Abstract
Background and aims
Although most cases of hereditary haemochromatosis are associated with homozygosity for the C282Y mutation of the HFE gene, clinical penetrance varies and other genes may modify disease expression. If so, relatives from clinically affected families, by inheriting such genes, may accumulate more iron. To seek evidence for this, we compared iron status and morbidity in unselected first degree relatives of two groups of index cases from South Wales, namely asymptomatic C282Y homozygotes identified by genetic screening of blood donors (n = 56) and C282Y homozygous haemochromatosis patients presenting clinically (n = 60).
Methods
All participating relatives had a structured interview, clinical assessment, and laboratory investigations. Health related quality of life was measured (SF‐36 version 2).
Results
In total, 92% of 180 eligible first degree relatives were interviewed in the “screened” family group and 85% of 143 eligible relatives in the “patient” group. Of 59 relatives homozygous for C282Y, 76% of men and 32% of women had the “iron phenotype” (raised transferrin saturation and serum ferritin). Logistic regression modelling of the iron phenotype risk showed that 42% of the initial model deviance could be explained by homozygosity for C282Y, another 6% by lifestyle factors, and 6% by being male. Family group membership was not a significant risk factor. Morbidity and SF‐36 scores did not differ significantly either between C282Y homozygotes and relatives lacking C282Y, or between C282Y homozygotes from the “screened” and “patient” groups. Serious morbidity (including cirrhosis) was low in both groups of relatives.
Conclusions
HFE C282Y homozygosity has a high penetrance for iron accumulation but a low clinical penetrance. Lack of excess morbidity among C282Y homozygous relatives of index cases who presented clinically suggests that residual unknown genetic or environmental factors do not greatly influence clinical outcome among C282Y homozygotes.
Keywords: haemochromatosis, iron metabolism, clinical penetrance, HFE, genetic modifiers
Hereditary haemochromatosis (HH) is an autosomal recessive condition in which too much iron is absorbed from the diet. Undetected progressive iron accumulation may have serious clinical consequences, including arthritis, diabetes, heart disease, and cirrhosis. Premature illness and death from the disease can be avoided by phlebotomy, provided treatment begins before cirrhosis has developed.1 In the UK, over 90% of HH patients are homozygous for the Cys282Tyr (C282Y) mutation of the HFE gene on chromosome 6p.2,3 Another 4% are compound heterozygotes, with C282Y on one chromosome and a second HFE mutation (mostly His63Asp, H63D) on the other. Rarer causes of HH include mutations within the HAMP,4,5TFR2,6SLC11A3,7,8,9 and HJV10 genes.
The availability of effective treatment, high frequency of the C282Y mutation among individuals of northern European descent,11 and the introduction of a diagnostic test for HFE gene mutations has led some to advocate HH population screening.12,13 However, recent research based population screening has revealed that the clinical penetrance of homozygous HFE gene mutations is lower than previously thought.14,15,16 There is therefore uncertainty and controversy about the overall clinical significance of HFE C282Y homozygosity17,18,19,20 and the overall cost effectiveness of genetic screening for HH.
In contrast with C282Y homozygotes identified by population screening, the prevalence of additional disease predisposing factors may be higher in first degree relatives of C282Y homozygotes who have presented with clinical symptoms and signs of haemochromatosis. Studies of twins21 and of several strains of HFE knockout mice indeed suggest that genetic factors other than HFE play a role in HH aetiology.22,23 Most C282Y homozygous relatives of HH patients have biochemical evidence of iron accumulation (that is, raised levels of transferrin saturation), and about 50% of men and a lower proportion of women were reported to manifest at least one clinical feature of the disease.24,25 Relatives in these families who lacked HFE mutations were not investigated, which limits the conclusions that can be drawn. As well as endogenous genetic factors, some exogenous exposures also contribute to the development of serious disease in some patients. These include alcohol26 and chronic hepatitis C infection.27
The aim of the present study was to define further the relative contributions of the HFE gene, genetic influences other than HFE, and some exogenous factors on the iron storage phenotype. To test the possibility that the ancestral haplotype for HFE is associated with more severe iron overload,28,29,30 microsatellite markers around the HFE gene were analysed in family members. To minimise the possibility of ascertainment bias, we studied first degree relatives of two groups of index cases, namely C282Y homozygotes ascertained through a genetic screening programme among healthy blood donors,31 and C282Y homozygous patients who presented with clinical HH. We hypothesised that, if genes other than HFE were of significance in determining the phenotype, then iron overload (that is, high transferrin saturation and serum ferritin) would be displayed by a higher proportion of relatives of clinically affected index cases than of cases detected by genetic screening.
Methods
Ascertainment of index cases
Identification of 72 C282Y homozygotes among 10 556 blood donors has been described elsewhere.31,32 To define the incidence of HH in South Wales, a rigorous search for cases was undertaken in the Bro Taf and Gwent Health Authorities, which serve a population of 1.3 million.16 Extension of the study to Iechyd Morgannwg Health Authority allowed identification of a total of 69 HH patients who were homozygous for HFE C282Y, and who had been treated by venesection. The study ran from January 1998 to December 1999. Patients selected as index cases for the current study were those who had presented clinically (rather than by family or population screening) and were confirmed to be C282Y homozygous. All were under 70 years of age, the same age range for the C282Y homozygotes detected by blood donor screening.
Contacting first degree relatives
All prospective index cases were informed by mail about the current project and asked to consider discussing the study with their first degree relatives (parents and siblings over the age of 18 years), even if they had already been tested for haemochromatosis. Once written consent to contact a family member had been obtained, a letter was sent to the relative in question, outlining the study and inviting their participation. Those agreeing to participate were then interviewed personally by one of us (AMcC) between July 2001 and December 2002, after written consent was obtained.
Questionnaire
All participants answered a structured interview to record their demographic details, family history (including details of deceased first degree relatives), ancestry, medical history, and current health status. Enquiry was made into all factors known to affect body iron stores, including details of past and current blood donation or bleeding disorder, iron/vitamin C supplementation, and non‐steroidal anti‐inflammatory drug use. Additional information was sought from females about menstrual history, contraceptive pill use, number of pregnancies, breast feeding, and fertility. Detailed enquiry was made into diet, current and past alcohol consumption, tea and coffee intake, together with full details of their medication use (both prescribed and over the counter).
All participating relatives were invited to complete a validated quality of life (QOL) questionnaire. The SF‐36 (version 2) health survey, which has been used extensively to compare health status of different groups,33 was selected because it provides a generic measure of health status and yields quantitative scores, as well as summary scores of physical and mental well being.
The interview usually took place in the individual's home and, in most cases, neither physician nor participant were aware of any genotyping or iron test results. Among 286 first degree relatives, only 13 had been previously diagnosed as having HH. Test results recorded for these cases were those taken at presentation (that is, before venesection). They were encouraged to respond to questions as best they could recall from the periods before or after diagnosis, as appropriate. Clinical notes for these cases were also scrutinised for inaccuracies arising from recall bias. Any discrepancies were further clarified with the respondent.
