values above the median value for the whole PBC population (>40)) compared with 8/28 (29%) with low fatigue scores (defined as FIS values <40) (p<0.005). In contrast, proportions of surviving and non‐surviving patients with pruritus at study outset (VAS >0) were similar (NS) (table 1). Survival over the study follow up period was significantly lower by Kaplan‐Meier analysis for the high fatigue patients at study outset than for the low fatigue patients (fig 3B). In contrast, the presence of pruritus was not associated with significantly decreased survival by Kaplan‐Meier analysis (data not shown). Association between a high fatigue score (FIS >40) at study outset and risk of mortality on follow up was independent of other well established PBC prognosis associated variables (age, bilirubin, and albumin which were found, along with FIS, to be associated with prognosis in this cohort on univariate analysis (data not shown)) and on Cox proportional hazard regression analysis (table 2).
Figure 3 (A) Comparison of the fatigue impact score (FIS) values at study outset for patients who survived to follow up and in those who died during follow up (horizontal bar denotes median value). (B) Survival in patients in the 2000 study cohort with fatigue scores above and below 40 (median score for primary biliary cirrhosis patients in this cohort).4
Table 2 Baseline biological parameters showing significant associations with subsequent survival on proportional hazards regression.
| Variable | B | SE (B) | p Value |
|---|---|---|---|
| FIS | 0.91 | 0.43 | 0.03 |
| Age | 0.095 | 0.023 | <0.001 |
| Albumin | −0.095 | 0.045 | 0.04 |
| Bilirubin | 0.027 | 0.01 | 0.01 |
FIS, fatigue impact score.
No association was seen between age or any parameter of liver disease severity (including serum albumin, bilirubin, or prothrombin time) and fatigue severity in the whole study cohort at enrolment.4 This observation remained true in retrospect for the group who died during follow up (table 3). Moreover, the vast majority of patients in both the fatigued and non‐fatigued groups at study outset who died during follow up had normal liver synthetic function (table 3). In terms of more general parameters of health, no correlation was seen between self reported general health status (which patients were asked to distinguish from fatigue), numbers of medications received, blood glucose and thyroid stimulating hormone levels, and fatigue severity at study outset in subjects who died during follow up (table 3).
Table 3 Baseline biological parameters and frequencies of abnormal values for key parameters and fatigue associated diagnoses in patients with high (FIS >40) and low (FIS <40) fatigue scores at study enrolment who died during follow up.
| Variable | FIS>40 n = 20 | FIS<40 n = 8 | p Value |
|---|---|---|---|
| Albumin (g/l) [n (%) <35 g/l] | 40 (36–45) [0 (0)] | 38 (35.5–42) [0 (0)] | NS (NS) |
| Bilirubin (μmol/l) [n (%) >17 μmol/l] | 12 (4–69) [3 (15)] | 10 (8–47) [1 (12.5)] | NS (NS) |
| PT (s) [n (%) >15 s] | 13 (12–15) [0 (0)] | 13 (12–18) [2 (25)] | NS (NS) |
| TSH (mu/l) [n (%) diagnosed thyroid disease] | 2.3 (0.5–3.8) [1 (5)] | 1.7 (1.2–2.8) [1 (12.5)] | NS (NS) |
| Random blood glucose (mmol/l) [n (%) diagnosed type II DM] | 7.0 (4.4–9.0) [2 (10)] | 8.0 (4.4–9.1) [0 (0)] | NS (NS) |
| Age (y) | 75 (54–86) | 71 (53–86) | NS |
| Health perception score (1–4) | 2 (1–4) | 2 (1–3) | NS |
| No of medications received | 2 (0–5) | 2 (0–6) | NS |
FIS, fatigue impact score; PT, prothrombin time; TSH, thyroid stimulating hormone; DM, diabetes mellitus.
Health perception score was a self reported score of general health, with values ranging from 1 (very poor health) to 4 (very good health). Subjects completing this scale were asked to consider the aspects of their health other than their fatigue.
