Abstract
Background and aims
Histamine is known as a regulator of gastrointestinal functions, such as gastric acid production, intestinal motility, and mucosal ion secretion. Most of this knowledge has been obtained from animal studies. In contrast, in humans, expression and distribution of histamine receptors (HR) within the human gastrointestinal tract are unclear.
Methods
We analysed HR expression in human gastrointestinal tissue specimens by quantitative reverse transcription‐polymerase chain reaction and immunostaining.
Results
We found that H1R, H2R, and H4R mRNA were expressed throughout the gastrointestinal tract, while H3R mRNA was absent. No significant differences in the distribution of HR were found between different anatomical sites (duodenum, ileum, colon, sigma, and rectum). Immunostaining of neurones and nerve fibres revealed that H3R was absent in the human enteric nervous system; however, H1R and H2R were found on ganglion cells of the myenteric plexus. Epithelial cells also expressed H1R, H2R and, to some extent, H4R. Intestinal fibroblasts exclusively expressed H1R while the muscular layers of human intestine stained positive for both H1R and H2R. Immune cells expressed mRNA and protein for H1R, H2R, and low levels of H4R. Analysis of endoscopic biopsies from patients with food allergy and irritable bowel syndrome revealed significantly elevated H1R and H2R mRNA levels compared with controls.
Conclusions
We have demonstrated that H1R, H2R and, to some extent, H4R, are expressed in the human gastrointestinal tract, while H3R is absent, and we found that HR expression was altered in patients with gastrointestinal diseases.
Keywords: histamine, intestine, irritable bowel syndrome, inflammatory bowel disease, food allergy
Histamine is known to be a major mediator of acute anaphylactic reactions. Since its discovery in 1910,1 numerous other physiological functions have been attributed to this biogenic amine which exerts its biological actions through four different receptors (H1R–H4R), identified to date.2 In the gastrointestinal tract, histamine is considered to regulate at least three major functions: (1) enhancement of gastric acid production,2,3 (2) modulation of gastrointestinal motility,4,5,6,7 and (3) alteration of mucosal ion secretion.8,9 Data confirming the last two concepts were mainly generated in animal models whereas attempts to transfer the findings to the human system often had limited success.10 Hitherto it is not even clear which of the four HR are expressed in the human gastrointestinal tract. Expression and function of H3R in particular, which is the subject of a controversial debate,10,11 remains to be examined in human tissue. An inhibitory role of H3R on neurotransmitter release from intestinal nerves has been repeatedly suggested12 but not yet documented in the human gastrointestinal tract.
Recent studies have identified histamine as a potent modulator of immune functions.13 Considering that the gastrointestinal tract represents the largest immune organ of the human body, hosting large numbers of mucosal mast cells (MC),14 histamine may be of particular relevance for MC dependent immune regulation. In the present study, we raised three questions: (1) Which HR are expressed in the human intestine at different anatomical sites? (2) Does the enteric nervous system (ENS) express HR? (3) Is the expression pattern of HR altered in patients with functional or immunological gastrointestinal disorders, such as irritable bowel syndrome (IBS) or allergic inflammation? To address these questions, we examined expression of HR using quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) and immunostaining in surgical specimens of normal intestinal tissue. Furthermore, we measured HR mRNA expression levels in endoscopic tissue samples from patients with IBS or food allergies (FA) and control patients. Different cell populations were isolated from human intestinal mucosa, including intestinal MC, intestinal fibroblasts (FB), and lamina propria mononuclear cells (LPMC), and analysed for HR mRNA expression.
Material and methods
Patients
Thirty three individuals undergoing bowel resection due to gastrointestinal cancer were recruited for the HR expression study. Samples from the resectates were taken from macroscopically unaffected tissue both from the mucosa/submucosa and the muscular layer and subjected to quantitative RT‐PCR analysis, primary cell isolation, or histological analysis. HR expression in intestinal mucosa from patients with gastrointestinal disorders was measured in homogenates of endoscopic biopsies.
Thirty patients with IBS fulfilling the Rome II criteria with an additional history of allergy (aged 37 (11) years) and 14 control patients (aged 38 (15) years) were recruited. In 19 of the IBS patients, diagnosis of intestinal FA was confirmed based on a history of adverse reactions to food combined with provocation tests such as open oral challenges and the colonoscopic allergen provocation test, as described previously.15 The remaining 11 patients were classified as having IBS according to the Rome II criteria. All medications related to the intestinal and/or allergic symptoms were stopped 10 days before colonoscopy. Control patients had no history of allergy or food intolerance and underwent colonoscopy for diverse reasons (that is, surveillance, polyps, blood in stool). Only patients with no endoscopic or histological evidence of mucosal inflammation were included in the control group. All patients underwent colonoscopy (videocoloscope; Olympus Optical Company, Hamburg, Germany). Endoscopic biopsies were taken from the terminal ileum (IBS, n = 10; FA, n = 7; controls, n = 5), the caecum (IBS, n = 11; FA, n = 19; controls, n = 14), and the rectum (IBS, n = 10; FA, n = 7; controls, n = 8). Biopsies were immediately incubated in RNAlater buffer (Qiagen, Hilden, Germany) and stored for 24 hours at 4°C and subsequently homogenised in RLT buffer (Qiagen) supplemented with β‐mercaptoethanol.