Laboratory analysis
Serum iron (sFe) and unsaturated iron binding capacity (UIBC) were determined by a microtitre plate assay.34 Total iron binding capacity (TIBC) and transferrin saturation (TS) were calculated from sFe and UIBC values.34 Serum ferritin (sFn) levels were measured on an Elecsys 2010 immunoanalyser (Roche, Lewes, UK). Full blood count and reticulocyte count (Advia 2120, Maidenhead, UK) and liver function tests (Aeroset analyser; Abbott Diagnostics) were also arranged.
DNA was extracted from whole blood. HFE alleles (wild‐type, C282Y, and H63D) were identified by heteroduplex analysis performed using a capillary electrophoresis system (GeneScan 310; Applied Biosystems, Warrington, UK).35,36 The “ancestral” haplotype was identified by microsatellite markers around the HFE gene.37 One polymerase chain reaction primer of each reaction was labelled with a fluorescent dye in a multiplex reaction and DNA fragment sizes were measured on the Applied Biosystems GeneScan 310.
Medical follow up of relatives
First degree relatives found to be C282Y homozygous, but with normal iron indices, were given their results, offered counselling, and provided with a written summary of the findings. Arrangements were made for annual follow up review. Those with iron abnormalities were sent an explanatory letter. Arrangements were made for a follow up appointment so that the findings could be discussed and also for any additional clinical assessments that were required. In accordance with UK Guidelines,38 no separate provision was made to arrange liver biopsy for C282Y homozygotes provided they had normal liver function tests, no hepatomegaly, and sFn <1000 μg/l, as the risk of significant fibrosis/cirrhosis is low. Therapeutic intervention, when needed, was applied in accordance with UK National Guidelines.38 The degree of iron overload was evaluated retrospectively by quantitative phlebotomy.
Statistical analysis
The data set had 80% power at the 5% significance level to unravel a decrease in iron overload prevalence from 50% among the homozygous relatives of patients to 20% among the homozygous relatives of blood donors. Measurements of sFe, TIBC, and TS were expressed as mean (SD). Data that were not normally distributed, such as age and sFn, were summarised as median (range). Comparisons between groups were made using a two sample t test or a Mann‐Whitney U test, as appropriate. Frequency differences between groups were assessed for statistical significance by means of a χ2 or Fisher's exact test and the results expressed as odds ratio (95% confidence intervals (CI)). Logistic regression was used to assess the association between HFE genotype, iron phenotype, and the recorded environmental and physiological parameters. Where appropriate, environmental and medical measurements were combined in a propensity function for use in the logistic regression analysis. Model construction was performed using the “Akaike” procedure of the statistical programme R39 which allows minimisation of the model deviance by stepwise inclusion or exclusion of variables, beginning with a null model. The presence of the iron phenotype was defined as TS >50% and sFn >300 μg/l (sFn >200 μg/l for premenopausal women), and entered into the procedure as a dependent variable. Multiple linear regression analysis was used to predict the SF‐36 physical component score from genetic, demographic, and environmental covariates. All statistical analyses were performed using SPSS version 10 and Statistica 5.1 customised programmes.
Ethics approval
Ethics approval for the study was obtained from Bro Taf, Gwent, and Iechyd Morgannwg regional ethics committees.
Results
Recruitment of first degree relatives of C282Y homozygous probands
The iron and health status of C282Y homozygous blood donors has been described previously.31 Family details were available for 66 of the 72 originally identified probands. A total of 56 probands (median age at screening 43 years) were finally available for inclusion in the study. These told us about 180 eligible relatives of whom 165 (92%) agreed to take part (96 siblings, 69 parents).
The iron and health status of most of the 69 C282Y homozygous patients with haemochromatosis identified in Bro Taf and Gwent have been described elsewhere.16 For the 60 probands, median age at diagnosis was 51 years for males and 52 years for females. Median time interval since diagnosis was 2.7 years (range <1 to 27 years). In total, 121 (85%) of the 143 identified first degree relatives (95 siblings, 26 parents) were included in the study.
All blood donor siblings took part in the survey (or had died). However, from the somewhat older families of patients, 20 siblings did not reply or did not wish to take part.
Demographics
Parents and siblings in the clinical group were significantly older than their respective counterparts in the screened group (table 1). The latter were significantly more likely to have a history of blood donation, although the mean number of units of blood donated in a lifetime was not significantly different. Siblings reported consuming more units of alcohol per week than their parents but the differences were not statistically significant when analysed within proband groups.
Table 1 Demographic characteristics of first degree relatives (n = 286) of C282Y homozygous probands.
| First degree relatives of C282Y homozygotes detected by genetic screening (n = 165) | First degree relatives of C282Y homozygotes presenting with clinical haemochromatosis (n = 121) | |||
|---|---|---|---|---|
| Parents | Siblings | Parents | Siblings | |
| No of cases | 69 | 96 | 26 | 95 |
| Age (y)† | 66 (44–89) | 43 (14–73) | 72 (45–88)* | 53 (20–82)*** |
| Sex ratio (F:M) | 35:34 | 49:47 | 16:10 | 56:39 |
| History of blood donation (%) | 21 (30.4) | 42 (43.8) | 9 (34.6) | 24 (25.3)** |
| Units of blood donated in lifetime, all relatives | 5.3 (12.6) | 5.2 (8.4) | 5.4 (11.3) | 3.6 (9.1) |
| 0.0 (0–60)† | 0.0 (0–30) | 0.0 (0–48) | 0.0 (0–63) | |
| Units of blood donated in lifetime, blood donors only | 17.3 (17.8) | 11.4 (9.2) | 15.6 (14.8) | 13.6 (13.6) |
| 10.0 (1–60)† | 10.0 (1–30) | 12.0 (1–48) | 9.5 (1–63) | |
| Units of alcohol consumed per week | 8.3 (14.8) | 12.5 (17.1) | 6.9 (9.8) | 11.6 (23.2) |
Measurements are given as mean (SD) and †median (range).
Note: one unit of alcohol contains 8 g alcohol.
For each variable, comparisons were made separately between the two parent and the two sibling groups, respectively: *p⩽0.05, **p⩽0.01, ***p⩽0.0001.
A total of 277 relatives (97%) provided data on the ancestry of all four grandparents. All except two families described themselves as being of Northern European descent and 244 (85%) claimed some “Celtic” descent, defined as Welsh, Scottish, or Irish. A total of 169 relatives (59%) had at least 75% Celtic ancestry, 44% had four Celtic grandparents, and 35% had four Welsh grandparents.