Number of medications received relates to prescription medications.
Despite the independent association between both albumin and bilirubin at enrolment and subsequent survival, liver disease was attributed as a direct cause of death in only four patients (variceal bleed n = 2 and liver failure n = 2). In contrast, nine died of histologically confirmed malignant disease (carcinoma of the bronchus (n = 3), oesophagus (n = 2), breast (n = 1), colon (n = 1), and pancreas (n = 1), and non‐Hodgkin's lymphoma (n = 1)). Of the remaining 15 patients, two died of respiratory system sepsis not directly linked to liver disease (that is, not following endoscopic procedures) and 13 were ascribed a cardiac cause of death (sudden cardiac events occurring in the absence of previous symptoms (all investigated with post mortem) n = 5, myocardial infarction n = 5, and heart failure n = 3). A principally cardiac cause of death was seen significantly more frequently in the group of patients with high outset fatigue scores who died during follow up (12/20, 60%) than in the group of patients with low outset fatigue scores who died during follow up (1/8, 12.5%; p<0.05).
Discussion
In this study, we set out to explore how the impact of fatigue evolves over time in PBC. The study population was a well characterised and representative cohort of patients, defined by geography of residence, who had participated in an earlier study of fatigue impact in PBC.4 The follow up assessment reported in the current study took place four years after the original assessment. Full follow up information was available for 93% of the original study cohort (99 subjects who were alive and participated in the follow up study and 28 who had died during the follow up period).
Fatigue severity, as determined by FIS, was unchanged in 2000 compared with 2004 among patients not undergoing liver transplantation during follow up. One potential explanation for this finding would be that the FIS lacks the responsiveness21 to detect change in fatigue severity over time. The observations that the FIS had sufficient responsiveness to detect statistically significant improvement in fatigue severity in the small number of patients who did undergo liver transplantation in the follow up phase of this study, and that the FIS has been demonstrated to be able to detect significant changes in fatigue severity in response to therapy in other disease settings,22,23 would argue against this interpretation. The alternative explanation for the stability of FIS over time, and the one which we favour, is that little or no change in fatigue severity in fact occurred over the time period of the study. The conventional view to date in PBC has been that the condition goes through an initial asymptomatic phase which is followed by symptom development and, in due course, by the development of progressive disease.24,25,26,27,28 If our interpretation is correct, our observations would challenge this view. The findings of the current study suggest that patients fall into fatigued and non‐fatigued groups which, at least over four years, remain stable. Moreover, the use of UDCA, an agent demonstrated to slow the progression of liver disease and improve liver biochemistry in PBC,29 appears to have no identifiable effect on fatigue severity. These observations would be more supportive of fatigue in PBC resulting from pathological processes to which PBC in some way acts as a cofactor, and to which PBC patients are thus predisposed, than it occurring as a direct consequence of liver disease.
The mortality rate for patients who participated in the original fatigue study cohort was 20.6%. Death certificate data were available for all patients who died during follow up, allowing confirmation of the mortality rate. The annual mortality rate observed for the current cohort was very similar to that reported by our group for an earlier cohort of North Eastern UK PBC patients.20 The apparent differences in mortality rates between the two Newcastle studies and a recent French study30 are likely to reflect different patient demographics. Survival was significantly lower in patients with high fatigue scores at study outset, with a high FIS being independently associated with reduced survival on multivariate analysis (the B value on proportional hazards regression for FIS >40 at study outset was 0.91 (p<0.05), indicating that the presence of fatigue at study outset (defined as FIS >40) was independently associated with a relative risk of dying during follow up of 1.91 compared with the risk in patients in whom fatigue was absent (defined as FIS <40)). The other adverse prognostic indicators at enrolment for the current cohort were age, serum albumin, and serum bilirubin (parameters previously well established as markers of adverse prognosis in PBC). These observations suggest that the pathological processes responsible for fatigue expression in PBC are not benign.