RNA preparation; real‐time and conventional RT‐PCR
Total RNA was prepared from intestinal specimens (surgical and endoscopic) and subjected to reverse transcription, as described previously.16 Real time fluorescence monitored PCR reactions were performed using SybrGreen PCR Master Mix (Applied Biosystems, Warrington, UK) and a PE Applied Biosystems model 7700 Sequence Detection System. The following oligonucleotide primers were used for RT‐PCR reactions: glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Genebank access No: NM_002046) (5′‐CAG CCT CAA GAT CAT CAG CA‐3′), (5′‐TTA AGA CCA CAC AGA TGG CG‐3′), fragment size 140 bp; H1R (Genebank access No: NM_000861) (5′‐GTC TAA CAC AGG CCT GGA TT‐3′), (5′‐GGA TGA AGG CTG CCA TGA TA‐3′), fragment size 140 bp; H2R (Genebank access No: NM_022304) (5′‐ATT AGC TCC TGG AAG GCA GC‐3′), (5′‐CTG GAG CTT CAG GGG TTT CT‐3′), fragment size 374 bp; H3R (Genebank access No: NM_007232) (5′‐TCG TGC TCA TCA GCT ACG AC‐3′), (5′‐AAG CCG TGA TGA GGA AGT AC‐3′), fragment size 214 bp; H4R (Genebank access No: NM_021624) (5′‐GGC TCA CTA CTG ACT ATC TG‐3′), (5′‐CCT TCA TCC TTC CAA GAC TC‐3′), fragment size 197 bp. PCR products were loaded on 1% agarose gel to check the specificity of the reactions. Comparison of two different runs with the same primer pair showed strong reproducibility (correlation coefficient rs = 0.91, p<0.001, n = 76 for example with GAPDH primers). Real time PCR data were expressed as a ratio of GAPDH mRNA expression.
Western blot analysis
Tissue samples were homogenised in extraction buffer containing 25 mM Tris‐HCl, pH 7.5, 0.5 mM EDTA, 0.5 mM EGTA, 0.05% Triton X‐100, supplemented with the protease inhibitor cocktail Complete Mini (Roche Diagnostics, Basel, Switzerland). Protein concentration in the homogenates was determined using Bio‐Rad protein assay (Bio‐Rad, Hercules, California, USA). Protein extracts (10–20 μg protein each) were separated on a 12% sodium dodecyl sulphate (SDS)‐polyacrylamide gel and blotted onto a nitrocellulose membrane (Schleicher and Schuell, Einbeck, Germany) in 0.1% SDS, 20% methanol, 400 mmol/l glycine, and 50 mmol/l Tris‐HCl, pH 8.3, 300 mA by electroblotting using Trans‐Blot Cell (Bio‐Rad). Membranes were blocked with 5% skim milk in phosphate buffered saline containing 0.025% Tween for one hour. Membranes were probed with anti‐H1R (Alpha Diagnostic International, San Antonio, Texas, USA; rabbit polyclonal antibody recognises a peptide highly conserved in humans, fish, and monkeys, but no other HR), anti‐H2R (Alpha Diagnostic International; rabbit polyclonal antibody recognises a peptide highly conserved in humans and fish, but no other HR), anti‐H3R (Acris Antibodies GmbH, Hiddenhausen, Germany; rabbit polyclonal antibody specific to the human H3R), or with anti‐H4R (Alpha Diagnostic International; rabbit polyclonal antibody specific to the human H4R). The bands were visualised using an electrochemiluminescence detection system, as described by the manufacturer (NEN Life science, Boston, Massachusetts, USA).
Immunohistochemistry and immunofluorescence
Tissue samples of 20 gut resectates (four each of duodenum, ileum, colon, sigma, and rectum) were fixed in paraformaldehyde 4%, embedded in paraffin wax, and sectioned at 4 μm. For immunostaining, a Histostain Plus Broad spectrum (AEC) kit (Zymed Laboratories Inc., San Francisco, California, USA) was used following the manufacturer's instructions. Sections were incubated overnight at 4°C with the following primary antibodies (all from Acris Antibodies GmbH) at these concentrations: H1R rabbit polyclonal antibody at 10 μg/ml, H2R rabbit polyclonal antibody at 5 μg/ml, H3R rabbit polyclonal antibody at 10 μg/ml, and H4R rabbit polyclonal antibodies at 10 μg/ml; protein gene product 9.5 (PGP 9.5) sheep polyclonal antibody (cross reacts with most mammals) was used at a dilution of 1:100. In addition, tyrosine hydroxylase mouse monoclonal antibody (Immunostar Inc., Hudson, Wisconsin, USA; cross reacts with most mammals) was used at a dilution of 1:1000. Secondary antibodies were supplied with the Histostain Plus kit and used according to the manufacturer's instructions, except for rabbit antisheep biotinylated antibodies (Zymed Laboratories Inc.) which were used at a concentration of 15 μg/ml for 30 minutes. For counterstaining, sections were exposed to hemalaun for 10 seconds.