Causes of death among relatives
The observed number of C282Y homozygous siblings of probands (n = 50, 26.4%) was close to the number expected from the population frequency of HFE C282Y. This suggests that there is no major excess of sibling deaths arising from haemochromatosis, an assertion that is further corroborated indirectly by assessment of the causes and circumstances of death among deceased first degree relatives. Four deaths (all male) had occurred among the 51 male and 49 female siblings from the blood donor families, three of which occurred neonatally or during infancy (stillbirth, “convulsions”, and pneumonia). The fourth had died at the age of 38 years of carcinoma of the pancreas. Of the 69 male and 77 female siblings of the clinical cases, 22 and nine had died, respectively. There were eight neonatal/infant deaths, four accidental deaths, eight deaths due to cancer, three from myocardial infarction, three from respiratory disease, three from unknown causes, and two from liver disease. Twelve were aged 60 years or over.
Genotypes and haematology
The observed genotype frequencies for siblings of C282Y homozygous probands (table 2) did not differ from those expected from the population frequencies31 of the three most common HFE variants, namely wild‐type, C282Y, and H63D.40 Mean corpuscular volume (MCV) and mean cell haemoglobin (MCH) were significantly higher in C282Y homozygous compared with “wild‐type” homozygous relatives (table 2). MCH, but not MCV, was also significantly higher in compound heterozygotes for C282Y and H63D. No significant differences were observed for haemoglobin, red blood cell, count and haematocrit (data not shown) between individuals grouped according to HFE genotype.
Table 2 HFE genotype and haematology for 282 first degree relatives of C282Y homozygous probands.
| Genotype (C282Y/H63D) | Parents (%) (n = 92) | Siblings (%) (n = 189) | Hb (g/dl) | MCV (fl) | MCH (pg) |
|---|---|---|---|---|---|
| −−/−− | 0 | 38 (20.1) | 14.0 (1.00) | 90 (5) | 30.0 (2.0) |
| −−/+− | 0 | 20 (10.6) | 14.2 (1.52) | 89 (7) | 29.9 (2.6) |
| −−/++ | 0 | 2 (1.1) | 13.8 (0.85) | 88 (1) | 29.7 (0.3) |
| +−/−− | 68 (73.9) | 65 (34.4) | 14.1 (1.42) | 91 (5) | 30.3 (2.0) |
| +−/+− | 15 (16.3) | 14 (7.4) | 14.6 (1.50) | 93 (6) | 31.3 (2.3)* |
| ++/−− | 9 (9.8) | 50 (26.4) | 14.3 (1.59) | 94 (5)*** | 31.5 (1.8)*** |
Measurements are given as mean (SD).
Hb, haemoglobin; MCV, mean corpuscular volume; MCH, mean cell haemoglobin.
Comparisons of blood parameters were made between individual genotypes and wild‐type genotype (−−/−−).
*p⩽0.05, ***p⩽0.001.
Note: data for one parent had to be omitted as DNA analysis revealed non‐paternity.
Genotypes and iron status
Compared with relatives lacking mutations (table 3), a statistically significant increase in TS and sFn was observed only in C282Y homozygotes. Thirty four (12%) of the first degree relatives were discovered to have the “iron phenotype”, defined as both raised TS and sFn. Thirty of these were C282Y homozygous (11 females, 19 males), one lacked C282Y and H63D, one was heterozygous for C282Y, and two were compound heterozygotes. Thus 32% of female homozygotes and 76% of male homozygotes showed biochemical evidence of iron overload (Fisher's exact test for sex difference: p = 0.001). Serum ferritin was significantly higher for men than women in all except the group lacking C282Y and H63D (data not shown). A total of 25 C282Y homozygotes (median age 50 years) were identified among relatives of screened cases. Their mean TS was 51% and median sFn was 251 μg/l. TS above 50% was found in five women (33%) and six men (60%). Three women (20%) and six men (60%) had the biochemical iron phenotype (p = 0.03). Among first degree relatives of clinical cases, we identified 34 C282Y homozygotes (median age 53 years) with a mean TS of 60% and a median sFn of 427 μg/l. TS above 50% was found in 12 women (63%) and 12 men (80%). Eight women (42%) and 12 men (80%) had the iron phenotype (p = 0.04).
Table 3 Iron status and HFE genotype of first degree relatives of C282Y homozygous probands.
| Genotype (C282Y/H63D) | Proband group | Age (y)† | Sex ratio (F:M) | sFe (μmol/l) | TIBC (μmol/l) | TS (%) | sFn† (μg/l) |
|---|---|---|---|---|---|---|---|
| −−/−− | S | 39 (16–73) | 9:13 | 16 (9) | 62 (8) | 27 (15) | 57 (4–381) |
| C | 57 (39–80) | 9:8 | 12 (7) | 59 (8) | 21 (12) | 79 (26–175) | |
| −−/+− | S | 50 (35–69)* | 6:4 | 16 (9) | 62 (6) | 26 (14) | 46 (6–1368) |
| C | 46 (38–68)* | 5:5 | 15 (6) | 61 (7) | 25 (10) | 47 (10–396) | |
| −−/++ | S | 41 (39–42) | 1:1 | 13 (5) | 57 (5) | 25 (11) | 40 (22–57) |
| +−/−− | S | 57 (17–89)**** | 45:43 | 16 (7) | 59 (8) | 27 (11) | 64 (5–451) |
| C | 65 (35–88) | 32:13 | 15 (9) | 59 (6) | 26 (15) | 67 (6–714) | |
| +−/+− | S | 68 (43–82)**** | 7:7 | 21 (8) | 58 (8) | 36 (13) | 138 (6–490)* |
| C | 60 (28–76) | 7:8 | 19 (10)* | 59 (6) | 34 (18)* | 106 (11–723) | |
| ++/−− | S | 50 (14–73) | 15:10 | 24 (11)* | 50(5)**** | 51 (27)*** | 251 (6–2607)** |
| C | 53 (20–86) | 19:15 | 29 (11)**** | 50 (8)**** | 61 (24)**** | 427 (12–4000)**** |
Proband group: S, screened, C, clinical presentation.
sFe, serum iron concentration; TIBC, total iron binding capacity; TS, transferrin saturation; sFn, serum ferritin concentration.
Measurements are given as mean (SD) and †median (range).
Comparisons of iron parameters were made between individual genotypes and wild‐type genotype (−−/−−): *p⩽0.05, **p⩽0.01, ***p⩽0.001, ****p⩽0.0001.
Median age of all 286 relatives combined was 55 years (range 14–89 years) and varied with genotype (table 3). Compared with relatives lacking mutations (all of whom were siblings), C282Y heterozygotes and compound heterozygotes were significantly older. Moreover, a greater proportion in the latter two groups were parents. A small increase in sFn values was noted with increasing age for all genotypes although this trend reached statistical significance only for the relatives lacking mutations (coefficient of determination r2 = 0.171, p = 0.009) and C282Y heterozygotes (r2 = 0.088, p = 0.005). Relatives lacking mutations were significantly older in the clinical group (56.6 years) than in the screened group (38.7 years; p⩽0.0001). The same was true of C282Y heterozygotes. Despite these age differences, there were no significant differences in iron status between the two sets of relatives in these genotype groups. In contrast, median sFn concentrations in the two C282Y homozygous sibling groups differed significantly despite absence of a significant age difference between the two groups (clinical 427 μg/l; screened 251 μg/l; p = 0.03).