In this study cohort, cardiac, but not malignant or directly liver disease associated, deaths were significantly overrepresented in the subgroup of patients who had high fatigue severity scores at the outset of the study. There are several potential explanations for the apparent association between fatigue and subsequent cardiac death. The first would be incorrect mode of death assignment. We could find no evidence to support this possibility. Indeed, all cardiac death patients had positive diagnostic findings (ECG features of arrhythmia or myocardial infarction, or echocardiographic features of heart failure) or post mortem findings compatible with a cardiac cause of death. The difficulties inherent in cause of death assignment, particularly in the context of sudden cardiac death secondary to arrhythmia even following a post mortem, mean however that the possibility of error in cause of death assignment remains. A second potential explanation would be that the high fatigue group at the outset of the study contained a proportion of patients with pre‐existing atherosclerotic cardiovascular disease or heart failure, or risk factors for the development of such disease (such as diabetes), which predisposed to both their fatigue at the time of the study and their subsequent death. The limitations of the original study protocol mean however that although the available records confirm that diagnosed heart disease and risk factors for heart disease were absent from the vast majority of patients who subsequently died of cardiac causes, we cannot exclude the possibility that occult heart disease was present in a number of fatigued patients who died of cardiac causes during follow up. If this were the case (and future prospective studies addressing cardiac risk together with fatigue assessment in PBC will be required to answer this question), it would have implications for our understanding of fatigue pathogenesis in PBC (implying, as it does, multifactorial aetiology with the implication that a single agent approach to therapy is less likely to be effective) and would add to the ongoing debate regarding atherosclerotic disease risk in PBC.31,32
A third explanation for the apparent links between fatigue in PBC and cardiovascular mortality might be autonomic dysfunction. Our group, and others, have recently identified a high prevalence of abnormalities of autonomic function in PBC patients (including reduced heart rate variability and blood pressure instability).33,34,35,36 Autonomic dysfunction of this type has previously been demonstrated (in the non‐liver setting) to be associated with an increased risk of cardiac mortality (including sudden cardiac death).37,38,39 Furthermore, degree of autonomic dysfunction has previously been demonstrated to show an association with fatigue severity in both PBC35 and other fatigue associated conditions, such as multiple sclerosis,40 autonomic failure,41 and chronic fatigue syndrome.42,43,44 One explanation for our findings might therefore be that PBC predisposes to autonomic dysfunction which results in fatigue but which also puts affected patients at risk of cardiac death via mechanisms (such as sudden cardiac death) which may have been under‐detected in earlier studies of cardiac risk in PBC which typically focused on atherosclerotic disease.
In this study, we have demonstrated that, in PBC patients affected by fatigue (and not all patients and populations are so affected), the symptom is a chronic one with, seemingly, little evidence of spontaneous or therapy related resolution. This symptom constancy, together with the emerging evidence regarding the degree to which it can impair quality of life, means that further efforts to identify therapies able to ameliorate fatigue in PBC are warranted. Our observations regarding the apparent associations between fatigue, reduced survival, and apparent risk of cardiac related death add further complexity to our current understanding of the pathogenesis of fatigue in PBC. Additional prospective studies in this area are again warranted.
Acknowledgements
This work was supported by grants from the Newcastle Hospital Special Trustees and Liver North.
Abbreviations
FIS - fatigue impact score
PBC - primary biliary cirrhosis
UDCA - ursodeoxycholic acid
VAS - visual analogue scale
Footnotes
Conflict of interest: None declared.