For immunofluorescence, sections were blocked in 10% horse native serum (HNS) for 30 minutes and the primary antibodies were added at the concentrations given above together with 5% HNS overnight at room temperature. The following secondary antibodies were added for 30 minutes at room temperature: Alexa Fluor goat antimouse 488 (3 μg/ml), Alexa Fluor donkey antirabbit 594 (3 μg/ml), and Alexa Fluor donkey antisheep 488 (3 μg/ml). Alexa Fluor streptavidin 594 (3 μg/ml) was used for detection of biotinylated rabbit antisheep. Confocal fluorescence microscopy was performed using a LSM 510 META microscope (Carl Zeiss AG, Oberkochen, Germany).
Cells and cell culture
Isolation of LPMC
LPMC were isolated from the intestinal mucosa of surgical specimen by mechanical and enzymatic digestion, as described elsewhere.17 The cell suspension gathered from the isolation procedure was then separated on Ficoll‐Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradients. Cells were washed in phosphate buffered saline and resuspended in RPMI 1640 containing 10% fetal calf serum and 50 ng/ml interleukin 2 at a density of 5×105/ml.
Isolation of human intestinal MC
Human intestinal MC were isolated from surgical tissue specimens. The methods of mechanical and enzymatic tissue dispersion yielding single cell preparations containing 4 (2)% (mean (SD)) MC have been described in detail elsewhere.16 After overnight incubation in culture medium (RPMI 1640 supplemented with 10 % heat inactivated fetal calf serum, 25 mM HEPES, 2 mM glutamine, 100 μg/ml streptomycin, 100 μg/ml gentamycin, 100 U/ml penicillin, and 0.5 μg/ml amphotericin; all cell culture reagents were from Gibco Invitrogen, Paisley, UK), MC were enriched by positive selection of c‐kit expressing cells using magnetic cell separation (MACS system; Miltenyi Biotech, Bergisch‐Gladbach, Germany) and the monoclonal antibody YB5.B8 (Pharmingen, Hamburg, Germany), as described previously.16 The fraction containing the c‐kit positive cells (mast cell purity 60 (25)%) was cultured at a density of 2×105 MC/ml for up to 28 days in medium supplemented with 50 ng/ml of recombinant human stem cell factor (Amgen, Thousand Oaks, California, USA) until a purity of 97–100% MC was achieved.
Isolation and culture of human intestinal FB
Human intestinal FB were isolated from surgical specimens from patients undergoing bowel resection. Isolation and culture methods have been described in detail recently.18 FB preparations of at least 95% purity where used for mRNA extraction and subsequent expression studies.
Isolation of PBMC
Blood from five healthy donors was separated on Ficoll‐Hypaque gradients. The ring fraction was washed and subjected to mRNA isolation.
Isolation of human umbilical vein endothelial cells
Human umbilical vein endothelial cells were isolated from human cords, as described previously.19 Cells were harvested when a confluent monolayer was achieved and subjected to RNA isolation.
Culture of MHH‐NB‐11
This human neuroblastoma cell line was purchased from the DSZM (Braunschweig, Germany). Cells were cultured in medium containing 90% RPMI 1640 plus 10% fetal bovine serum (Biochrom AG, Berlin, Germany) and 2 ml L‐glutamine + MEM non‐essential amino acids. Adherent cells were harvested at a density of 106 cells/80 cm2 and RNA was extracted.
Statistical analysis
Statistical analysis was performed using an unpaired (Mann‐Whitney) two tailed, non‐parametric test; p values less than 0.05 were considered significant.
Results
HR mRNA expression in the human intestinal tract
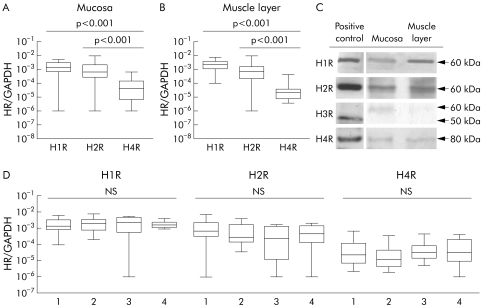
Analysis of surgical specimens of 33 patients revealed that H1R, H2R, and H4R were expressed in the human gastrointestinal tract (fig 1A, B); H3R was absent in most samples but was detected at low levels in five of the 66 samples (8.3%, one mucosa/submucosa and four muscle layer samples, from four different patients, data not shown). HR mRNA proved to be equally distributed between the mucosa/submucosa and muscular layer (fig 1A, B). Overall mRNA expression of HR, displayed as the HR‐GAPDH ratio, was low in intestinal samples compared with, for example, H2R expression in gastric mucosa (n = 2; H2R/GAPDH ratio median 2.4×10−2) or H1R expression in umbilical veins (n = 3; H1R/GAPDH ratio median 1.3×10−1) used as positive controls (data not shown). H1R and H2R were expressed at higher levels than H4R, both in the mucosa and in the muscular layer (p<0.001). H1R, H2R, and H4R expression was confirmed at the protein level by western blot analysis (fig 1C). There were no substantial differences in HR mRNA expression at different anatomical sites in the intestine (fig 1D).