Iron deficiency
Iron deficiency, defined as sFn <15 μg/l, was observed in 18 women (age range 34–78 years) and two men (46 and 54 years). For further analysis see appendix.
Quantitative phlebotomy
To date, 26 of 59 C282Y homozygous relatives have completed a venesection programme, 24 have been assessed as currently not requiring venesection, and assessment is ongoing in six. Two homozygotes have defaulted on their follow up appointments and no further details are available for them. One C282Y homozygote (a 67 year old male sibling, TS 69%, sFn 596 μg/l) from the clinical group died during the study period of an unrelated pneumonia complicating fibrosing alveolitis.
Among the 26 venesected homozygotes, 12 (five females, seven males) were from the screened family group and 14 (four females, 10 males) were from the clinical group. Median amount of iron requiring removal for sFn and TS to fall to recommended levels was 2.3 g (range 0.4–5.2) for the screened group members and 3.5 g (range 1–8) for the relatives of clinical patients (p = 0.09). More iron needed to be removed from men (3.0 g, range 1–8) than from women (2.3 g, range 0.4–4), although the difference was also not statistically significant (p = 0.11). Only 10 homozygotes required removal of more than 4 g of iron (seven from the clinical group, three from the screened group).
Risk factors for iron overload
Univariate analysis revealed that C282Y homozygosity was the greatest risk factor compared with all other genotypes (odds ratio (OR) 56.6 (95% CI 17.3–206)) (table 4). First degree homozygous relatives of clinical cases were at greater risk of iron overload than relatives of blood donors (OR 2.76 (95% CI 1.24–6.23)). Possession of at least one H63D mutation appeared to be protective (OR 0.25 (95% CI 0.04–1.14)), probably because the two mutations hardly ever occur on the same chromosome.41,42 Sex was also identified as a significant cofactor, with males having a significantly greater risk for iron overload (OR 2.50 (95% CI 1.12–5.63)). That age was not identified as a significant risk factor (p>0.5), despite the observed age dependence of sFn, is explicable by the latter effect being confined mainly to the non‐pathological sFn range for relatives lacking mutations or heterozygous for C282Y. From data collected at interview about medical history, dietary history, and lifestyle, seven potential parameters modifying iron accumulation were identified in the univariate analysis as being either significant (p⩽0.05) or of borderline significance (0.05<p⩽0.10), namely H63D carriership, history of liver disease, current blood donorship, having been previously deferred as a blood donor, fresh fruit consumption, alcohol consumption, and regular aspirin intake. For the purpose of estimating iron overload risks, corrected for lifestyle and health status, associated with homozygosity for C282Y, sex, and proband group, the seven parameters listed above were collapsed into a single propensity score and the outcome used as a single explanatory variable in a multivariate logistic regression model (table 5). The resulting model showed that while homozygosity for C282Y explains 42% of the model deviance, and sex and the propensity score each explain 6%, proband group was no longer a significant risk factor for iron overload. No significant interaction was observed between any factors. In total, 57% of the initial model deviance could be explained by the selected variables.
Table 4 Univariate analysis of potential risk factors for iron overload.
| Parameter | Iron overload (yes:no) | p Value* | OR | 95% CI |
|---|---|---|---|---|
| HFE genotype (homozygous C282Y v other) | 30.29 v 4.219 | ⩽0.001 | 56.6 | 17.3–205.6 |
| Proband group (clinical v screened) | 22.99 v 12.49 | 0.009 | 2.76 | 1.24–6.23 |
| Sex (M v F) | 22.105 v 12.143 | 0.017 | 2.50 | 1.12–5.63 |
| Fresh fruit per week (⩽7 v >7) | 29.66 v 4.75 | 0.024 | 3.28 | 1.05–11.42 |
| Units of alcohol per week (>5 v ⩽5) | 22.113 v 11.130 | 0.040 | 2.30 | 1.01–5.31 |
OR, odds ratio; 95% CI, 95% confidence interval.
*Only risk factors with a two tailed Fisher p value ⩽0.05 are listed. All other recorded risk factors were either of borderline significance, including H63D carriership, liver disease, current blood donorship, previously deferred blood donorship, and aspirin intake (all 0.05<p⩽0.10), or not significant, including age, diabetes, thyroid disease, heart disease, history of bleeding disorder or anaemia, iron/vitamin C consumption, red meat intake, coffee, tea, other dietary/mineral supplements, smoking, and all female specific factors such as menorrhagia and pregnancy (all p>0.10).
Table 5 Logistic regression modelling of iron phenotype risk.
| Coefficient | Standard error | Deviance explained (%) | p Value* | |
|---|---|---|---|---|
| Intercept | −5.17 | 0.89 | – | – |
| Homozygous C282Y | 4.86 | 0.79 | 84.15 (41.7%) | ⩽0.001 |
| Sex | −1.79 | 0.61 | 12.72 (6.3%) | 0.003 |
| Propensity score | 7.31 | 2.33 | 11.38 (5.6%) | 0.002 |
| Proband group | 0.51 | 0.57 | 6.66 (3.3%) | 0.189† |
*p value from a z approximation of the maximum likelihood estimate of each regression coefficient.
All p values are two sided except (†).
Use of a one sided test for the proband group was justified by the prior expectation that relatives in the clinical group should be at a higher risk than relatives in the screened group.
Iron status and ancestral haplotype
The “ancestral” haplotype was defined as D6S265‐1, D6S105‐8, D6S1260‐4 (37). For the 54 C282Y homozygotes that could be typed, nine (16.7%) had two copies of the ancestral haplotype, 30 (55.5%) carried alleles suggesting the presence of one copy, and 15 (27.8%) lacked marker alleles from the ancestral haplotype. There were no significant differences in age, sFn, or TS between the three groups (data not shown).
Morbidity in relatives
Compound heterozygotes (C282Y/H63D) had significantly higher rates of diabetes, hypertension, and heart disease than relatives lacking the C282Y mutation (table 6).