References
- 1.Jones D E J. Pathogenesis of primary biliary cirrhosis. J Hepatol 200339639–648. [DOI] [PubMed] [Google Scholar]
- 2.Jones D E J. Primary biliary cirrhosis. Horiz Med 2005. (in press)
- 3.Prince M I, James O F W, Holland N P.et al Validation of a fatigue impact score in primary biliary cirrhosis: towards a standard for clinical and trial use. J Hepatol 200032368–373. [DOI] [PubMed] [Google Scholar]
- 4.Goldblatt J, Taylor P J S, Lipman T.et al The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 20021221235–1241. [DOI] [PubMed] [Google Scholar]
- 5.Cauch‐Dudek K, Abbey S, Stewart D E.et al Fatigue in primary biliary cirrhosis. Gut 199843705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huet P M, Deslauriers J, Tran A.et al Impact of fatigue on the quality of life in patients with primary biliary cirrhosis. Am J Gastroenterol 200095760–767. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z M, Kiwi M L, Boporai N.et al Cholestatic liver diseases and health‐related quality of life. Am J Gastroenterol 200095497–502. [DOI] [PubMed] [Google Scholar]
- 8.Stanca C M, Bach N, Krause C.et al Evaluation of fatigue in US patients with primary biliary cirrhosis. Am J Gastroenterol 20051001104–1109. [DOI] [PubMed] [Google Scholar]
- 9.Poupon R E, Chretien Y, Chazouilleres O.et al Quality of life in patients with primary biliary cirrhosis. Hepatology 200440489–494. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby A, Rannard A, Buck D.et al Development, validation and evaluation of the PBC‐40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut 2005541622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornsson E, Simren M, Olsson R.et al Fatigue is not a specific symptom in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol 200517351–357. [DOI] [PubMed] [Google Scholar]
- 12.Jones E A, Bergasa N V. The pathogenesis and treatment of pruritus in patients with PBC. Eur J Gastroenterol Hepatol 199911623–631. [DOI] [PubMed] [Google Scholar]
- 13.Jones D E J. Complications of cholestasis. Medicine 20023067–68. [Google Scholar]
- 14.Prince M, Mitchison H, Ashley D.et al Oral antioxidant supplementation for fatigue associated with primary biliary cirrhosis: results of a multi‐centre randomised placebo‐controlled crossover trial. Aliment Pharmacol Therap 200317137–143. [DOI] [PubMed] [Google Scholar]
- 15.ter Borg P C J, van Os E, van den Broek W W.et al Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised control trial. BMC Gastroenterol 200448–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swain M G, Maric M. Improvement in cholestasis‐associated fatigue with a serotonin receptor agonist using a novel rat model of fatigue assessment. Hepatology 199725492–494. [DOI] [PubMed] [Google Scholar]
- 17.Swain M G, Le T. Chronic cholestasis in rats induces anhedonia and loss of social interest. Hepatology 1998286–10. [DOI] [PubMed] [Google Scholar]
- 18.Swain M G, Beck P, Rioux K.et al Augmented interleukin‐1 beta‐induced depression of locomotor activity in cholestatic rats. Hepatology 1998281561–1565. [DOI] [PubMed] [Google Scholar]
- 19.Forton D, Patel N, Prince M.et al Fatigue and primary biliary cirrhosis: association of globus pallidus magnetization transfer ratio measurements with fatigue seveirty and blood manganese levels. Gut 200453587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince M, Chetwynd A, Newman W L.et al Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow‐up for up to 28 years. Gastroenterology 20021231044–1051. [DOI] [PubMed] [Google Scholar]
- 21.Rannard A C, Buck D, Jones D E J.et al Assessing quality of life in primary biliary cirrhosis. Clin Gastroenterol Hepatol 20042164–174. [DOI] [PubMed] [Google Scholar]
- 22.Romani A, Bergamaschi R, Candeloro E.et al Fatigue in multiple sclerosis: Multidimensional assessment and response to symptomatic treatment. Mult Scler 200410462–468. [DOI] [PubMed] [Google Scholar]