Figure 1 Histamine receptor (HR) mRNA expression in 66 human intestinal tissue samples taken from surgical specimens from 33 individuals. Data were generated by quantitative reverse transcription‐polymerase chain reaction and are displayed as glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH)/HR ratio (p values as indicated). (A, B) HR expression in the mucosa/submucosa (A) and the muscular layer (B). H3R was not detected in >90% of samples. (C) Protein expression of HR in intestinal tissue homogenates, shown by immunoblot. Mucosa and muscular layer samples of colonic tissue were probed with H1R, H2R, and H4R antibodies. Human umbilical vein endothelial cells, gastric mucosa homogenate, human neuroblastoma cells MHH‐NB 11, and purified human intestinal mast cells were used as positive controls for H1R, H2R, H3R, and H4R, respectively. As indicated, H3R antibodies detected an unspecific protein band of approximately 60 kDa in lysates of human colon while a specific band migrated at the expected weight of 50 kDa. (D) Comparison of HR expression at different intestinal sites: 1 = duodenum, 2 = colon, 3 = sigmoid, 4 = rectum.
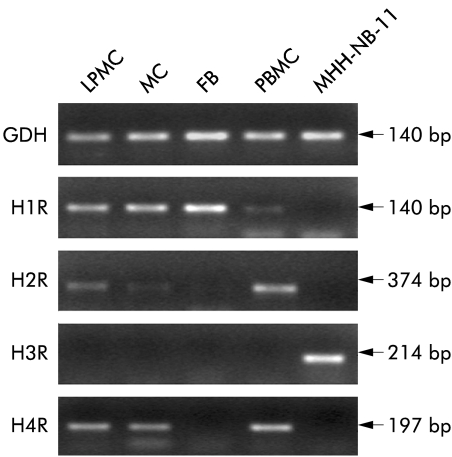
When analysing distinct cell populations of the human intestinal mucosa—namely LPMC, MC, and FB—we found different patterns of HR expression (fig 2). LPMC, the fraction of cells consisting of lymphocytes, macrophages, and dendritic cells, mainly expressed H1R, H2R, and H4R while H3R was absent. Mucosal MC expressed H1R, H2R, and H4R also, but not H3R. FB, representing connective tissue cells of the intestine found to interact with MC,18 expressed exclusively H1R at high levels.
Figure 2 Histamine receptor (HR) mRNA expression in lamina propria mononuclear cells (LPMC), mast cells (MC), and fibroblasts (FB) isolated and purified from surgical specimens of normal intestinal tissue. Peripheral blood mononuclear cells (PBMC) expressing H1R, H2R, and H4R and the human neuroblastoma cell line MHH‐NB‐11 expressing H3R were used as controls. One representative experiment out of five is shown.
Histological analysis of human intestinal tissue
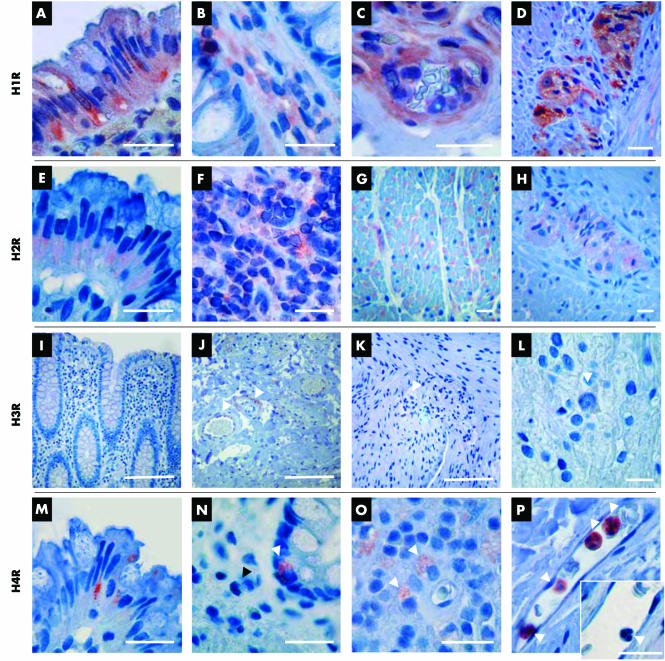
In order to analyse expression of HR in further detail, immunostaining of paraffin embedded human small and large intestine was performed. The staining pattern of H1R, H2R, and H4R corresponded to mRNA expression. H1R and H2R were both found to be ubiquitously expressed throughout the gut wall. Enterocytes as well as connective tissue cells, immune cells, blood vessels, myocytes, and enteric nerves were all shown to express H1R (fig 3A–D). H2R was also found to be expressed on enterocytes, although staining appeared less pronounced, and on immune cells, such as lymphocytes in Payer's patches (fig 3E, F). Myenteric ganglia along with smooth muscle cells also stained positive for H2R (fig 3G, H).