Table 6 Morbidity and HFE genotype in first degree relatives (n = 282) of C282Y homozygous probands.
| Genotype (C282Y/H63D) | ||||||
|---|---|---|---|---|---|---|
| −−/−− | −−/+− | −−/++ | +−/−− | +−/+− | ++/−− | |
| No (%) | 39 (13.8) | 20 (7.1) | 2 (0.7) | 133 (47.2) | 29 (10.3) | 59 (20.9) |
| Sex ratio (F:M) | 18:21 | 11:9 | 1:1 | 77:56 | 14:15 | 34:25 |
| Age (y) | 44 (16–80) | 49 (35–69) | 41 (39–42) | 58 (17–89)** | 63 (29–82)** | 52 (14–86) |
| Iron phenotype | 1 | 0 | 0 | 1 | 2 | 30 |
| Diabetes | 0 | 1 (5.0) | 0 | 10 (7.5) | 4 (13.8)* | 1 (1.7) |
| Raised AST | 0 | 1 (5.0) | 0 | 2 (1.5) | 1 (3.5) | 5 (8.5) |
| Liver disease | 0 | 0 | 0 | 2 (1.5) | 0 | 2 (3.4) |
| Any arthralgia | 23 (59.0) | 13 (65.0) | 2 (100.0) | 83 (62.4) | 22 (75.9) | 36 (61.0) |
| Distal arthralgia | 14 (35.9) | 7 (35.0) | 2 (100.0) | 38 (28.6) | 14 (48.3) | 23 (39.0) |
| Severe arthralgia | 5 (12.8) | 5 (25.0) | 1 (50.0) | 23 (17.3) | 8 (27.6) | 9 (15.3) |
| Joint investigation (x ray) | 15 (38.5) | 10 (50.0) | 0 | 43 (32.3) | 9 (31.0) | 18 (30.5) |
| Lethargy | 14 (35.9) | 9 (45.0) | 1 (50.0) | 41 (30.8) | 12 (41.4) | 25 (42.3) |
| Thyroid disease | 3 (7.7) | 2 (10.0) | 0 | 10 (7.5) | 3 (10.3) | 8 (13.6) |
| Hypertension | 4 (10.3) | 5 (25.0) | 0 | 30 (22.6) | 10 (34.5)* | 7 (11.9) |
| Heart disease | 1 (2.6) | 2 (10.0) | 0 | 18 (13.5) | 7 (24.1)** | 4 (6.8) |
| Coeliac disease | 1 (2.6) | 0 | 0 | 0 | 0 | 0 |
| Upper abdominal pain | 4 (10.3) | 0 | 0 | 11 (8.3) | 1 (3.5) | 3 (5.1) |
Age is given as median (range).
Aspartate aminotransferase (AST) reference range 5–45 IU/l.
Comparisons of measurements were made between relatives of a given genotype and those lacking the C282Y mutation (that is, genotypes −−/−−, −−/+− and −−/++) except for age where a comparison was made with wild type (−−/−−): *p⩽0.05, **p⩽0.01.
No significant differences were detected among C282Y carriers lacking H63D, both heterozygous and homozygous. The higher morbidity in compound heterozygotes did not relate to the degree of iron accumulation (table 3). Instead, the difference in morbidity may have resulted from a substantial difference in age as compound heterozygotes were significantly older than the wild‐type relatives (median 63 v 44 years), reflecting the high proportion of parents in this group.
Among C282Y homozygotes, when 13 morbidity comparisons were stratified by the presence or absence of the iron phenotype, a significant difference was detected only for the proportions with elevated aspartate aminotransferase (AST) levels. Of the C282Y homozygotes with iron overload, five (17%) had raised AST levels compared with none among those without the iron phenotype. All those with raised levels had a history of regular alcohol intake. When morbidity among C282Y homozygotes was stratified by proband group (screened v clinical), the only significant difference to emerge was a higher prevalence of hypertension among relatives in the clinical group (7/34 in the clinical group and 0/25 in the screened group; p<0.05).
Quality of life survey among relatives
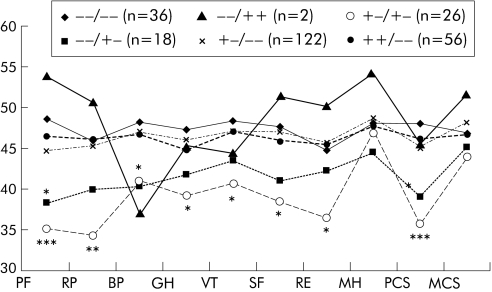
The SF‐36 QOL questionnaire was completed in full by 152 relatives (94%) from the screened and 109 (90%) from the clinical group. No significant score differences were observed between C282Y homozygotes and those lacking mutations (fig 1). However, the HDCY compound heterozygotes had significantly lower scores than relatives lacking mutations. No significant difference in the QOL profiles was observed between previously diagnosed C282Y homozygotes and newly identified C282Y homozygotes and relatives lacking mutations. Furthermore, there were no significant differences between C282Y homozygotes from the screened group and the clinical group. C282Y homozygotes with or without the iron phenotype also showed no significant difference in QOL scores (data not shown).
Figure 1 HFE genotype and quality of life (QOL) profile of first degree relatives of C282Y homozygous probands. Numbers relate to those who completed the QOL survey (261 of 282 genotyped, 92.6%). Norm based scoring: higher scores represent better health. For the US population, mean = 50, standard deviation = 10. PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary. Significance of difference from wild type (−−/−−):*p<0.05, **p<0.01, ***p = 0.001.
Relatives with raised AST levels
Nine relatives had elevated AST levels. Median amount of alcohol consumed per week by those with raised AST levels was 16 units (range 1–190) compared with 5 units (0–80) for those with normal AST levels (p<0.018). Five were C282Y homozygous of whom two had AST values higher than twice the upper normal limit (45 IU/l). All five C282Y homozygotes also had raised iron indices, and in all but one liver function tests returned to normal following lifestyle advice and venesection. Details of the remaining subjects are given in the appendix.
Relatives with a previous history of liver disease and liver biopsy results for newly identified C282Y homozygotes
Four relatives had a history of liver disease, of whom three had a history of heavy alcohol consumption. The two C282Y homozygotes had clinically evident cirrhosis. For further details see appendix. As a result of our investigations, a further three C282Y homozygous cases (two females, one male) had a liver biopsy. All had raised TS and sFn but normal AST levels. Histology in all three showed grade 2 iron, but no fibrosis or cirrhosis. There were another three homozygotes (all male) in whom the sFn level was above 1500 μg/l. They either did not wish to have a biopsy (one case) or their clinician did not deem it necessary. None had obvious clinical evidence of liver disease. Their clinical examination, synthetic liver function (albumin and prothrombin time), haematology (platelet count), and liver ultrasound scans were all normal.
Discussion
The potential public health impact of iron overload and the availability of effective treatment make it essential to assess more accurately the penetrance of HFE gene mutations. In order to identify evidence for possible genetic modifiers of the associated haemochromatosis disease phenotype, we have examined the relative contribution of genetic, environmental, and physiological factors to both iron phenotype and morbidity in two groups of first degree relatives of probands, genetically predisposed to haemochromatosis.
Studying relatives, rather than index cases, limits ascertainment bias, a drawback of many previous investigations. Studies of blood donors31 have been criticised18 as they represent a population preselected to be healthy and relatively young. In addition, blood donation may modify the iron phenotype although donation is usually followed by partial compensation through increased iron absorption.43 Our present study would also be rightly criticised if the relatives of blood donors had given more blood than those of clinical cases. This was not, however, the case. A mean of 5.2 and 3.6 units was donated in adult life by the screened and clinical siblings, respectively. In the UK, 6% of the general population are current blood donors (www.blooddonor.org.uk), as were 9.7% of the screened and 5.0% of the clinical group. Although a univariate analysis of our data suggested a protective effect against iron overload of blood donation, this did not reach statistical significance (p = 0.087).