- 23.Surawy C, Roberts J, Silver A. The effect of mindfulness training on mood and measures of fatigue, activity and quality of life in patients with chronic fatigue syndromeon a hospital waiting list: A series of exploratory studies. Behav Cogn Psychother 200533103–109. [Google Scholar]
- 24.Jones D E J, James O F W, Bassendine M F. Primary biliary cirrhosis: Clinical and associated autoimmune features and natural history. Clin Liver Dis 19982265–282. [DOI] [PubMed] [Google Scholar]
- 25.Christensen E, Crowe J, Doniach D.et al Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology 198078236–246. [PubMed] [Google Scholar]
- 26.Beswick D, Klatskin G, Boyer J. Asymptomatic primary biliary cirrhosis: A progress report on long‐term follow‐up and natural history. Gastroenterology 198589268–271. [PubMed] [Google Scholar]
- 27.Balasubramaniam K, Grambsh P M, Wiesner R H.et al Diminished survival in asymptomatic primary biliary cirrhosis. Gastroenterology 1990981707–1709. [DOI] [PubMed] [Google Scholar]
- 28.Prince M I, Jones D E J. Primary biliary cirrhosis: new perspectives in diagnosis and treatment. Postgrad Med J 200076199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poupon R E, Lindor K D, Cauch‐Dudek K.et al Combined analysis of randomized trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997113884–890. [DOI] [PubMed] [Google Scholar]
- 30.Corpechot C, Carrat F, Bahr A.et al The effect of ursodeoxycholic acid therapy on the natural history of primary biliary cirrhosis. Gastroenterology 2005128297–303. [DOI] [PubMed] [Google Scholar]
- 31.Crippin J S, Lindor K D, Jorgenson R.et al Hypercholesterolaemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology 199215858–862. [DOI] [PubMed] [Google Scholar]
- 32.Longo M, Crosignani A, Battezzati P M.et al Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 200251265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton J L, Bhala N, Jones D E J. Fatigue in primary biliary cirrhosis is associated with nocturnal hypotension. Hepatology 200440461A [Google Scholar]
- 34.Newton J L, Bhala N, Jones D E J. Autonomic dysfunction is common in both cirrhotic and precirrhotic primary biliary cirrhosis. Hepatology 200440466A [Google Scholar]
- 35.Newton J L, Bhala N, Jones D E J. Risk factors for sudden cardiac death in primary biliary cirrhosis. Hepatology 200440462A [Google Scholar]
- 36.Keresztes K, Istenes I, Folhoffer A.et al Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol 2004103030–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parati G, Di Renzo M, Mancia G. Dynamic modulation of baroreflex sensitivity in health and disease. Ann N Y Acad Sci 2001940469–487. [DOI] [PubMed] [Google Scholar]
- 38.Vanoli E, Cerati D, Pedretti R F. Autonomic control of heart rate: pharmacological and non‐pharmacological modulation. Basic Res Cardiol 199893133–142. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz P J. The autonomic nervous system and sudden death. Eur Heart J 199819F72–F80. [PubMed] [Google Scholar]
- 40.Flackenecker P, Rufer A, Bihler I.et al Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 200361851–853. [DOI] [PubMed] [Google Scholar]
- 41.Mathias C J, Mallipeddi R, Bleasdale‐Barr K. Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple system atrophy. J Neurol 1999246893–898. [DOI] [PubMed] [Google Scholar]
- 42.Peckerman A, La Mancha J J, Quereshi B.et al Baroreceptor reflex and integrative stress responses in chronic fatigue syndrome. Psychosom Med 200365889–895. [DOI] [PubMed] [Google Scholar]
- 43.Stewart J M. Autonomic nervous system dysfunction in adolescents with postural tachycardia syndrome and chronic fatigue syndrome is characterised by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res 200048218–226. [DOI] [PubMed] [Google Scholar]
- 44.Streeten D H, Thomas D, Bell D S. The roles of orthostatic hypotension, orthostatic tachycardia and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci 20003201–8. [DOI] [PubMed] [Google Scholar]