Figure 3 Immunohistochemical staining of the human large and small intestine. Bars indicate 20 μm, except in I–K where bars indicate 100 μm. (A‐D) Histamine 1 receptor (H1R) expression in the (A) epithelium, (B) cells of the lamina propria, (C) small submucosal blood vessel, and (D) myenteric nerve plexus located between the circular and longitudinal muscle layers. (E–H) Histamine 2 receptor (H2R) expression in the (E) epithelium, (F) Payer's patch, (G) smooth muscle in the longitudinal muscle layer, and (H) myenteric nerve plexus. (I‐L) Histamine 3 receptor (H3R) expression in the (I) colonic mucosa (epithelium and lamina propria), no positive cells, (J) submucosa, unspecific staining of fibres in and around the walls of blood vessels (arrowheads), (K) muscular layers and myenteric plexus (arrowhead), and (L) ganglion cells of the myenteric plexus (arrowhead). (M–P) Histamine 4 receptor (H4R) expression: (M) granular staining of H4R in enterocytes at the tip of a crypt of Lieberkühn, (N) H4R positive cell with morphological characteristics of a neuroendocrine cell (white arrowhead), no staining for H4R on a leucocyte in the lamina propria (black arrowhead), (O) mononuclear cells in the lamina propria, probably macrophages (arrowheads), and (P) pronounced staining of intravascular granulocytes (arrowheads); the isotype control is negative (arrowhead in the small image), excluding unspecific peroxidase reactions.
Only a few cells located within the human intestinal tissue expressed H4R (fig 3M–P). Mainly leucocytes inside the small mucosal and submucosal blood vessels were stained positive and exhibited a much stronger reactivity against H4R compared with tissue resident leucocytes (fig 3N, P). Moreover, intraepithelial cells with typical morphological characteristics of neuroendocrine cells (fig 3N) stained positive for H4R. Finally, enterocytes at the apical end of the crypts of Lieberkühn were found to be H4R positive (fig 3M).
Human intestinal tissue in general, and the ENS in particular, is devoid of H3R
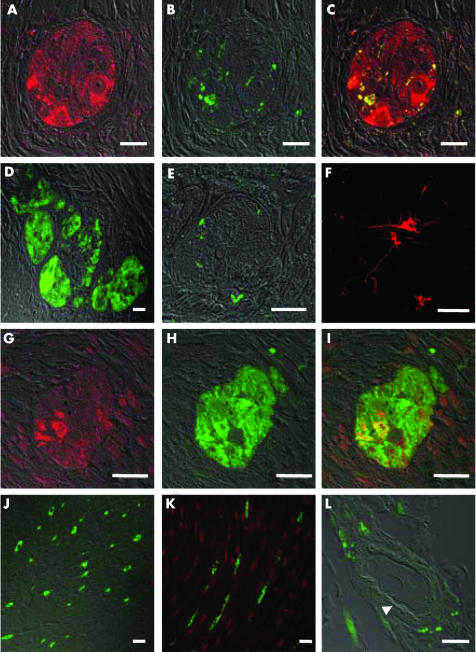
Confirming the mRNA expression data, H3R antibodies did not stain any cellular structures in human intestinal tissue. PGP 9.5, a pan‐neurone marker, was used to trace nerves within the gut wall in order to find out if such cells express H3R. Immunofluorescence double staining with anti‐H3R and PGP 9.5 antibodies in the human intestine revealed no co‐localisation of the two antigens (fig 4D, J), indicating that intestinal nerve fibres do not express H3R. Sympathetic nerve terminals, which have been reported to express H3R in guinea pigs, were specifically identified by anti‐tyrosine hydroxylase staining. However, they were also found to be devoid of H3R (fig 4E). In comparison, neurones in human brain cortex, included as positive controls, exhibited strong H3R expression (fig 4F). Notably, H3R antibodies exhibited some cross reactivity to perivascular fibres (fig 3J); however, employment of high resolution confocal microscopy revealed that H3R staining was nearly absent, once highly autofluorescent structures such as elastic fibres in blood vessels were subtracted (fig 4L). In contrast, H1R and PGP 9.5 double staining of myenteric ganglia showed a high degree of co‐localisation, indicating that enteric nerves indeed express H1R, but not H3R (fig 4). We therefore conclude that, despite contradictory findings from guinea pig studies, human large and small intestine does not express H3R under normal conditions.