Using genetic, environmental, and physiological factors recorded in the relatives of genetically predisposed probands, we have shown that by far the highest risk for iron overload is associated with C282Y homozygosity. Of the 34 relatives with both raised TS and sFn, 30 were C282Y homozygous. Two male compound heterozygotes (7%) showed biochemical evidence of iron overload confirming the much lower biochemical penetrance of this genotype31,44,45,46,47 in comparison with C282Y homozygosity. Only two lifestyle parameters were found to be significant risk factors for the iron phenotype—namely, high alcohol and low fresh fruit consumption. While the effect of alcohol on indicators of iron status appears straightforward, the protective effect of high fruit consumption is more difficult to explain. People who eat more fruit may also eat more vegetables and less red meat, but further examination of the data did not confirm this supposition.
The only HFE genotype associated with significantly higher morbidity was compound heterozygosity (C282Y/H63D). Diabetes, hypertension, and heart disease increase in frequency with age, and compound heterozygotes, being mostly parents, were indeed the oldest group. That age was the major determinant of morbidity for all relatives was also confirmed by a stepwise multiple regression analysis to predict the SF‐36 physical component score from demographic, genetic, and environmental covariates. In addition to the observed effect of compound heterozygosity, only age and (not surprisingly) aspirin intake were highly predictive (p⩽0.0001).
Only one difference in morbidity was observed when the C282Y homozygous relatives were stratified by the presence or absence of the iron phenotype. Five homozygotes with the iron phenotype also had raised AST activity, but none of the others had (p⩽0.05). This limited difference in morbidity supports a study from Ireland where the frequency of fatigue, arthropathy and impotence in C282Y homozygous relatives was not found to be related to iron overload.48 When morbidity was compared in our study between C282Y homozygotes from the screened and clinical group, only the frequency of hypertension was found to differ significantly. Neither stratification revealed a significant age difference.
Cirrhosis of the liver and hepatoma represent significant, albeit less common, complications associated with homozygosity for HFE C282Y.49,50 Only two of 59 C282Y homozygotes were found to have cirrhosis, and although we could not confidently exclude significant fibrosis in a further three homozygotes, none of the latter had clinical evidence of cirrhosis. The prevalence of cirrhosis is therefore low, as observed in the largest screening study of a general population to date14 and in Irish C282Y homozygotes identified through family screening.48 The two C282Y homozygotes with cirrhosis both had a longstanding history of excessive alcohol intake, consistent with an earlier report of a substantially higher risk of cirrhosis among HH patients who drink alcohol excessively.26
Our family based observations are consistent with large scale surveys of primary care patients15,47 where the incidence of arthritis, diabetes, and heart disease was no higher than in subjects lacking C282Y or H63D. However, in both surveys, there was an increased frequency of self reported liver disease in C282Y homozygotes. Previous observational studies have also revealed that patients with diabetes, heart disease, and arthritis do not have significantly increased frequencies of homozygosity or heterozygosity for C282Y.51 Furthermore, a study among subjects recruited prospectively from general practices in Oxfordshire, in which genetic testing was prompted by symptoms commonly associated with HH, failed to identify a single case with an iron loading susceptibility genotype.52
Our finding that C282Y homozygosity has a poor positive predictive value for morbidity would seem to weaken further the case for HH population screening based on HFE genotyping. As was advocated by some before the discovery of the HFE gene,53 as well as others more recently,54 screening based on biochemical testing of iron indices offers the benefit of detecting only those who are already accumulating iron, as well as avoiding the problem of identifying large numbers of people with C282Y homozygosity who may be at low risk of developing clinical complications from iron overload. Screening healthy subjects is desirable as inflammation and infection increase ferritin concentration and decrease transferrin saturation.55 Cases with iron overload detected in this way and found not to be homozygous for C282Y or compound heterozygous may then be tested for mutations in genes other than HFE, including HAMP and HJV.56
The main aim of the present study was to compare the relatives of index cases detected by a genetic screening programme with relatives of clinical cases. We hypothesised that biochemical expression of the iron phenotype would be higher in relatives of clinical cases and speculated that the presence of additional familial modifiers (most likely genetic) would explain any differences in expression found. Indeed, biochemical expression was found in 40% of C282Y homozygous relatives from the screening group and in 59% from the clinical group. The difference was statistically significant on univariate analysis. In a multivariate logistic regression analysis, however, only C282Y homozygosity, sex, and a collapsed propensity score remained important, but not the proband group. Its univariate effect may therefore be explicable mainly through confounding by other factors, although a trend towards a higher risk in relatives of clinical than screened probands remained discernible.
As only 57% of the variability in iron phenotype was explicable by the genetic, environmental, and physiological factors considered here, there must be other yet unidentified influential factors. These are however unlikely to be of great clinical importance as we have shown that significant morbidity is rare in both groups of relatives. Results of the microsatellite analysis to identify the haemochromatosis “ancestral” haplotype also suggest that modifier genes close to the HFE gene do not play an important role in altering biochemical expression in these families, confirming a recent report by Barton and colleagues.57
The clinical and biochemical observations among a cohort of C282Y homozygotes ascertained through family history revealed that HFE C282Y homozygosity has a high penetrance in terms of iron accumulation but has poor predictive value for morbidity. These observations further weaken the cases for HH population screening based primarily on HFE genotyping.
Acknowledgments
This study was funded by a grant awarded in July 2001 by the Wales Office of Research and Development.
Abbreviations
AST - aspartate aminotransferase
Hb - haemoglobin
HH - hereditary haemochromatosis
MCH - mean cell haemoglobin
MCV - mean corpuscular volume
PCP - physical component score
QOL - quality of life
sFe - serum iron concentration
sFn - serum ferritin concentration
TIBC - total iron binding capacity
UIBC - unsaturated iron binding capacity
TS - transferrin saturation
Appendix
IRON DEFICIENCY
Iron deficiency, defined as sFn <15 μg/l, was observed in 18 women (age range 34–78 years) and two men (46 and 54 years). This sex difference was statistically significant (Fisher's exact test, p = 0.008). The proportions with iron deficiency were similar in the two groups of relatives (screened group 8.7%; clinical group 5.0%). Fifteen of the 18 iron deficient women (83%) were premenopausal. The proportion of relatives with iron deficiency varied with HFE genotype, from 5% (n = 3) among C282Y homozygotes to 15% (n = 3) among individuals heterozygous for H63D, but none of the differences reached statistical significance. One of the iron deficient women had a recent diagnosis of coeliac disease, 11 had a history of menorrhagia, and 16 had one or more children. None was vegetarian, but nine had a history of blood donation (five were current donors). These women had donated a mean of 15.8 units of blood during adult life (two women had donated 30 units). Nine relatives (eight females, one male) had iron deficiency and anaemia.