Figure 4 Immunofluorescence staining of human intestinal tissue and human brain cortex, examined by confocal microscopy. (A–C) Myenteric ganglion cells in human colon stained with PGP 9.5 (A, red), tyrosine hydroxylase (B, green), or both (C—(A) and (B) merged). (D) PGP 9.5 (green) and histamine 3 receptor (H3R) (red) double staining of a myenteric ganglion in human colon; no H3R staining was observed. (E) Tyrosine hydroxylase (green) and H3R (red) double staining of a myenteric ganglion in human colon; sympathetic nerve endings did not express H3R. (F) H3R staining (red) of neurones in human brain cortex. (G–I) Myenteric ganglion cells in human colon stained with anti‐H1R antibody (G, red), PGP 9.5 (H, green), or both (I—(G) and (H) merged). Note the prominent co‐localisation of H1R and PGP 9.5 staining. (J) PGP 9.5 (green) and H3R (red) staining of the circular muscle layer; nerve fibres (green) are devoid of H3R. (K) PGP 9.5 (green) and H1R (red) staining of the circular muscle layer; H1R was expressed on smooth muscle but not on nerve fibres. (L) PGP 9.5 (green) and H3R (red) staining of a submucosal blood vessel; after subtraction of unspecific autofluorescence there remained a faint red staining of a circular structure (arrowhead), reminiscent of the basal lamina.
Elevated HR expression in patients with FA or IBS
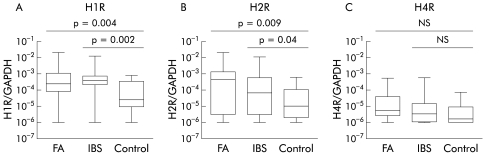
HR expression was examined in biopsies from patients with IBS and FA, respectively. Biopsies were taken from the terminal ileum, caecum, and rectum. As there were no substantial differences between the different anatomical regions, the results are presented cumulatively. Both patients with IBS and FA exhibited significantly higher levels of mRNA encoding for H1R and H2R in comparison with controls (fig 5A, B). Levels of H4R mRNA were not significantly changed in biopsies of patients compared with controls (fig 5C). H3R mRNA could only be detected in two samples (one IBS and one control patient), suggesting that this receptor is not upregulated in these disorders.
Figure 5 (A–C) Histamine 1 receptor (H1R) mRNA expression (A), H2R mRNA expression (B), and H4R mRNA expression (C) in biopsy samples from patients with irritable bowel syndrome (IBS) tested positive for food allergy (FA) (n = 19), tested negative for FA (n = 11), and from control subjects (n = 14), measured by quantitative reverse transcription‐polymerase chain reaction. H3R mRNA was only detected in two samples. (Methods and data presentation as in fig 1.)
Discussion
The present study shows for the first time that H1R, H2R, and H4R are expressed in the human gastrointestinal tract. In contrast, no H3R mRNA expression was seen in the normal human intestine, which was further confirmed at the protein level. The expression pattern of HR did not change at different anatomical sites along the intestine. Expression and distribution of HR in the human or animal gastrointestinal tract have not been examined in detail. However, the existence of HR in the gastrointestinal tract was anticipated as functional and pharmacological experiments in guinea pigs and other species revealed that histamine, acting through particular receptors, regulates multiple intestinal functions, such as mucosal secretion8,9 and motility.4,6,7 We show here that H1R and H2R are indeed expressed throughout the human gastrointestinal tract. To the best of our knowledge, expression of H1R and H2R on ganglion cells of the myenteric plexus has not been shown in humans to date, and the functional implications still need to be defined.
H4R was discovered five years ago and found to be expressed on leucocytes and in bone marrow.20,21 High expression was described in the spleen and liver,20 while rather low expression in other peripheral organs was reported, which is consistent with the results presented in our study. H4R mRNA levels were significantly lower compared with H1R and H2R mRNA levels. The immunohistochemical approach revealed that leucocytes inside blood vessels were highly positive for H4R, while tissue cells expressed H4R at lower levels. Recent reports have described a chemotactic property of histamine binding to H4R, enhancing leucocyte migration and recruitment from bone marrow.22,23,24,25 This observation could tempt speculation that H4R are downregulated after migration of leucocytes from the blood stream into the tissue. Interestingly, enterocytes stained positive for H4R at the apical tip of the crypts of Lieberkühn. According to epithelial cell dynamics, enterocytes migrate from the base of the crypts to the tip of the villi (or crypts in the colon) where they are fully mature before they undergo apoptosis. Thus functionality of H4R on these cells has to be questioned. Pronounced staining of cells with typical morphological characteristics of neuroendocrine cells was surprising, and it will be exciting to investigate possible histaminergic effects on these cells.
H3R, expressed predominantly on neurones, has been shown to function as an autoreceptor, inhibiting synthesis and release of histamine26 and other neurotransmitters.27,28 The effects of H3R activation on the ENS have been the subject of numerous studies in guinea pigs. These experiments revealed involvement of this receptor in the control of contractile responses of the intestine, mainly by modulating transmitter release from enteric neurones.5,12,29 Surprisingly, despite the fact that intestinal tissue contains neurones in considerable amounts (fig 4),30 we found no H3R mRNA expression in the human intestine. Specific staining of enteric nerves in the tissue revealed that H3R was also virtually absent at the protein level. It had been repeatedly proposed that H3R controls transmitter release from sympathetic nerve terminals. We double stained for tyrosine hydroxylase and H3R but found no co‐localisation. As mentioned before, cross reactivity of H3R to non‐neurogenic fibres occurred within circular structures in the walls of blood vessels. These were totally devoid of PGP 9.5 or tyrosine hydroxylase staining and showed a high degree of unspecific autofluorescence, as detected by confocal microscopy. Moreover, immunoblot staining revealed staining of unspecific protein bands in tissue lysates (fig 1C). Our data are in accordance with previous studies reporting a lack of H3R mRNA and function in human tissues.10,11,31 Thus substantial interspecies differences with regard to HR expression in the intestine and pharmacological properties of the different HR have to be considered.11,32,33
It is well known that histamine is involved in the regulation of intestinal secretion and motility, processes that, when dysregulated, cause clinical symptoms such as diarrhoea and abdominal pain.34 Therefore, we were interested to know if HR expression might be altered in patients with gastrointestinal diseases. We found that mRNA for H1R and H2R was significantly upregulated in patients with IBS with and without FA, which may be related to the IBS‐like symptoms these patients are suffering from. Indeed, Wantke et al have described a spectrum of intestinal symptoms that can be evoked by histamine in selected individuals.35 This histamine intolerance was attributed to impaired histamine metabolism.36,37 Alternatively, such symptoms could be a result of elevated HR expression, as found here in patients with IBS and FA which, similar to reduced histamine degradation, may cause histamine hypersensitivity. This hypothesis needs to be confirmed by showing that not only FA but also IBS and other gastrointestinal diseases characterised by secretory and motility disorders improve by treatment with antihistamines or a histamine free diet.
In summary, we have shown that H1R, H2R, and H4R are present in the human gastrointestinal tract and that expression of H1R and H2R is upregulated in patients with FA or IBS. In contrast with previous studies in guinea pigs, we conclude that H3R are not expressed in the human intestine.
Acknowledgements
The authors would like to express their thanks to Gisela Weier for excellent technical assistance with regards to the histological part of the study. This work was supported by the Deutsche Forschungsgemeinschaft (SFB621‐A8 to SCB).
Abbreviations
ENS - enteric nervous system
FA - food allergy
FB - fibroblasts
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
HNS - horse native serum
HR - histamine receptor
IBS - irritable bowel syndrome
LPMC - lamina propria mononuclear cells
MC - mast cells
PBMC - peripheral blood mononuclear cells
PGP - protein gene product
RT‐PCR - reverse transcription‐polymerase chain reaction
SDS - sodium dodecyl sulphate
Footnotes
Conflict of interest: None declared.
References
- 1.Barger G, Dale H H. Chemical structure and sympathomimetic actions of amines. J Physiol Pharmacol 19104119–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka S, Hamada K, Yamada N.et al Gastric acid secretion in L‐histidine decarboxylase‐deficient mice. Gastroenterology 2002122145–155. [DOI] [PubMed] [Google Scholar]
- 3.Tari A, Yamamoto G, Sumii K.et al Roles of histamine 2 receptor in increased expression of rat gastric H+‐K+ ATPase‐alpah‐subunit induced by omeprazole. Am J Physiol 1993265G752–G758. [DOI] [PubMed] [Google Scholar]
- 4.Bennett A, Whitney B. A pharmacological study of the motility of the human gastrointestinal tract. Gut 19667307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertaccini G, Coruzzi G. An update on histamine H3 receptors and gastrointestinal functions. Dig Dis Sci 1995402052–2063. [DOI] [PubMed] [Google Scholar]
- 6.Bolton T B, Clark J P, Kitamura K.et al Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea‐pig ileum. J Physiol 1981320363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harry J. The action of drugs on the circular muscle strip from guinea‐pig isolated ileum. Br J Pharmacol 196320399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly S J, Stack W A, O'Donoghue D P.et al Regulation of ion transport by histamine in human colon. Eur J Pharmacol 199512203–209. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y Z, Cooke H J, Su H C.et al Histamine augments colonic secretion in guinea pig distal colon. Am J Physiol 1990258G432–G439. [DOI] [PubMed] [Google Scholar]