RELATIVES WITH RAISED ASPARTATE AMINOTRANSFERASE (AST) LEVELS
Nine relatives had elevated AST levels. Five were C282Y homozygous. The remaining subjects included two C282Y heterozygotes (one male, one female) with normal iron indices and minor derangement of AST (57/53 IU/l) of no obvious cause, a 50 year old obese female compound heterozygote (normal iron indices, AST 152 IU/l), subsequently diagnosed with non‐alcoholic fatty liver disease, and a 57 year old male H63D heterozygote (TS 41%; sFn 1368 μg/l; AST 115 IU/l; MCV 100 fl) with a history of heavy alcohol consumption. Our investigations also revealed type II diabetes. After management of his diabetes and a reduced alcohol intake, AST level returned to normal and his sFn fell to 425 μg/l.
RELATIVES WITH A PREVIOUS HISTORY OF LIVER DISEASE
Four relatives had a history of liver disease. Two were C282Y heterozygous and two were C282Y homozygous. One heterozygote was the 60 year old father of a blood donor index case. He had finished a course of chemotherapy for carcinoma of the colon with liver metastases prior to interview. The other C282Y heterozygote was a 53 year old female sibling from the clinical group with normal iron indices. She had a history of heavy alcohol consumption and was previously diagnosed with alcoholic hepatitis (no liver biopsy). Her liver function tests returned to normal with abstinence and remained normal at interview. Her brother is currently being treated for haemochromatosis and another brother died, possibly of liver cirrhosis, at the age of 31 years. Both C282Y homozygotes are siblings of clinical cases and were detected through family screening. One, a 47 year old male with a history of heavy alcohol consumption, was found at liver biopsy to have grade 4 haemosiderosis with fibrosis and possible early cirrhosis. He had 5 g of iron removed at venesection and is currently well on a maintenance venesection programme. The other, a 48 year old female, had a clinical diagnosis of chronic liver disease and a history of postoperative acute hepatic failure (TS 91%; sFn 4000 μg/l at review). She was a heavy drinker (>190 units alcohol per week) and had clinical signs of chronic liver disease. Her liver function tests were abnormal at presentation (AST 125 IU/l, alkaline phosphatase 273 IU/l). She was an inconsistent attendee and did not have liver biopsy.
Footnotes
Conflict of interest: None declared
References
- 1.Niederau C, Fischer R, Purschel A.et al Long‐term survival in patients with hereditary hemochromatosis. Gastroenterology 19961101107–1119. [DOI] [PubMed] [Google Scholar]
- 2. The UK Haemochromatosis Consortium, Worwood M. A simple genetic test identifies 90% of UK patients with haemochromatosis. Gut 199741841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feder J N, Gnirke A, Thomas W.et al A novel MHC class I‐like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 199613399–408. [DOI] [PubMed] [Google Scholar]
- 4.Roetto A, Papanikolaou G, Politou M.et al Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 20033321–22. [DOI] [PubMed] [Google Scholar]
- 5.Merryweather‐Clarke A T, Cadet E, Bomford A.et al Digenic inheritance of mutations in HAMP and HFE results in different types of haemochromatosis. Hum Mol Genet 2003122241–2247. [DOI] [PubMed] [Google Scholar]
- 6.Roetto A, Daraio F, Alberti F.et al Hemochromatosis due to mutations in transferrin receptor 2. Blood Cells Mol Dis 200229465–470. [DOI] [PubMed] [Google Scholar]
- 7.Montosi G, Donovan A, Totaro A.et al Autosomal‐dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest 2001108619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devalia V, Carter K, Walker A P.et al Autosomal dominant reticuloendothelial iron overload associated with a 3‐base pair deletion in the ferroportin 1 gene (SLC11A3). Blood 2002100695–697. [DOI] [PubMed] [Google Scholar]
- 9.Njajou O T, Vaessen N, Joosse M.et al A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet 200128213–214. [DOI] [PubMed] [Google Scholar]
- 10.Papanikolaou G, Samuels M E, Ludwig E H.et al Mutations in HFE2 cause iron overload in chromosome 1q‐linked juvenile hemochromatosis. Nat Genet 20043677–82. [DOI] [PubMed] [Google Scholar]
- 11.Distante S, Robson K J H, Graham‐Campbell J.et al The origin and spread of the HFE‐C282Y haemochromatosis mutation. Hum Genet 2004115269–279. [DOI] [PubMed] [Google Scholar]
- 12.Witte D L, Crosby W H, Edwards C Q.et al Hereditary hemochromatosis. Clin Chim Acta 1996245139–200. [DOI] [PubMed] [Google Scholar]
- 13.Allen K, Williamson R. Screening for hereditary haemochromatosis should be implemented now. BMJ 2000320183–184. [PMC free article] [PubMed] [Google Scholar]
- 14.Asberg A, Hveem K, Thorstensen K.et al Screening for hemochromatosis: High prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol 2001361108–1115. [DOI] [PubMed] [Google Scholar]
- 15.Beutler E, Felitti V J, Koziol J A.et al Penetrance of 845G → A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002359211–218. [DOI] [PubMed] [Google Scholar]
- 16.McCune C A, Al Jader L N, May A.et al Hereditary haemochromatosis: only 1% of adult HFE C282Y homozygotes in South Wales have a clinical diagnosis of iron overload. Hum Genet 2002111538–543. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E. The HFE Cys282Tyr mutation as a necessary but not sufficient cause of clinical hereditary hemochromatosis. Blood 20031013347–3350. [DOI] [PubMed] [Google Scholar]
- 18.Ajioka R S, Kushner J P. Clinical consequences of iron overload in hemochromatosis homozygotes. Blood 20031013351–3354. [DOI] [PubMed] [Google Scholar]
- 19.Beutler E. Clinical consequences of iron overload in hemochromatosis homozygotes—Rebuttal to Ajioka and Kushner. Blood 20031013354–3357. [DOI] [PubMed] [Google Scholar]
- 20.Ajioka R S, Kushner J P. Clinical consequences of iron overload in hemochromatosis homozygotes—Rebuttal to Beutler. Blood 20031013358. [DOI] [PubMed] [Google Scholar]
- 21.Whitfield J B, Cullen L M, Jazwinska E C.et al Effects of HFE C282Y and H63D polymorphisms and polygenic background on iron stores in a large community sample of twins. Am J Hum Genet 2000661246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy J E, Montross L K, Andrews N C. Genes that modify the hemochromatosis phenotype in mice. J Clin Invest 20001051209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupic F, Fruchon S, Bensaid M.et al Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut 200251648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley L A, Haddow J E, Palomaki G E. Population screening for hemochromatosis: expectations based on a study of relatives of symptomatic patients. J Med Screen 19963171–177. [DOI] [PubMed] [Google Scholar]
- 25.Bulaj Z J, Ajioka R S, Phillips J D.et al Disease‐related conditions in relatives of patients with hemochromatosis. N Engl J Med 20003431529–1535. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher L M, Dixon J L, Purdie D M.et al Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology 2002122281–289. [DOI] [PubMed] [Google Scholar]
- 27.Diwakaran H H, Befeler A S, Britton R S.et al Accelerated hepatic fibrosis in patients with combined hereditary hemochromatosis and chronic hepatitis C infection. J Hepatol 200236687–691. [DOI] [PubMed] [Google Scholar]
- 28.Crawford D H G, Powell L W, Leggett B A.et al Evidence that the ancestral haplotype in Australian hemochromatosis patients may be associated with a common mutation in the gene. Am J Hum Genet 199557362–367. [PMC free article] [PubMed] [Google Scholar]
- 29.Piperno A, Arosio C, Fargion S.et al The ancestral hemochromatosis haplotype is associated with a severe phenotype expression in Italian patients. Hepatology 19962443–46. [DOI] [PubMed] [Google Scholar]
- 30.Barton J C, Harmon L, Rivers C.et al Hemochromatosis: Association of severity of iron overload with genetic markers. Blood Cells Mol Dis 199622195–204. [DOI] [PubMed] [Google Scholar]
- 31.Jackson H A, Carter K, Darke C.et al HFE mutations, iron deficiency and overload in 10 500 blood donors. Br J Haematol 2001114474–484. [DOI] [PubMed] [Google Scholar]
- 32.McCune C A, Ravine D, Worwood M.et al Screening for hereditary haemochromatosis within families and beyond. Lancet 20033621897–1898. [DOI] [PubMed] [Google Scholar]
- 33.Ware J E. Using generic measures of functional health and well‐being to increase understanding of disease burden. Spine 2000251467. [DOI] [PubMed] [Google Scholar]
- 34.Worwood M. Iron deficiency anaemia and iron overload. In: Lewis SM, Bain BJ, Bates I, eds. Dacie and Lewis practical haematology. London: Churchill Livingstone, 2001115–128.
- 35.Jackson H A, Bowen D J, Worwood M. Rapid genetic screening for haemochromatosis using heteroduplex technology. Br J Haematol 199798856–859. [DOI] [PubMed] [Google Scholar]
- 36.Worwood M, Jackson H A, Feeney G P.et al A single tube heteroduplex PCR for the common HFE genotypes. Blood 199994405a [Google Scholar]
- 37.Raha‐Chowdhury R, Bowen D J, Stone C.et al New polymorphic microsatellite markers place the haemochromatosis gene telomeric to D6S105. Hum Mol Genet 199541869–1874. [DOI] [PubMed] [Google Scholar]
- 38.Dooley J, Worwood M.Guidelines on diagnosis and therapy: Genetic haemochromatosis. British Committee for Standards in Haematology. Oxford, Darwin Medical Communications Ltd, 2000 http://www.bcshguidelines.com/pdf/chpt9B.pdf
- 39.Ihaka R, Gentleman R. A language for data analysis and graphics. J Comput Graph Stat 19965299–314. [Google Scholar]
- 40.Adams P C, Walker A P, Acton R T. A primer for predicting risk of disease in HFE‐linked hemochromatosis. Genet Test 20015311–316. [DOI] [PubMed] [Google Scholar]
- 41.Best L G, Harris P E, Spriggs E L. Hemochromatosis mutations C282Y and H63D in ‘cis' phase. Clin Genet 20016068–72. [DOI] [PubMed] [Google Scholar]
- 42.Thorstensen K, Asberg A, Kvitland M.et al Detection of an unusual combination of mutations in the HFE gene for hemochromatosis. Genet Test 20004371–376. [DOI] [PubMed] [Google Scholar]
- 43.Garry P J, Koehler K M, Simon T L. Iron stores and iron absorption: Effects of repeated blood donations. Am J Clin Nutr 199562611–620. [DOI] [PubMed] [Google Scholar]
- 44.Bacon B R, Olynyk J K, Brunt E M.et al HFE genotype in patients with hemochromatosis and other liver diseases. Ann Intern Med 1999130953–962. [DOI] [PubMed] [Google Scholar]
- 45.Crawford D H G, Jazwinska E C, Cullen L M.et al Expression of HLA‐linked hemochromatosis in subjects homozygous or heterozygous for the C282Y mutation. Gastroenterology 19981141003–1008. [DOI] [PubMed] [Google Scholar]
- 46.de Juan M D, Reta A, Castiella A.et al HFE gene mutations analysis in Basque hereditary haemochromatosis patients and controls. Eur J Hum Genet 20019961–964. [DOI] [PubMed] [Google Scholar]
- 47.Adams P C, Reboussin D M, Barton J C.et al Hemochromatosis and iron‐overload screening in a racially diverse population. N Engl J Med 20053521769–1778. [DOI] [PubMed] [Google Scholar]
- 48.Ryan E, Byrnes V, Coughlan B.et al Underdiagnosis of hereditary haemochromatosis: lack of presentation or penetration? Gut 200251108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willis G, Wimperis J Z, Lonsdale R.et al Haemochromatosis gene mutation in hepatocellular cancer. Lancet 1997350565–566. [DOI] [PubMed] [Google Scholar]
- 50.Willis G, Wimperis J Z, Lonsdale R.et al Incidence of liver disease in people with HFE mutations. Gut 200046401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worwood M. HFE mutations a risk factors for disease. Best Pract Res Clin Haematol 200215295–314. [PubMed] [Google Scholar]
- 52.Emery J, Rose P, Harcourt J.et al Hereditary haemochromatosis: pilot study of case‐finding approach to early diagnosis in primary care. Am J Hum Genet 2001691455 [Google Scholar]
- 53.Edwards C Q, Kushner J P. Screening for hemochromatosis. N Engl J Med 19933281616–1620. [DOI] [PubMed] [Google Scholar]
- 54. EASL International Consensus Conference on Haemochromatosis. Co‐sponsored by the World Health Organization. Supported by the National Institutes of Health and Centers for Disease Control and Prevention (USA). J Hepatol 200033485–504. [DOI] [PubMed] [Google Scholar]
- 55.Distante S. Phenotypic expression of the HFE gene mutauion (C282Y) among the hospitalised population. Gut 200047575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pietrangelo A. Medical progress—hereditary hemochromatosis—A new look at an old disease. N Engl J Med 20043502383–2397. [DOI] [PubMed] [Google Scholar]
- 57.Barton J C, Wiener H W, Acton R T.et al HLA haplotype A*03‐B*07 in hemochromatosis probands with HFE C282Y homozygosity: frequency disparity in men and women and lack of association with severity of iron overload. Blood Cells Mol Dis 20053438–47. [DOI] [PubMed] [Google Scholar]