- 10.Hemedah M, Loiacono R, Coupar I M.et al Lack of evidence for histamine H3 receptor function in rat ileum and human colon. Naunyn Schmiedebergs Arch Pharmacol 2001361133–138. [DOI] [PubMed] [Google Scholar]
- 11.Lovenberg T W, Roland B L, Wilson S J.et al Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol 2000551101–1107. [PubMed] [Google Scholar]
- 12.Blandizzi C, Tognetti M, Colucci R.et al Histamine H(3) receptors mediate inhibition of noradrenaline release from intestinal sympathetic nerves. Br J Pharmacol 20001291387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jutel M, Watanabe T, Akdis M.et al Immune regulation by histamine. Curr Opin Immunol 200214735–740. [DOI] [PubMed] [Google Scholar]
- 14.Atkins F M. Intestinal mucosal mast cells. Ann Allergy 19875944–53. [PubMed] [Google Scholar]
- 15.Bischoff S C, Mayer J, Wedemeyer J.et al Colonoscopic allergen provocation (COLAP): a new diagnostic approach for gastrointestinal food allergy. Gut 199740745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff S C, Sellge G, Lorentz A.et al IL‐4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci U S A 1999968080–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonsky R, Deem R L, Young H A.et al CD2 mediates activation of the IFN‐gamma intronic STAT binding region in mucosal T cells. Eur J Immunol 2003331152–1162. [DOI] [PubMed] [Google Scholar]
- 18.Sellge G, Lorentz A, Gebhardt T.et al Human intestinal fibroblasts prevent apoptosis in human intestinal mast cells by a mechanism independent of stem cell factor, IL‐3, IL‐4, and nerve growth factor. J Immunol 2004172260–267. [DOI] [PubMed] [Google Scholar]
- 19.Mierke C T, Ballmaier M, Werner U.et al Human endothelial cells regulate survival and proliferation of human mast cells. J Exp Med 2000192801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coge F, Guenin S P, Rique H.et al Structure and expression of the human histamine H4‐receptor gene. Biochem Biophys Res Commun 2001284301–309. [DOI] [PubMed] [Google Scholar]
- 21.Oda T, Morikawa N, Saito Y.et al Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem 200027536781–36786. [DOI] [PubMed] [Google Scholar]
- 22.Ling P, Ngo K, Nguyen S.et al Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol 2004142161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama T, Kato Y, Hieshima K.et al Liver‐expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J Immunol 20041732078–2083. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita K, Sakai K, Bacon K.et al Critical role of histamine H4 receptor in leukotriene B4 production and mast cell dependent neutrophil recruitment induced by zymosan. J Pharmacol Exp Ther 20033071072–1078. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita K, Bacon K B, Gantner F. Critical role of L‐selectin and histamine H4 receptor in zymosan‐induced neutrophil recruitment from the bone marrow: comparison with carrageenan. J Pharmacol Exp Ther 2004310272–280. [DOI] [PubMed] [Google Scholar]
- 26.Arrang J M, Garbarg M, Schwartz J C. Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983302832–837. [DOI] [PubMed] [Google Scholar]
- 27.Schlicker E, Fink K, Hinterthaner M.et al Inhibition of noradrenaline release in the rat brain cortex via presynaptic H3 receptors. Naunyn Schmiedebergs Arch Pharmacol 1989340633–638. [DOI] [PubMed] [Google Scholar]
- 28.Schlicker E, Fink K, Detzner M.et al Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J Neural Transm Gen Sect 1993931–10. [DOI] [PubMed] [Google Scholar]
- 29.Blandizzi C, Tognetti M, Colucci R.et al H3 receptor‐mediated inhibition of intestinal acetylcholine release: pharmacological characterization of signal transduction. Naunyn Schmiedebergs Arch Pharmacol 2001363193–202. [DOI] [PubMed] [Google Scholar]
- 30.Wood J D. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 2004127635–657. [DOI] [PubMed] [Google Scholar]
- 31.Pozzoli C, Poli E, Costa A.et al Absence of histamine H3‐receptors in the rabbit colon: species difference. Gen Pharmacol 199729217–221. [DOI] [PubMed] [Google Scholar]
- 32.Ireland‐Denny L, Parihar A S, Miller T R.et al Species‐related pharmacological heterogeneity of histamine H(3) receptors. Eur J Pharmacol. 2001;21433141–150. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Wilson S J, Kuei C.et al Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther 2001299121–130. [PubMed] [Google Scholar]
- 34.Barbara G, Stanghellini V, De Giorgio R.et al Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004126693–702. [DOI] [PubMed] [Google Scholar]
- 35.Wantke F, Gotz M, Jarisch R. Histamine‐free diet: treatment of choice for histamine‐induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 199323982–985. [DOI] [PubMed] [Google Scholar]
- 36.Kufner M A, Ulrich P, Raithel M.et al Determination of histamine degradation capacity in extremely small human colon samples. Inflamm Res 200150(suppl 2)S96–S97. [DOI] [PubMed] [Google Scholar]
- 37.Wantke F, Gotz M, Jarisch R. The red wine provocation test: intolerance to histamine as a model for food intolerance. Allergy Proc 19941527–32. [DOI] [PubMed] [Google Scholar]